Effects of Dietary Fibre from the Traditional Indonesian Food, Green Cincau (Premna oblongifolia Merr.) on Preneoplastic Lesions and Short Chain Fatty Acid Production in an Azoxymethane Rat Model of Colon Cancer
Abstract
:1. Introduction
2. Results
2.1. Body and Liver Weight Changes, Food and Water Intake
2.2. Short Chain Fatty Acids (SCFAs)
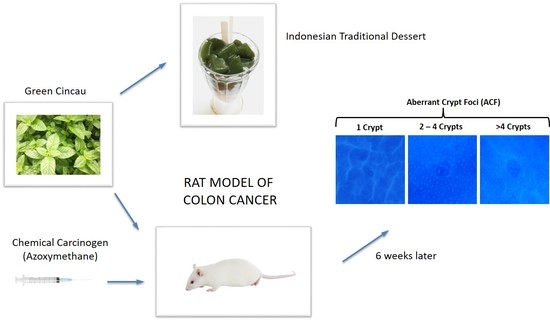
2.3. Aberrant Crypt Foci (ACF) Formation
2.4. Cell Proliferation in Distal Colon
2.5. Lipid Peroxidation in Liver
2.6. Microbial Profile of the Colon Digesta
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Green Cincau Leaf Preparation
4.3. Animals and Diet
4.4. Determination of SCFA Composition of Faecal and Caecal Digesta Samples
4.5. Measuring ACF Number and Multiplicity
4.6. Proliferating Cell Nuclear Antigen (PCNA) Staining
4.7. Measuring Lipid Peroxidation in Rat Liver
4.8. Measuring Faecal Bacterial Community
4.8.1. Extraction of Bacterial DNA from Rat Faecal Samples
4.8.2. Bacterial 16S rDNA Amplification
4.8.3. Denaturing Gradient Gel Electrophoresis (DGGE)
4.8.4. Excision, Cloning, and Sequencing of Selected Bands from DGGE Gels
4.9. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Aberrant crypt foci |
| AOM | Azoxymethane |
| CRC | Colorectal cancer |
| DDGE | Denaturing gradient gel electrophoresis |
| EGCG | Epigallocatechin-3-gallate |
| TBARS | Thiobarbituric acid reactive substances |
| SCFAs | Short chain fatty acids |
References
- Center, M.M.; Jemal, A.; Ward, E. International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A.; Smith, R.A.; Ward, E. Worldwide variations in colorectal cancer. CA Cancer J. Clin. 2009, 59, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Gingras, D.; Beliveau, R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011, 4, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; de Boeck, G.; Becker, K. Dietary roles of non-starch polysachharides in human nutrition: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit and cancer. II. Mechanisms. Cancer Causes Control 1991, 2, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Siersema, P.D.; Overvad, K.; Tjonneland, A.; Olsen, A.; Boutron-Ruault, M.C.; Bastide, N.; Fagherazzi, G.; Katzke, V.; Kuhn, T.; et al. Subtypes of fruit and vegetables, variety in consumption and risk of colon and rectal cancer in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2015, 137, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the prostate, lung, colorectal, and ovarian cancer screening trial. Am. J. Clin. Nutr. 2015, 102, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, M.; Yang, F.G.; Cui, L.H.; Lu, X.Y.; Chen, C. Dietary fibre and the risk of colorectal cancer: A case—control study. Asian Pac. J. Cancer Prev. 2015, 16, 3747–3752. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Perez-Jimenez, J.; Tourino, S.; Serrano, J.; Fuguet, E.; Torres, J.L.; Goni, I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol. Nutr. Food Res. 2010, 54, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, S.U.; Zuidar, S.A.; Suharyono. Dried extract from green cincau leaves as potential fibre sources for food enrichment. Afr. Crop Sci. J. 2005, 7, 655–658. [Google Scholar]
- Nurdin, S.U.; Hwang, J.K.; Hung, P. Effect of green cincau leaf (Premna oblongifolia Merr.) water extracts on cytokines production in whole cell culture of mouse splenocytes. Indones. Food Nutr. Prog. 2003, 10, 70–74. [Google Scholar]
- Nurdin, S.U. Evaluation of laxative effect and fermentability of gel forming component of green cincau leaves (Premna oblongifolia Merr.). Teknologi dan Industri Pangan 2007, 18, 10–16. [Google Scholar]
- Nurdin, S.U.; Le Leu, R.; Young, G.P.; Stangoulis, J.C.; Christophersen, C.T.; Abbott, C.A. Analysis of the anti-cancer effects of cincau extract (Premna oblongifolia Merr.) and other types of non-digestible fibre using faecal fermentation supernatants and caco-2 cells as a model of the human colon. Nutrients 2017, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Jimenez, A.; Guillen, R. Composition of plant cell walls. Z. Lebensmittel Untersuch. Forsh. 1995, 200, 24–31. [Google Scholar] [CrossRef]
- Rao, C.V.; Chou, D.; Simi, B.; Ku, H.; Reddy, B.S. Prevention of colonic aberrant crypt foci and modulation of large bowel microbial activity by dietary coffee fiber, inulin and pectin. Carcinogenesis 1998, 19, 1815–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vince, A.J.; McNeil, N.I.; Wager, J.D.; Wrong, O.M. The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br. J. Nutr. 1990, 63, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, U.; Nyman, M.; Ahrne, S.; Sullivan, E.O.; Fitzgerald, G. Bifidobacterium lactis Bb-12 and Lactobacillus salivarius UCC500 modify carboxylic acid formation in the hindgut of rats given pectin, inulin, and lactitol. J. Nutr. 2006, 136, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Morris, A.P.; Kourouma, F.; Sellin, J.H. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif. 2003, 36, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Otani, S.; Nanjo, F. Antioxidative activity of microbial metabolites of (−)-epigallocatechin gallate produced in rat intestines. Biosci. Biotechnol. Biochem. 2011, 75, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Shimizu, M.; Tsurumi, H.; Hara, Y.; Tanaka, T.; Moriwaki, H. EGCG and polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DSS. Mol. Med. Rep. 2008, 1, 355–361. [Google Scholar] [PubMed]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin gallate hinders human hepatoma and colon cancer sphere formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ren, G.; Xu, X.; Yuan, H.; Wang, Z.; Kang, L.; Yu, W.; Tian, K. Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kishimoto, Y.; Miura, N.; Shiota, G.; Kohri, T.; Hara, Y.; Hasegawa, J.; Isemura, M. Synergistic effects of (−)-epigallocatechin gallate with sulindac against colon carcinogenesis of rats treated with azoxymethane. Cancer Lett. 2002, 177, 49–56. [Google Scholar] [CrossRef]
- Hu, Y.; McIntosh, G.H.; Le Leu, R.K.; Nyskohus, L.S.; Woodman, R.J.; Young, G.P. Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PLoS ONE 2013, 8, e64362. [Google Scholar] [CrossRef] [PubMed]
- Caderni, G.; De Filippo, C.; Luceri, C.; Salvadori, M.; Giannini, A.; Biggeri, A.; Remy, S.; Cheynier, V.; Dolara, P. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in f344 rats. Carcinogenesis 2000, 21, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Ghosh, S.; Das, S. Tea can protect against aberrant crypt foci formation during azoxymethane induced rat colon carcinogenesis. J. Exp. Clin. Cancer Res. 2003, 22, 185–191. [Google Scholar] [PubMed]
- Carter, O.; Dashwood, R.H.; Wang, R.; Dashwood, W.M.; Orner, G.A.; Fischer, K.A.; Lohr, C.V.; Pereira, C.B.; Bailey, G.S.; Williams, D.E. Comparison of white tea, green tea, epigallocatechin-3-gallate, and caffeine as inhibitors of PhiP-induced colonic aberrant crypts. Nutr. Cancer 2007, 58, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Duncan, S.H.; Louis, P.; Flint, H.J. Nutritional influences on the gut microbiota and the consequences for gastrointestinal health. Biochem. Soc. Trans. 2011, 39, 1073–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Bellion, P.; Hofmann, T.; Pool-Zobel, B.L.; Will, F.; Dietrich, H.; Knaup, B.; Richling, E.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of phenolic apple juice extracts and their gut fermentation products in the human colon carcinoma cell line caco-2. J. Agric. Food Chem. 2008, 56, 6310–6317. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, M.; Calani, L.; Tedeschi, M.; Jechiu, L.; Brighenti, F.; Del Rio, D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition 2012, 28, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrom, S. Improved bioavailability of dietary phenolic acids in whole grain barley and oat groat following fermentation with probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Pinto, S. Protein thiols and thiobarbituric acid reactive substance status in colon cancer patients. Scand. J. Gastroenterol. 2007, 42, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, P.; Ramanathan, M. Effect of kaempferol on lipid peroxidation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur. J. Pharmacol. 2011, 654, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Giftson, J.S.; Jayanthi, S.; Nalini, N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Investig. New Drugs 2010, 28, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Iino, T.; Mori, K.; Tanaka, K.; Suzuki, K.; Harayama, S. Oscillibacter valericigenes gen. nov., sp. nov., a valerate-producing anaerobic bacterium isolated from the alimentary canal of a Japanese Corbicula clam. Int. J. Syst. Evol. Microbiol. 2007, 57, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Sgouras, D.N.; Panayotopoulou, E.G.; Martinez-Gonzalez, B.; Petraki, K.; Michopoulos, S.; Mentis, A. Lactobacillus johnsonii La1 attenuates helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin. Diagn. Lab. Immunol. 2005, 12, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Bohak, I.; Back, W.; Richter, L.; Ehrmann, M.; Ludwig, W.; Schleifer, K.H. Lactobacillus amylolyticus sp. nov., isolated from beer malt and beer wort. Syst. Appl. Microbiol. 1998, 21, 360–364. [Google Scholar] [CrossRef]
- Murray, W.D.; Khan, A.W.; van den Berg, L. Clostridium saccharolyticum sp. Nov., a saccharolytic species from sewage sludge. Int. J. Syst. Evol. Microbiol. 1982, 32, 132–135. [Google Scholar] [CrossRef]
- Jang, Y.S.; Woo, H.M.; Im, J.A.; Kim, I.H.; Lee, S.Y. Metabolic engineering of clostridium acetobutylicum for enhanced production of butyric acid. Appl. Microbiol. Biotechnol. 2013, 97, 9355–9363. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.M.; Young, G.P.; Topping, D.L.; Bird, A.R.; Cobiac, L.; Scherer, B.L.; Winkler, J.G.; Lockett, T.J. Butyrate delivered by butyrylated starch increases distal colonic epithelial apoptosis in carcinogen-treated rats. Carcinogenesis 2012, 33, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Esterman, A.; Young, G.P. Suppression of azoxymethane-induced colon cancer development in rats by dietary resistant starch. Cancer Biol. Ther. 2007, 6, 1621–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velmurugan, B.; Singh, R.P.; Agarwal, R.; Agarwal, C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol. Carcinog. 2010, 49, 641–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, R.A.; Abdul Hamid, A.; Mohamed, S.; Bakar, F.A. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Torres, C.; Diaz-Maroto, M.C.; Hermosin-Gutierrez, I.; Perez-Coello, M.S. Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal. Chim. Acta 2010, 660, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Cieslik, E.; Filipiak-Florkiewicz, A.; Leszczynska, T. Effect of hydrothermal processing on phenolic acids and flavonols contents in selected brassica vegetables. Acta Sci. Pol. Technol. Aliment. 2012, 11, 45–51. [Google Scholar] [PubMed]
- Balder, H.F.; Vogel, J.; Jansen, M.C.; Weijenberg, M.P.; van den Brandt, P.A.; Westenbrink, S.; van der Meer, R.; Goldbohm, R.A. Heme and chlorophyll intake and risk of colorectal cancer in the netherlands cohort study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, F.P.; Guth, E.P. Carbohydrate and chlorophyll content of leaves of Digitalis purpurea L. and Digitalis lutea L. after freeze-drying and oven-drying. J. Am. Pharm. Assoc. 1954, 43, 268–269. [Google Scholar] [CrossRef]
- Barry, J.L.; Hoebler, C.; Macfarlane, G.T.; Macfarlane, S.; Mathers, J.C.; Reed, K.A.; Mortensen, P.B.; Nordgaard, I.; Rowland, I.R.; Rumney, C.J. Estimation of the fermentability of dietary fibre in vitro: A european interlaboratory study. Br. J. Nutr. 1995, 74, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Lin, Y.-M.; Wang, Y.-C. Comparative effects of cellulose and soluble fibers (pectin, konjac glucomannan, inulin) on fecal water toxicity toward caco-2 cells, fecal bacteria enzymes, bile acid, and short-chain fatty acids. J. Agric. Food Chem. 2010, 58, 10277–10281. [Google Scholar] [CrossRef] [PubMed]
- Avivi-Green, C.; Madar, Z.; Schwartz, B. Pectin-enriched diet affects distribution and expression of apoptosis-cascade proteins in colonic crypts of dimethylhydrazine-treated rats. Int. J. Mol. Med. 2000, 6, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ohkami, H.; Tazawa, K.; Yamashita, I.; Shimizu, T.; Murai, K.; Kobashi, K.; Fujimaki, M. Effects of apple pectin on fecal bacterial enzymes in azoxymethane-induced rat colon carcinogenesis. Jpn. J. Cancer Res. 1995, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Remesy, C.; Demigne, C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, 5239–5328. [Google Scholar]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, A.; Oikawa, S.; Murata, M.; Hiraku, Y.; Kawanishi, S. (−)-Epigallocatechin gallate causes oxidative damage to isolated and cellular DNA. Biochem. Pharmacol. 2003, 66, 1769–1778. [Google Scholar] [CrossRef]
- Kim, M.; Murakami, A.; Ohigashi, H. Modifying effects of dietary factors on (−)-epigallocatechin-3-gallate-induced pro-matrix metalloproteinase-7 production in ht-29 human colorectal cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Jacobasch, G.; Dongowski, G.; Florian, S.; Muller-Schmehl, K.; Raab, B.; Schmiedl, D. Pectin does not inhibit intestinal carcinogenesis in APC-deficient Min/+ mice. J. Agric. Food Chem. 2008, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Hino, A.; Morita, M.; Une, M.; Fujimura, K.; Kuramoto, T. Effects of deoxycholic acid and its epimers on lipid peroxidation in isolated rat hepatocytes. J. Biochem. 2001, 129, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Calderon, P.B.; Taper, H.S.; Roberfroid, M.B. Comparative hepatotoxicity of cholic acid, deoxycholic acid and lithocholic acid in the rat: In vivo and in vitro studies. Toxicol. Lett. 1992, 61, 291–304. [Google Scholar] [CrossRef]
- Juskiewicz, J.; Zdunczyk, Z.; Zary-Sikorska, E.; Krol, B.; Milala, J.; Jurgonski, A. Effect of the dietary polyphenolic fraction of chicory root, peel, seed and leaf extracts on caecal fermentation and blood parameters in rats fed diets containing prebiotic fructans. Br. J. Nutr. 2011, 105, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrné, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, e80476. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Hu, Y.; Young, G.P. Effects of resistant starch and nonstarch polysaccharides on colonic luminal environment and genotoxin-induced apoptosis in the rat. Carcinogenesis 2002, 23, 713–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Adachi, S.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (−)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev. Res. 2008, 1, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Bird, A.R.; Jackson, M.; Esterman, A.; Young, G.P. A synbiotic combination of resistant starch and bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J. Nutr. 2005, 135, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987, 37, 147–151. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16s rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [PubMed]
- Kadali, K.K.; Simons, K.L.; Skuza, P.P.; Moore, R.B.; Ball, A.S. A complementary approach to identifying and assessing the remediation potential of hydrocarbonoclastic bacteria. J. Microbiol. Methods 2012, 88, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Perez-Leblic, M.I.; Turmero, A.; Hernandez, M.; Hernandez, A.J.; Pastor, J.; Ball, A.S.; Rodriguez, J.; Arias, M.E. Influence of xenobiotic contaminants on landfill soil microbial activity and diversity. J. Environ. Manag. 2012, 95, S285–S290. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Type | Cellulose Only (Control Diet) | Pectin | Cellulose + EGCG | Pectin + EGCG | Cincau Extract | Cincau Leaves |
|---|---|---|---|---|---|---|
| Digesta | ||||||
| Total SCFA | 45.9 ± 8.4 ab | 107.3 ± 12.2 c | 69.1 ± 5.3 b | 65.8 ± 7.1 b | 44.9 ± 5.2 a | 64.5 ± 10.5 ab |
| Acetate | 18.2 ± 3.2 a | 40.0 ± 3.8 c | 28.2 ± 2.1 b | 26.2 ± 2.8 ab | 19.1 ± 2.0 ab | 28.3 ± 5.3 b |
| Propionate | 5.0 ± 1.0 ab | 16.0 ± 1.9 d | 7.8 ± 0.8 bc | 9.2 ± 1.0 c | 4.0 ± 0.5 a | 5.7 ± 1.1 ab |
| Butyrate | 4.2 ± 0.9 a | 9.8 ± 1.4 c | 7.5 ± 0.7 bc | 6.9 ± 1.7 abc | 5.0 ± 0.8 ab | 6.5 ± 1.0 abc |
| pH | 6.9 ± 0.06 a | 6.7 ± 0.08 b | 6.7 ± 0.05 b | 6.7 ± 0.07 b | 6.7 ± 0.05 b | 6.7 ± 0.05 b |
| Faeces | ||||||
| Total SCFA | 14.8 ± 2.0 a | 44.4 ± 8.3 cd | 17.5 ± 2.4 ab | 51.9 ± 9.0 d | 30.8 ± 3.9 bc | 33.7 ± 4.2 c |
| Acetate | 5.8 ± 0.6 a | 14.5 ± 3.0 c | 7.2 ± 0.9 ab | 14.5 ± 2.2 c | 12.6 ± 1.1 bc | 13.7 ± 1.2 c |
| Propionate | 1.4 ± 0.2 a | 8.0 ± 1.3 b | 1.9 ± 0.3 a | 7.3 ± 1.0 b | 2.3 ± 0.2 a | 2.8 ± 0.3a |
| Butyrate | 1.6 ± 0.2 a | 4.7 ± 0.5 b | 2.4 ± 0.3 a | 6.3 ± 1.0 b | 4.6 ± 0.5 b | 4.8 ± 0.5 b |
| pH | 6.7 ± 0.06a | 6.4 ± 0.05c | 6.4 ± 0.04c | 6.5 ± 0.05bc | 6.6 ± 0.05 b | 6.4 ± 0.05c |
| Type/Location | Cellulose Only (Control Diet) | Pectin | Cellulose + EGCG | Pectin + EGCG | Cincau Extract | Cincau Leaves |
|---|---|---|---|---|---|---|
| ACF Incidence | 12/12 | 12/12 | 12/12 | 12/12 | 12/12 | 12/12 |
| Total No. ACF | 73.2 ± 12.1 a | 110.7 ± 19.6 b | 64.7 ± 8.5 a | 114.5 ± 14.1 b | 79.7 ± 10.7 ab | 88.8 ± 10.7 ab |
| 1 Crypt | 32.9 ± 5.2 a | 53.6 ± 10.9 b | 31.5 ± 4.5 a | 52.3 ± 5.6 b | 43.4 ± 4.3 ab | 44.5 ± 5.0 ab |
| 2 Crypts | 26.8 ± 4.7 ac | 40.5 ± 6.7 ab | 23.7 ± 3.2 c | 41.3 ± 5.9 b | 23.5 ± 4.6 c | 30.2 ± 4.5 abc |
| 3 Crypts | 8.4 ± 1.7 a | 10.6 ± 1.9 ab | 7.1 ± 1.3 a | 14.5 ± 2.5 b | 7.7 ± 2.1 a | 9.7 ± 1.8 ab |
| <4 Crypts | 68.2 ± 11.2 a | 104.9 ± 18.5 b | 62.3 ± 8.8 a | 108.2 ± 13.0 b | 74.7 ± 9.3 ab | 84.4 ± 9.8 ab |
| ≥4 Crypts | 4.5 ± 1.0 ab | 5.8 ± 1.3 b | 2.4 ± 0.5 a | 6.4 ± 1.5 b | 4.3 ± 1.3 ab | 4.3 ± 0.9 ab |
| Proximal Colon | 1.6 ± 0.6 a | 2.0 ± 0.8 a | 0.6 ± 0.2 a | 3.6 ± 2.7 a | 3.2 ± 1.9 a | 2.4 ± 1.4 a |
| Middle Colon | 38.8 ± 6.4 a | 64.3 ± 14.3 a | 43.0 ± 7.2 a | 63.0 ± 11.3 a | 44.1 ± 9.7 a | 49.8 ± 8.1 a |
| Distal Colon | 32.8 ± 6.7 ac | 44.4 ± 6.5 ab | 21.2 ± 2.9 c | 48.0 ± 3.2 b | 32.4 ± 4.4 ac | 36.4 ± 6.8 ab |
| Band | Fragment Size | Closest Relative | Accession Number | Percent Similarity | Known Function |
|---|---|---|---|---|---|
| 1 | 196 | Lactobacillus johnsonii DPC 6026 | NC017477.1 | 100 | Probiotic [43] |
| 2 | 196 | Lactobacillus amylolyticus | ADNY01000006.1 | 99 | Lactic acid producer [44] |
| 3 | 173 | Oscillibacter valericigenes | NC_016048 | 97 | Valerat producer [42] |
| 4 | 170 | Clostridiales sp. SM4/1 | FP929060.1 | 96 | Butyrate producer (genomesonline.org) |
| 5 | 173 | Clostridium saccharolyticum | NC_014376.1 | 97 | Acetic acid [45] |
| 6 | 171 | Clostridiales sp. SM4/1 | FP929060.1 | 98 | Butyrate producer (genomesonline.org) |
| 7 | 177 | Lactobacillus amylolyticus | ADNY01000006.1 | 100 | Lactic acid producer [44] |
| (g/1000 g in Diet) | Cellulose Only (Control Diet) | Pectin | Cellulose + EGCG | Pectin + EGCG | Cincau Extract | Cincau Leaves |
|---|---|---|---|---|---|---|
| Casein | 190 | 190 | 190 | 190 | 190 | 190 |
| Corn Starch | 430 | 430 | 430 | 430 | 430 | 430 |
| Cellulose | 50 | - | 50 | - | - | |
| Pectin | - | 50 | - | 50 | - | - |
| Cincau extract | - | - | - | - | 50 | - |
| Cincau leave powder | - | - | - | - | - | 50 |
| Corn oil | 180 | 180 | 180 | 180 | 180 | 180 |
| Sucrose | 109 | 109 | 109 | 109 | 109 | 109 |
| dl-Methionine | 3 | 3 | 3 | 3 | 3 | 3 |
| Choline | 1 | 1 | 1 | 1 | 1 | 1 |
| Mineral Mix * | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamin Mix * | 10 | 10 | 10 | 10 | 10 | 10 |
| EGCG in water ** | - | - | 0.1% | 0.1% | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurdin, S.U.; Le Leu, R.K.; Aburto-Medina, A.; Young, G.P.; Stangoulis, J.C.R.; Ball, A.S.; Abbott, C.A. Effects of Dietary Fibre from the Traditional Indonesian Food, Green Cincau (Premna oblongifolia Merr.) on Preneoplastic Lesions and Short Chain Fatty Acid Production in an Azoxymethane Rat Model of Colon Cancer. Int. J. Mol. Sci. 2018, 19, 2593. https://doi.org/10.3390/ijms19092593
Nurdin SU, Le Leu RK, Aburto-Medina A, Young GP, Stangoulis JCR, Ball AS, Abbott CA. Effects of Dietary Fibre from the Traditional Indonesian Food, Green Cincau (Premna oblongifolia Merr.) on Preneoplastic Lesions and Short Chain Fatty Acid Production in an Azoxymethane Rat Model of Colon Cancer. International Journal of Molecular Sciences. 2018; 19(9):2593. https://doi.org/10.3390/ijms19092593
Chicago/Turabian StyleNurdin, Samsu U., Richard K. Le Leu, Arturo Aburto-Medina, Graeme P. Young, James C. R. Stangoulis, Andy S. Ball, and Catherine A. Abbott. 2018. "Effects of Dietary Fibre from the Traditional Indonesian Food, Green Cincau (Premna oblongifolia Merr.) on Preneoplastic Lesions and Short Chain Fatty Acid Production in an Azoxymethane Rat Model of Colon Cancer" International Journal of Molecular Sciences 19, no. 9: 2593. https://doi.org/10.3390/ijms19092593