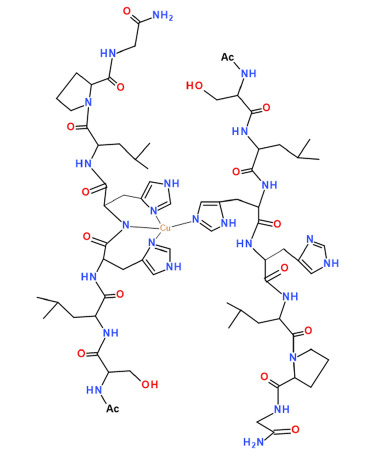
Copper Binding Features of Tropomyosin-Receptor-Kinase-A Fragment: Clue for Neurotrophic Factors and Metals Link
Abstract
:1. Introduction
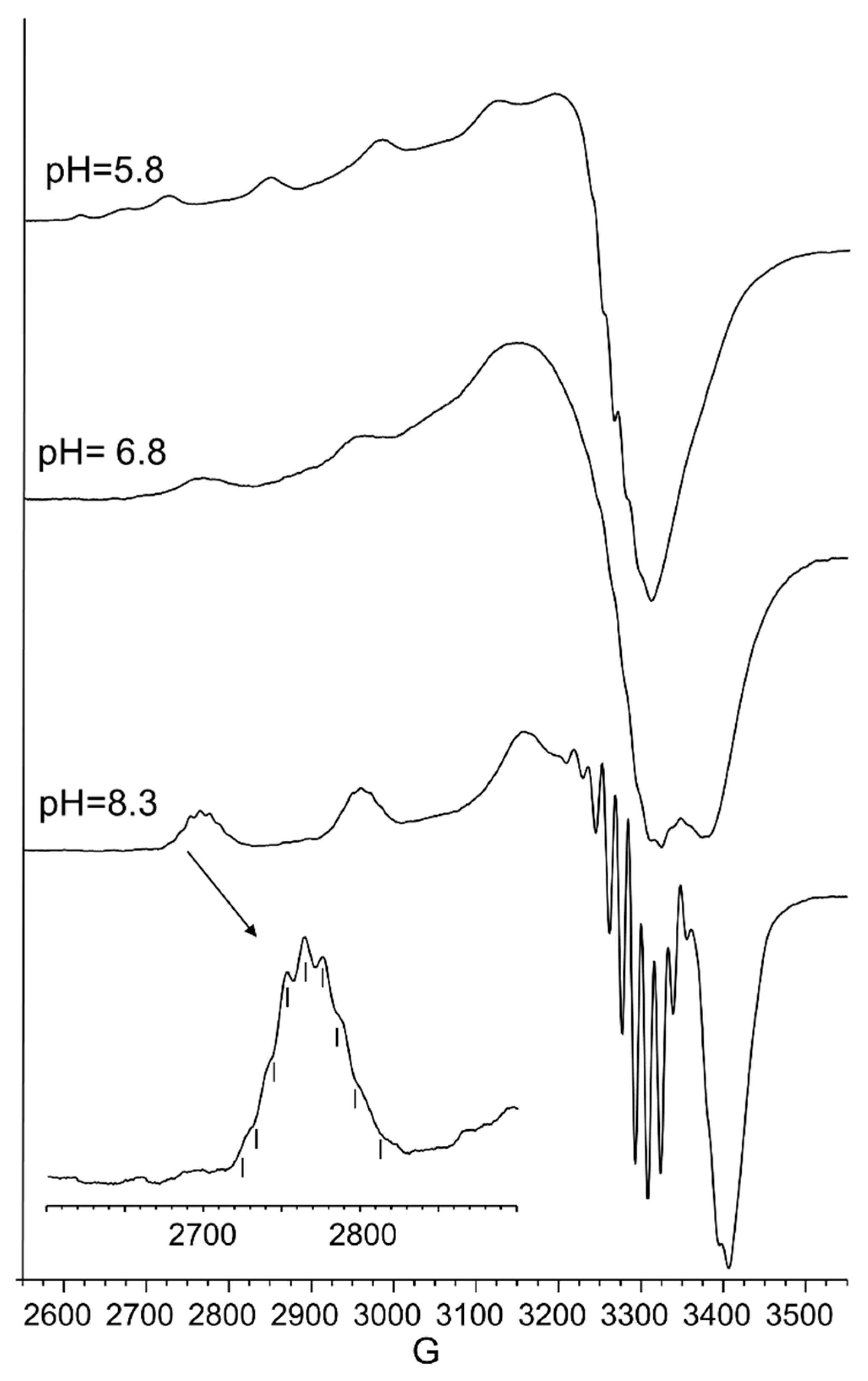
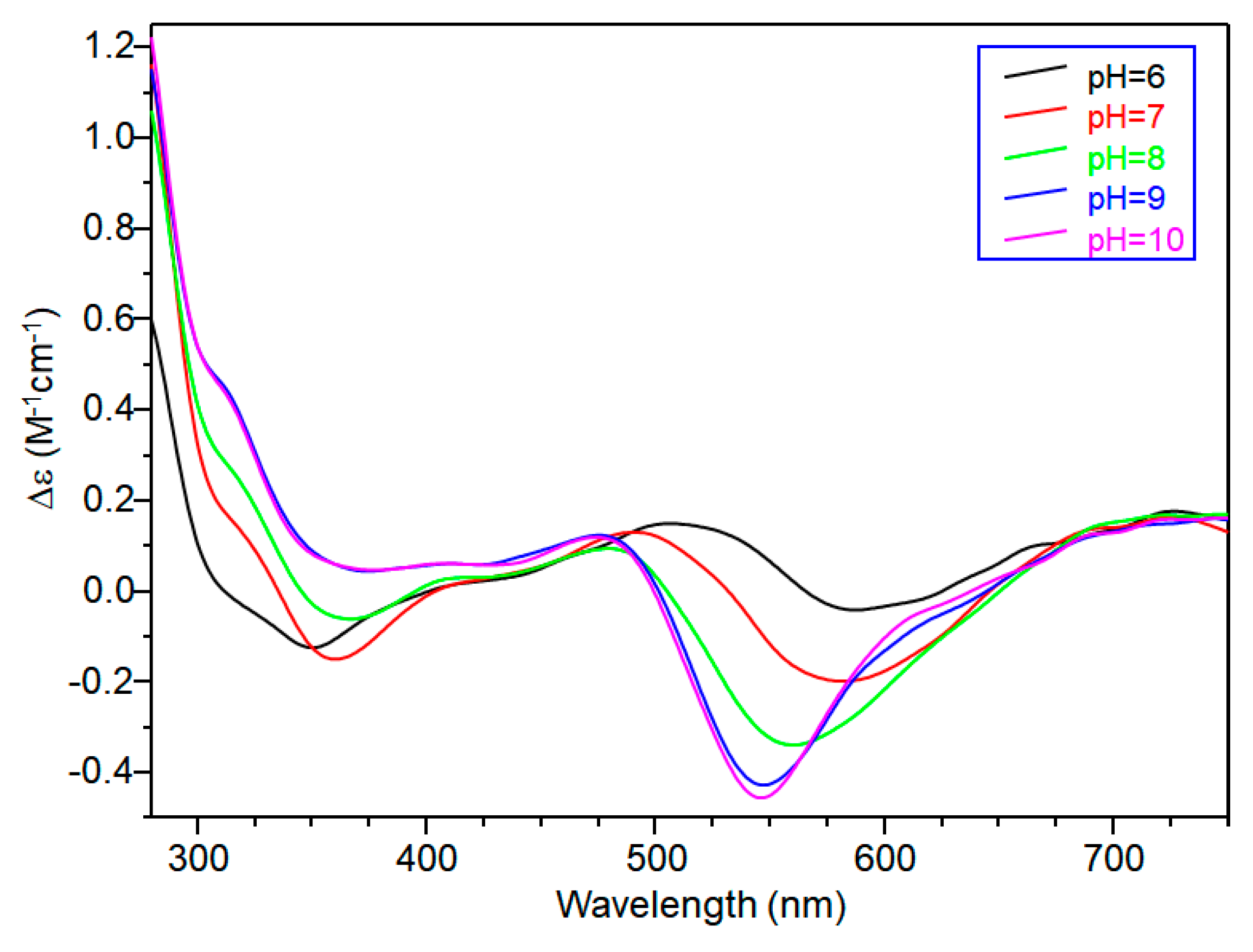
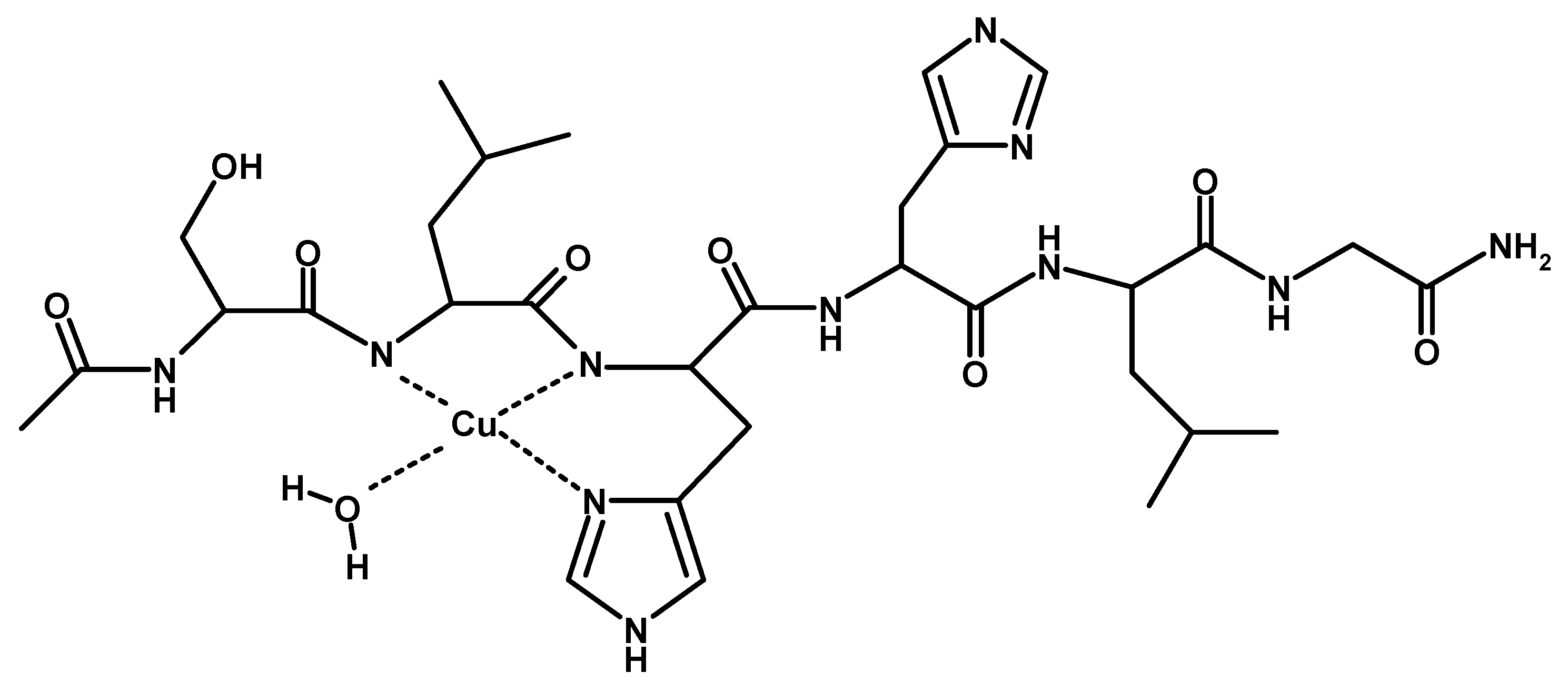
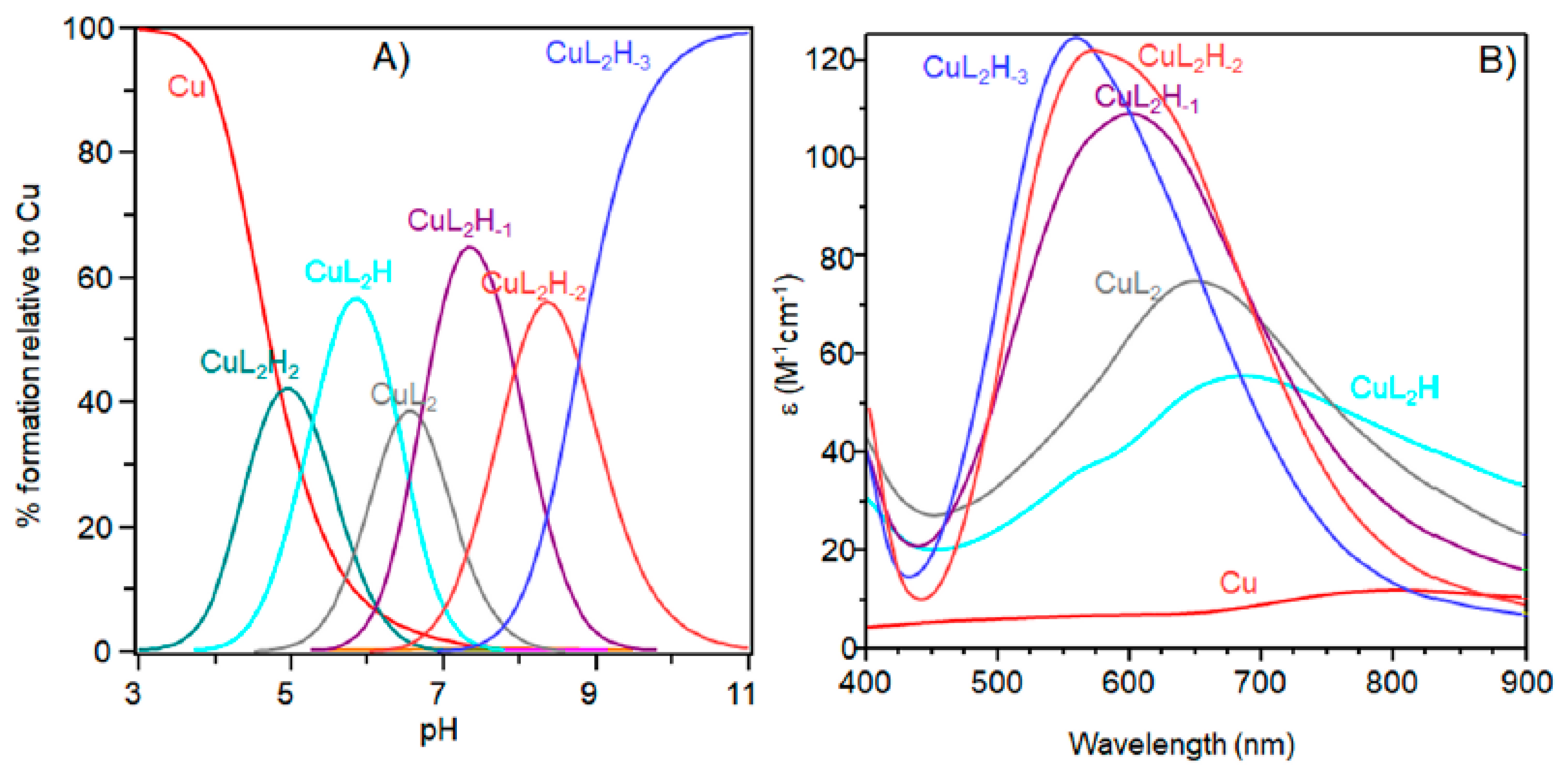
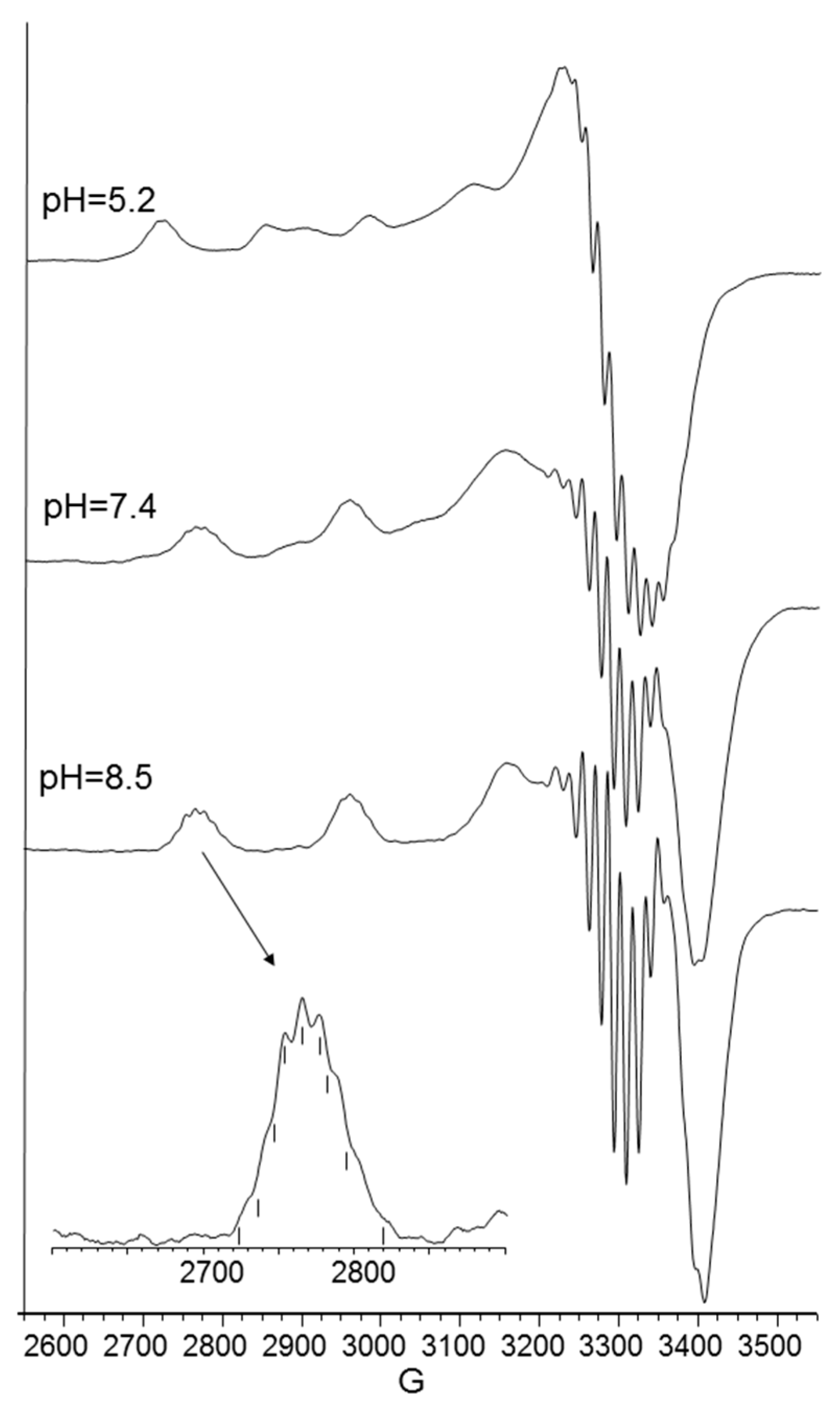
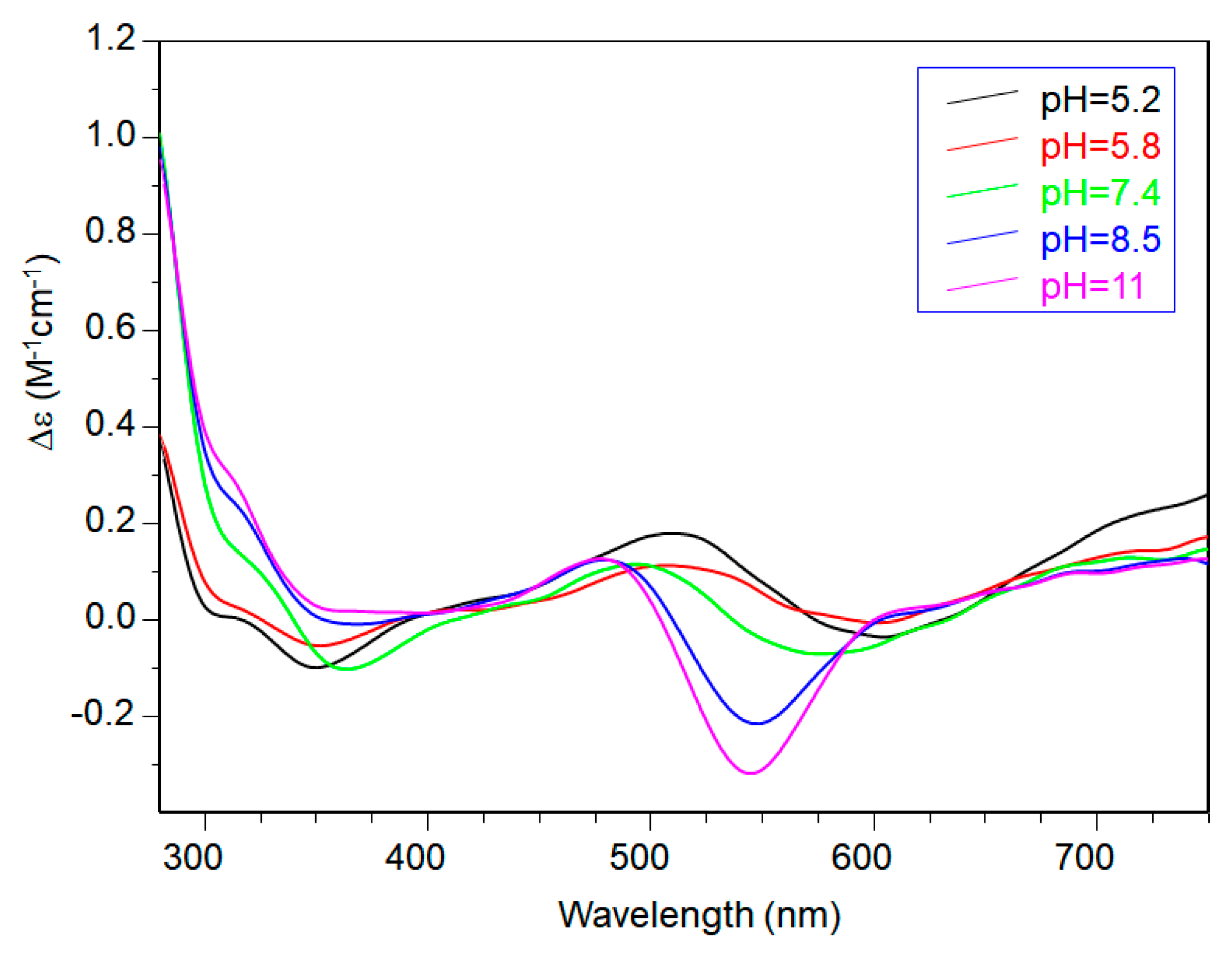
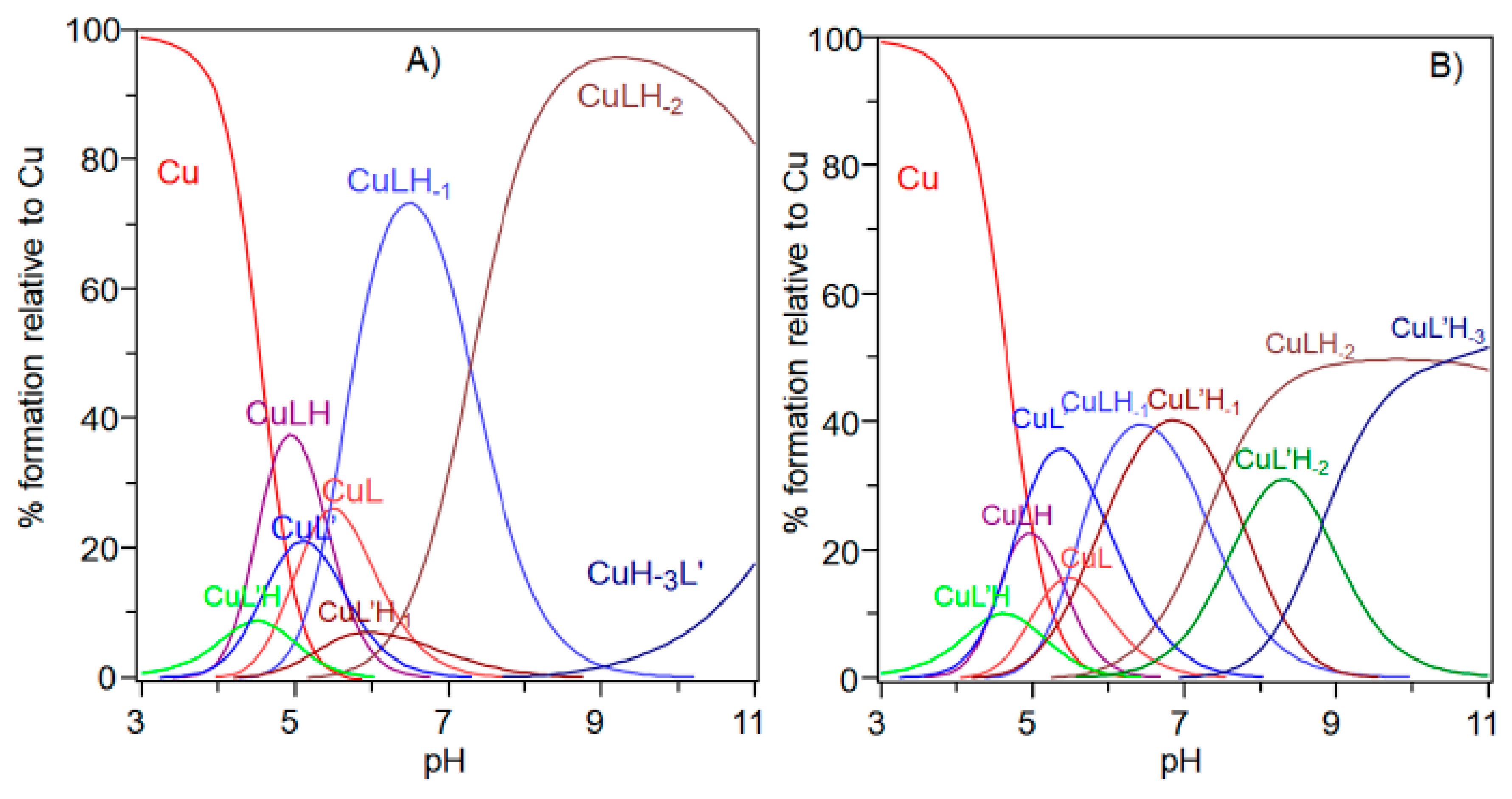
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis and Purification
4.3. Potentiometric Titrations
4.4. Spectroscoscopic Studies
4.4.1. UV-Vis Measurements
4.4.2. CD Measurements
4.4.3. EPR Measurements
Author Contributions
Funding
Conflicts of Interest
References
- Huang, J.E.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Ann. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Twiss, J.L.; Chang, J.H.; Schanen, N.C. Pathophysiological Mechanisms for Actions of the Neurotrophins. Brain Pathol. 2006, 16, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Neurotrophic Factors: An Overview. Methods Mol. Biol. 2018, 1727, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Haddad, Y.; Adam, V.; Heger, Z. Trk Receptors and Neurotrophin Cross-Interactions: New Perspectives Toward Manipulating Therapeutic Side-Effects. Front. Mol. Neurosci. 2017, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Campenot, R.B. NGF uptake and retrograde signaling mechanisms in sympathetic neurons in compartmented cultures. Results Probl. Cell Differ. 2009, 48, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Iulita, M.F.; Cuello, A.C. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and down syndrome. Trends Pharmacol. Sci. 2014, 35, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Triaca, V.; Calissano, P. Impairment of the nerve growth factor pathway driving amyloid accumulation in cholinergic neurons: the incipit of the Alzheimer’s disease story? Neural Regen Res. 2016, 11, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Wang, J.L.; Jin, W.L. The Emerging Therapeutic Role of NGF in Alzheimer’s Disease. Neurochem. Res. 2016, 41, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. Drug Development Based on the Metals Hypothesis of Alzheimer’s Disease. J. Alzheimers Dis. 2008, 15, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Bush, A.I. Metals in Alzheimer’s and Parkinson’s diseases. Curr. Opin. Chem. Biol. 2008, 12, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Mathys, Z.K.; White, A.R. Copper and Alzheimer’s Disease. Adv. Neurobiol. 2017, 18, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.; Shamovsky, I.L.; Woo, S.B.; Post, J.I.; Vrkljan, P.N.; Lawrence, G.; Solc, M.; Dostaler, S.M.; Neet, K.E.; Riopelle, R.J. The binding of zinc and copper ions to nerve growth factor is differentially affected by pH: implications for cerebral acidosis. J. Neurochem. 2001, 78, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travaglia, A.; Pietropaolo, A.; La Mendola, D.; Nicoletti, V.G.; Rizzarelli, E. The inorganic perspectives of neurotrophins and Alzheimer’s disease. J. Inorg. Biochem. 2012, 111, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Birkaya, B.; Aletta, J.M. NGF promotes copper accumulation required for optimum neurite outgrowth and protein methylation. J. Neurobiol. 2005, 63, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, G.M.; Shamovsky, I.L.; Lawrance, G.; Solc, M.; Dostaler, S.M.; Jimmo, S.L.; Weaver, D.F.; Riopelle, R.J. Zinc alters conformation and inhibits biological activities of nerve growth factor and related neurotrophins. Nat. Med. 1997, 3, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Post, J.I.; Dow, K.E.; Shin, S.H.; Riopelle, R.J.; Ross, G.M. Zinc and copper inhibit nerve growth factor-mediated protection from oxidative stress-induced apoptosis. Neurosci. Lett. 1999, 259, 115–118. [Google Scholar] [CrossRef]
- Allington, C.; Shamovsky, I.L.; Ross, G.M.; Riopelle, R.J. Zinc inhibits p75NTR-mediated apoptosis in chick neural retina. Cell Death Differ. 2001, 8, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travaglia, A.; Arena, G.; Fattorusso, R.; Isernia, C.; La Mendola, D.; Malgeri, G.; Nicoletti, V.G.; Rizzarelli, E. The inorganic perspective of nerve growth factor: interactions of Cu2+ and Zn2+ with the N-terminus fragment of nerve growth factor encompassing the recognition domain of the TrkA receptor. Chem. Eur. J. 2011, 17, 3726–3738. [Google Scholar] [CrossRef] [PubMed]
- Pandini, G.; Satriano, C.; Pietropaolo, A.; Gianì, F.; Travaglia, A.; La Mendola, D.; Nicoletti, V.G.; Rizzarelli, E. The Inorganic Side of NGF: Copper(II) and Zinc(II) Affect the NGF Mimicking Signaling of the N-Terminus Peptides Encompassing the Recognition Domain of TrkA Receptor. Front. Neurosci. 2016, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tamano, H. Significance of the degree of synaptic Zn²⁺ signaling in cognition. Biometals 2016, 29, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sindreu, C.; Storm, D.R. Modulation of neuronal signal transduction and memory formation by synaptic zinc. Front. Behav. Neurosci. 2011, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Grubman, A.; White, A.R. Copper as a key regulator of cell signalling pathways. Expert Rev. Mol. Med. 2014, 16, e11. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Park, M.H.; Choi, S.Y.; Koh, J.Y. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J. Biol. Chem. 2005, 280, 11995–12001. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, A.; Magrì, A.; Greco, V.; Losasso, V.; la Mendola, D.; Sciuto, S.; Carloni, P.; Rizzarelli, E. Binding of Zn(II) to Tropomyosin Receptor Kinase A in Complex with Its Cognate Nerve Growth Factor: Insights from Molecular Simulation and in Vitro Essays. ACS Chem. Neurosci. 2018, 9, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Politano, R.; Rowinska-Zyrek, M.; Guerrini, R.; Remelli, M.; Kozlowski, H. The Coordination of NiII and CuII Ions to the Polyhistidyl Motif of Hpn Protein: Is It as Strong as We Think? Chem. Eur. J. 2012, 18, 11088–11099. [Google Scholar] [CrossRef] [PubMed]
- Pontecchiani, F.; Simonovsky, E.; Wieczorek, R.; Barbosa, N.; Rowinska-Zyrek, M.; Potocki, S.; Remelli, M.; Miller, Y.; Kozlowski, H. The unusual binding mechanism of Cu(II) ions to the poly-histidyl domain of a peptide found in the venom of an African viper. Dalton Trans. 2014, 43, 16680–16689. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guzmán, J.; Sun, L.; Mehta, A.K.; Dong, J.; Lynn, D.G.; Warncke, K. Copper(II)-bis-Histidine Coordination Structure in a Fibrillar Amyloid β-Peptide Fragment and Model Complexes Revealed by Electron Spin Echo Envelope modulation Spectroscopy. Chembiochem 2013, 14, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Mital, M.; Wezynfeld, N.E.; Frączyk, T.; Wiloch, M.Z.; Wawrzyniak, U.E.; Bonna, A.; Tumpach, C.; Barnham, K.J.; Haigh, C.L.; Bal, W.; et al. A Functional Role for Aβ in Metal Homeostasis? N-Truncation and High-Affinity Copper Binding. Angew. Chem. Int. Ed. Engl. 2015, 54, 10460–10464. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; La Mendola, D.; Nicoletti, V.G.; Pappalardo, G.; Rizzarelli, E. New Insight in Copper-Ion Binding to Human Islet Amyloid: The Contribution of Metal-Complex Speciation to Reveal the Polypeptide Toxicity. Chem. Eur. J. 2016, 22, 13287–13300. [Google Scholar] [CrossRef]
- Sanna, D.; Micera, G.; Kallay, C.; Rigo, V.; Sovago, I. Copper(II) complexes of N-terminal protected tri- and tetra-peptides containing histidine residues. Dalton Trans. 2004, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Jancsó, A.; Kolozsi, A.; Gyurcsik, B.; Nagy, N.V.; Gajda, T. Probing the Cu2+ and Zn2+ binding affinity of histidine-rich glycoprotein. J. Inorg. Biochem. 2009, 103, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Matera, A.; Brasuń, J.; Cebrat, M.; Świątek-Kozłowska, J. The role of the histidine residue in the coordination abilities of peptides with a multi-histidine sequence towards copper(II) ions. Polyhedron 2008, 27, 1539–1555. [Google Scholar] [CrossRef]
- Timári, S.; Kállay, C.S.; Osz, K.; Sóvágó, I.; Várnagy, K. Transition metal complexes of short multihistidine peptides. Dalton Trans. 2009, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Kulon, K.; Valensin, D.; Kamysz, W.; Valensin, G.; Nadolski, P.; Porciatti, E.; Gaggelli, E.; Kozlowski, H. The His-His sequence of the antimicrobial peptide demegen P-113 makes it very attractive ligand for Cu2+. J. Inorg. Biochem. 2008, 102, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Myari, A.; Malandrinos, G.; Deligiannakis, Y.; Plakatouras, J.C.; Hadjiliadis, N.; Nagy, Z.; Sovago, I. Interaction of Cu2+ with His-Val-His and of Zn2+ with His-Val-Gly-Asp, two peptides surrounding metal ions in Cu,Zn-superoxide dismutase enzyme. J. Inorg. Biochem. 2001, 85, 253–261. [Google Scholar] [CrossRef]
- Valensin, D.; Luczkowski, M.; Mancini, F.M.; Legowska, A.; Gaggelli, E.; Valensin, G.; Rolka, K.; Kozlowski, H. The dimeric and tetrameric octarepeat fragments of prion protein behave differently to its monomeric unit. Dalton Trans. 2004, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Sóvágó, I.; Osz, K. Metal ion selectivity of oligopeptides. Dalton Trans. 2006, 3841–3854. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; Tabbì, G.; Breglia, R.; de Gioia, L.; Fantucci, P.; Bruschi, M.; Bonomo, R.P.; la Mendola, D. Copper ion interaction with the RNase catalytic site fragment of the angiogenin protein: An experimental and theoretical investigation. Dalton Trans. 2017, 46, 8524–8538. [Google Scholar] [CrossRef] [PubMed]
- La Mendola, D.; Magrì, A.; Santoro, A.M.; Nicoletti, V.G.; Rizzarelli, E. Copper(II) interaction with peptide fragments of histidine-proline-rich glycoprotein: Speciation, stability and binding details. J. Inorg. Biochem. 2012, 111, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hecel, A.; Wątły, J.; Rowińska-Zyrek, M.; Świątek-Kozłowska, J.; Kozłowski, H. Histidine tracts in human transcription factors: insight into metal ion coordination ability. J. Biol. Inorg. Chem. 2018, 23, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Jankowska, T.; Ruta, M.; Wiśniewska, K.; Lankiewicz, L. Coordination abilities of the 1-16 and 1-28 fragments of beta-amyloid peptide towards copper(II) ions: A combined potentiometric and spectroscopic study. J. Inorg. Biochem. 2003, 95, 270–282. [Google Scholar] [CrossRef]
- Amorini, A.M.; Bellia, F.; di Pietro, V.; Giardina, B.; la Mendola, D.; Lazzarino, G.; Sortino, S.; Tavazzi, B.; Rizzarelli, E.; Vecchio, G. Synthesis and antioxidant activity of new homocarnosine beta-cyclodextrin conjugates. Eur. J. Med. Chem. 2007, 42, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Rajković, S.; Kállay, C.; Serényi, R.; Malandrinos, G.; Hadjiliadis, N.; Sanna, D.; Sovago, I. Complex formation processes of terminally protected peptides containing two or three histidyl residues. Characterization of the mixed metal complexes of peptides. Dalton Trans. 2008, 5059–5071. [Google Scholar] [CrossRef] [PubMed]
- Potocki, S.; Valensin, D.; Kozlowski, H. The specificity of interaction of Zn2+, Ni2+ and Cu2+ ions with the histidine-rich domain of the TjZNT1 ZIP family transporter. Dalton Trans. 2014, 10215–10223. [Google Scholar] [CrossRef] [PubMed]
- La Mendola, D.; Bonomo, R.P.; Caminati, S.; Di Natale, G.; Emmi, S.S.; Hansson, Ö.; Maccarrone, G.; Pappalardo, G.; Pietropaolo, A.; Rizzarelli, E. Copper(II) complexes with an avian prion N-terminal region and their potential SOD-like activity. J. Inorg. Biochem. 2009, 103, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Remenyi, C.; Reviakine, R.; Kaupp, M. Density Functional Study of EPR Parameters and Spin-Density Distribution of Azurin and Other Blue Copper Proteins. J. Phys. Chem. B 2007, 111, 8290–8304. [Google Scholar] [CrossRef] [PubMed]

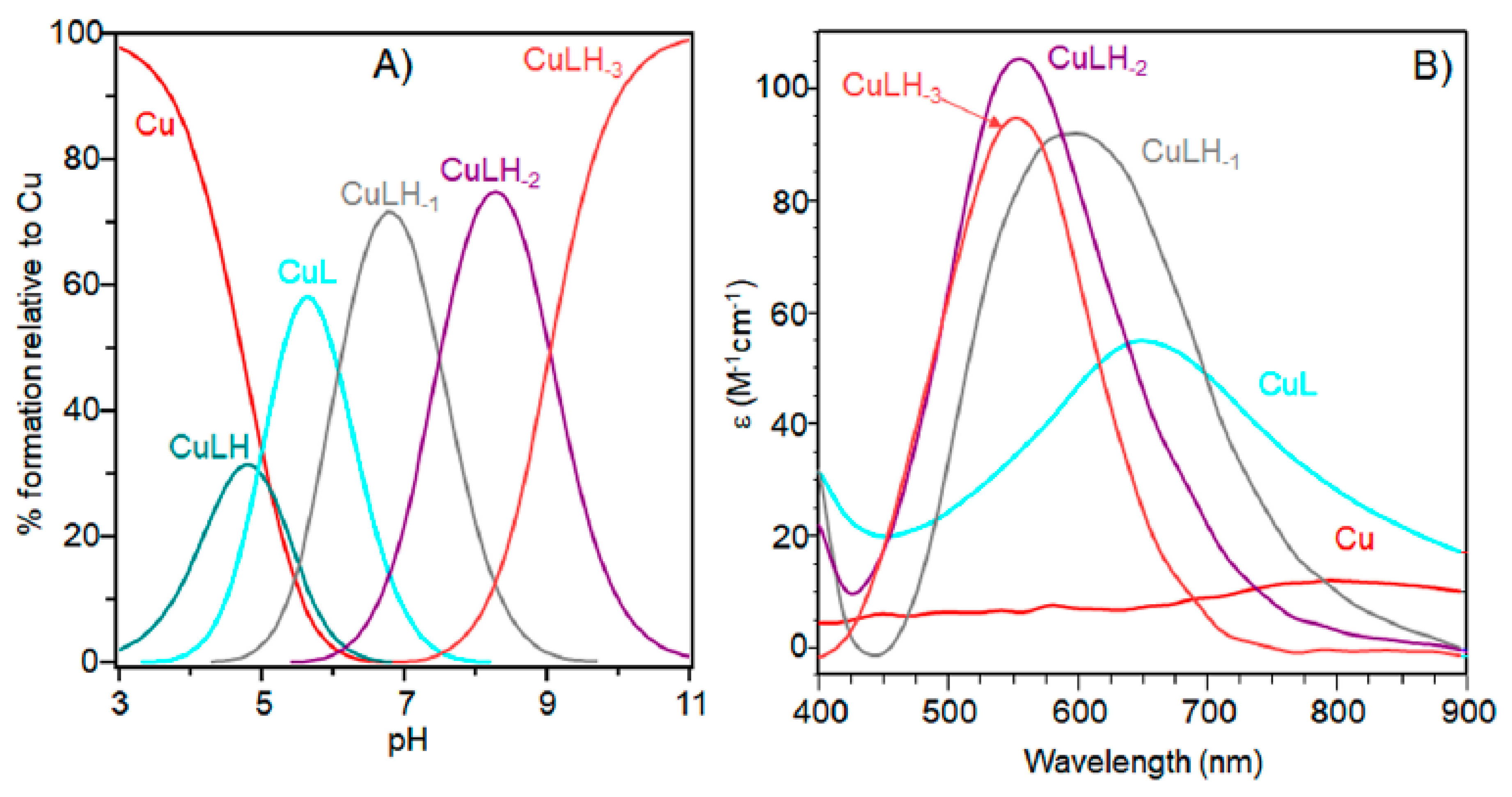
| Species (pqr) | logβ | pK (n/m) |
|---|---|---|
| CuLH | 10.61 (9) | - |
| CuL | 6.19 (3) | 4.42 |
| CuLH−1 | 0.23 (5) | 5.97 |
| CuLH−2 | −7.55 (4) | 7.78 |
| CuLH−3 | −16.36 (6) | 8.80 |
| pH | Species | UV-Vis | CD | EPR | ||
|---|---|---|---|---|---|---|
| λ (nm) (ε (M−1·cm−1)) | λ (nm) (Δε (M−1·cm−1)) | g║║ | A║║ (10−4 cm−1) | |||
| 5.8 | CuL | 650 | (50) | 280 (0.65), 350 (−0.30), 511 (0.30), 723 (0.25) | 2.315 (2) | 145 (3) |
| 6.8 | CuLH−1 | 595 | (95) | 280 (1.40), 362 (−0.22), 482 (0.17), 585 (−0.71), 723 (0.30) | 2.210 (3) | 194 (5) |
| 8.3 | CuLH−2 | 550 | (105) | 280 (1.52), 362 (−0.10), 474 (0.24), 549 (−0.80), 717 (0.19) | 2.206 (1) | 202 (2) |
| 9–11 | CuLH−3 | 550 | (95) | 280 (1.90), 477 (0.23), 547 (−0.78) | 2.206 (1) | 202 (2) |
| Species (pqr) | logβ | pK (n/m) |
|---|---|---|
| CuL2H2 | 22.12 (5) | - |
| CuL2H | 16.85 (3) | 5.27 |
| CuL2 | 10.46 (5) | 6.39 |
| CuL2H−1 | 3.77 (4) | 6.69 |
| CuL2H−2 | −4.17 (4) | 7.94 |
| pH | Species | UV-Vis | CD | EPR | ||
|---|---|---|---|---|---|---|
| λ (nm) (ε (M−1·cm−1)) | λ (nm) (Δε (M−1·cm−1)) | g║ | A║ (10−4 cm−1) | |||
| 5.2 | CuL2H2 | 675 | (55) | − | 2.310 (2) | 142 (3) |
| 5.8 | CuL2H | 650 | (75) | 280 (0.44), 349 (0.06), 516 (0.13), 600 (−0.03), 725 (0.13) | − | − |
| 7.4 | CuL2H−1 | 598 | (110) | 280 (1.16), 360 (−0.11), 491 (0.14), 568 (−0.09), 723 (0.12) | 2.249 (2) | 192 (3) |
| 8.5 | CuL2H−2 | 555 | (120) | 280 (1.20), 474 (0.15), 549 (−0.23), 723 (0.12) | 2.206 (1) | 202 (2) |
| 11 | CuL2H−3 | 550 | (125) | 280 (1.05), 481 (0.15), 547 (−0.35) | 2.206 (1) | 202 (2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magrì, A.; La Mendola, D. Copper Binding Features of Tropomyosin-Receptor-Kinase-A Fragment: Clue for Neurotrophic Factors and Metals Link. Int. J. Mol. Sci. 2018, 19, 2374. https://doi.org/10.3390/ijms19082374
Magrì A, La Mendola D. Copper Binding Features of Tropomyosin-Receptor-Kinase-A Fragment: Clue for Neurotrophic Factors and Metals Link. International Journal of Molecular Sciences. 2018; 19(8):2374. https://doi.org/10.3390/ijms19082374
Chicago/Turabian StyleMagrì, Antonio, and Diego La Mendola. 2018. "Copper Binding Features of Tropomyosin-Receptor-Kinase-A Fragment: Clue for Neurotrophic Factors and Metals Link" International Journal of Molecular Sciences 19, no. 8: 2374. https://doi.org/10.3390/ijms19082374