An Innovative Approach to Manganese-Substituted Hydroxyapatite Coating on Zinc Oxide–Coated 316L SS for Implant Application
Abstract
:1. Introduction
2. Result and Discussion
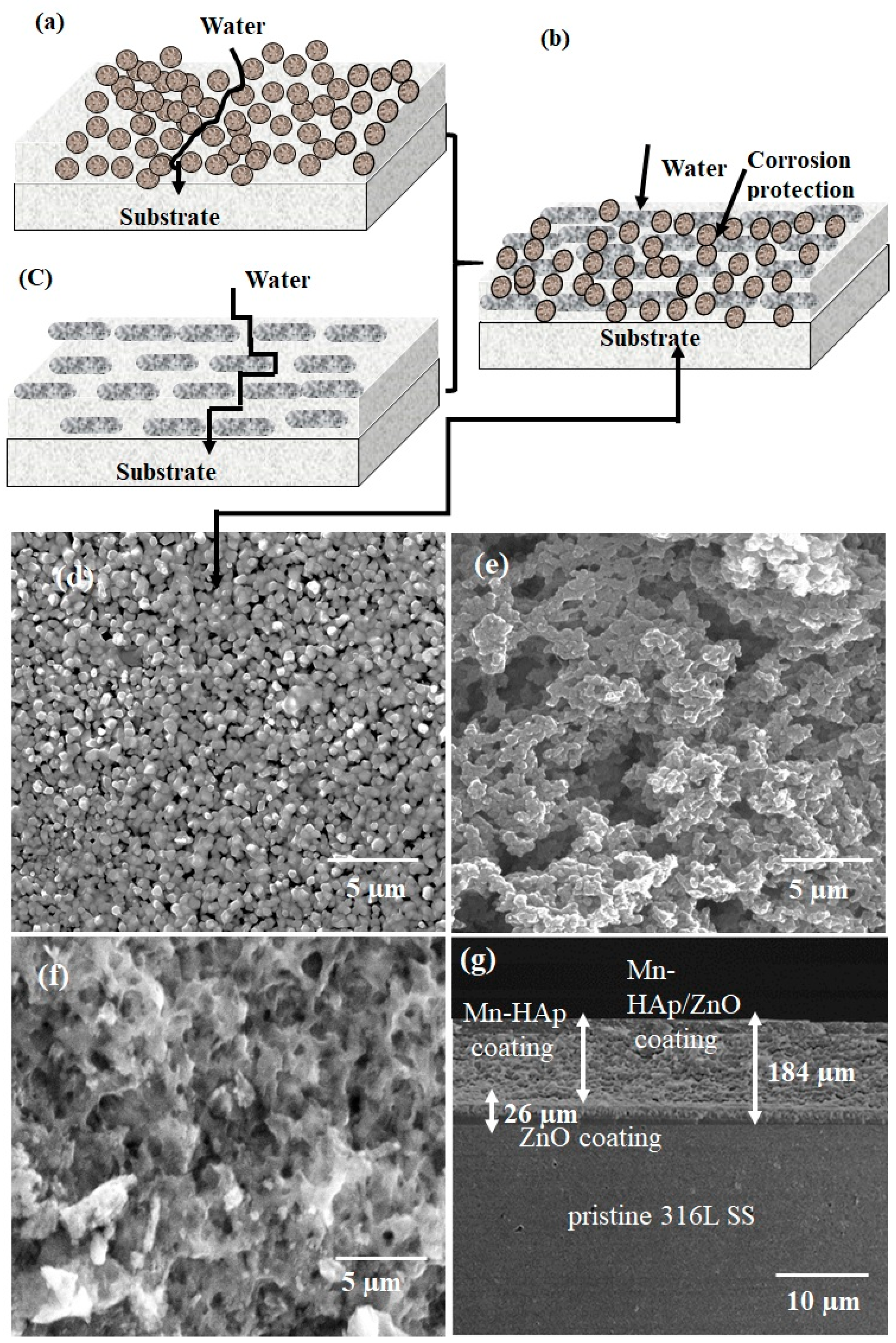
2.1. Field Emission Scanning Electron Microscopy (FE-SEM) Analysis
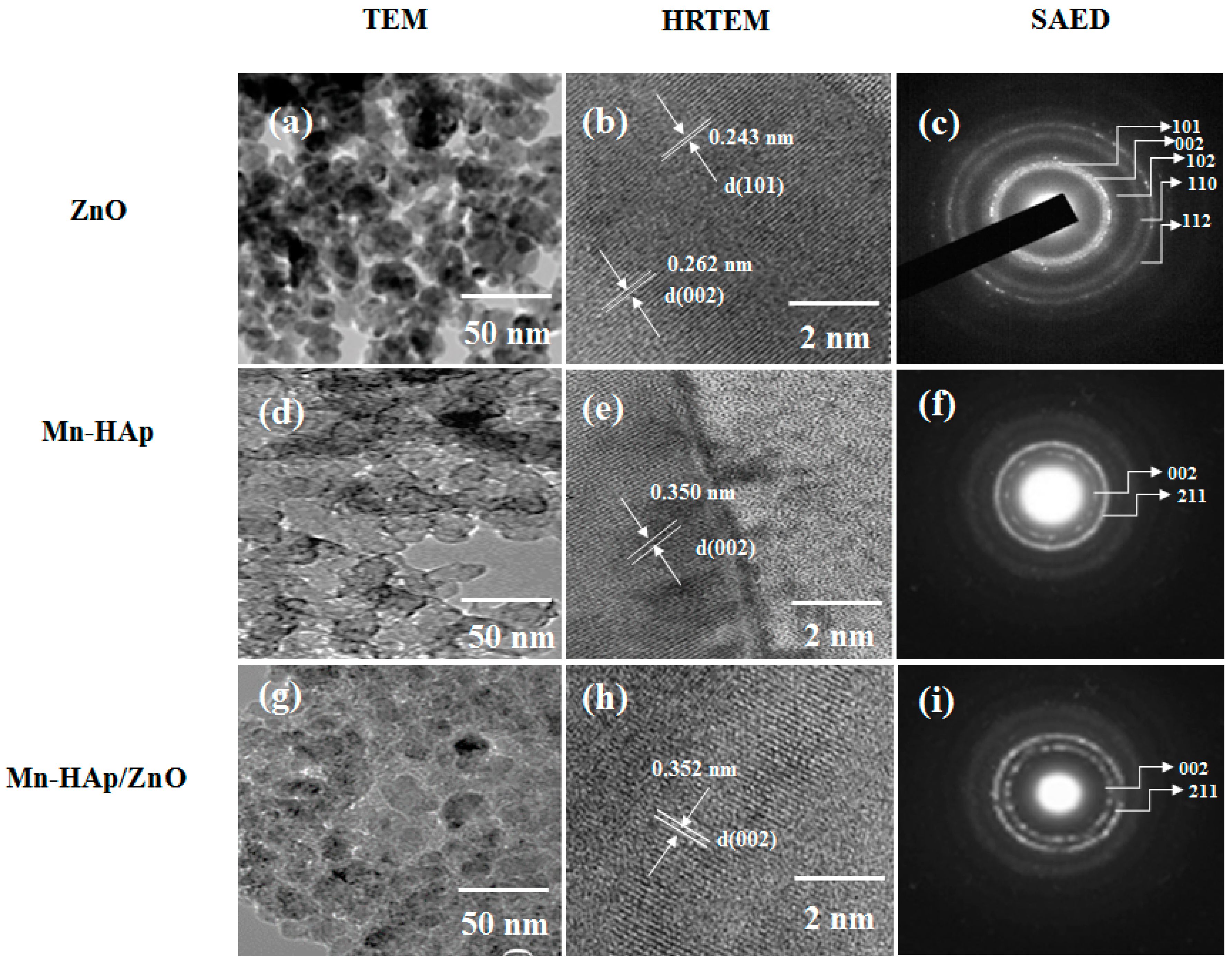
2.2. Transmission Electron Microscopy (TEM), High-Resolution Transmission Electron Microscopy (HRTEM), and Selected Aread Electron Difraction (SAED) Pattern
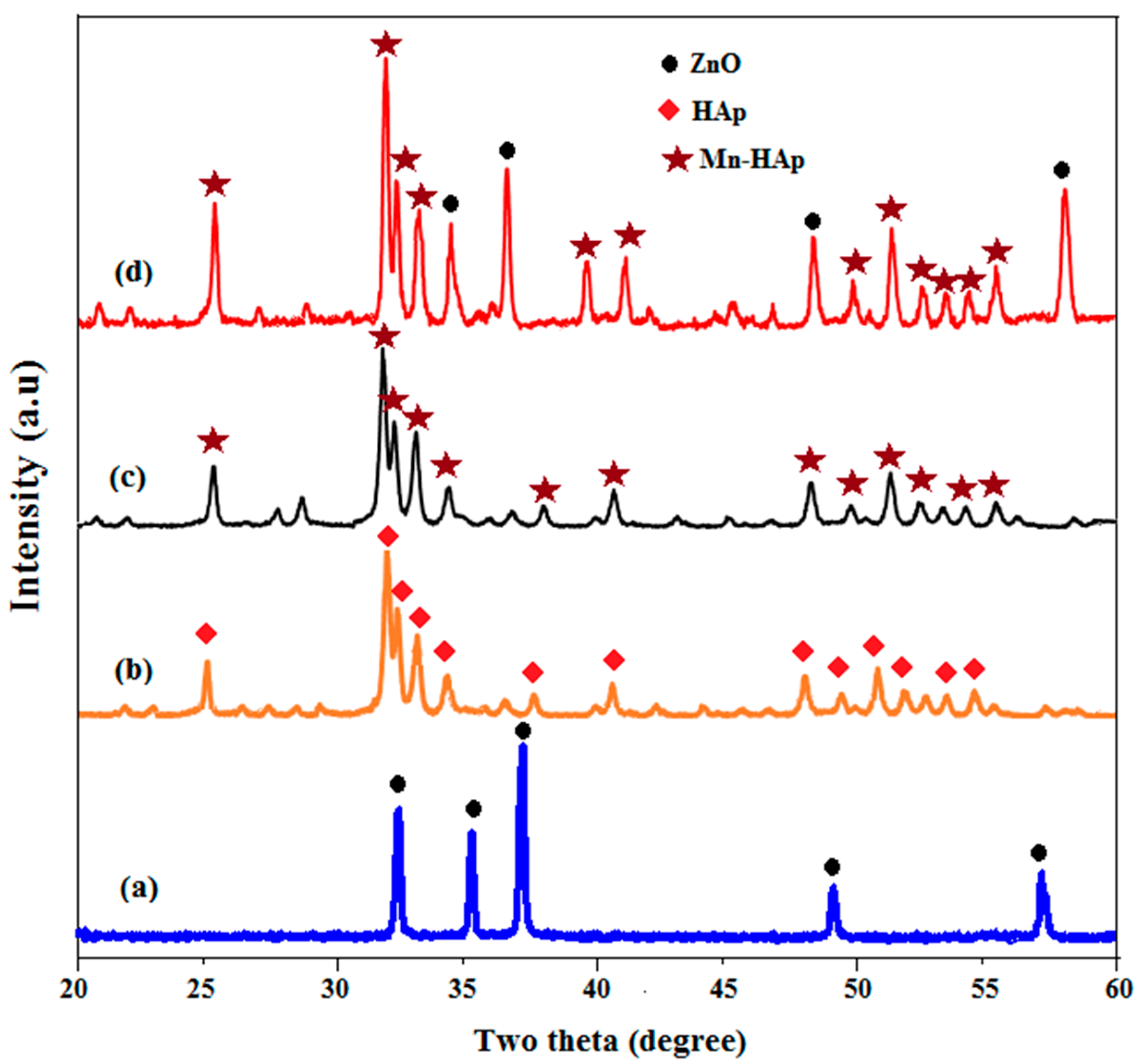
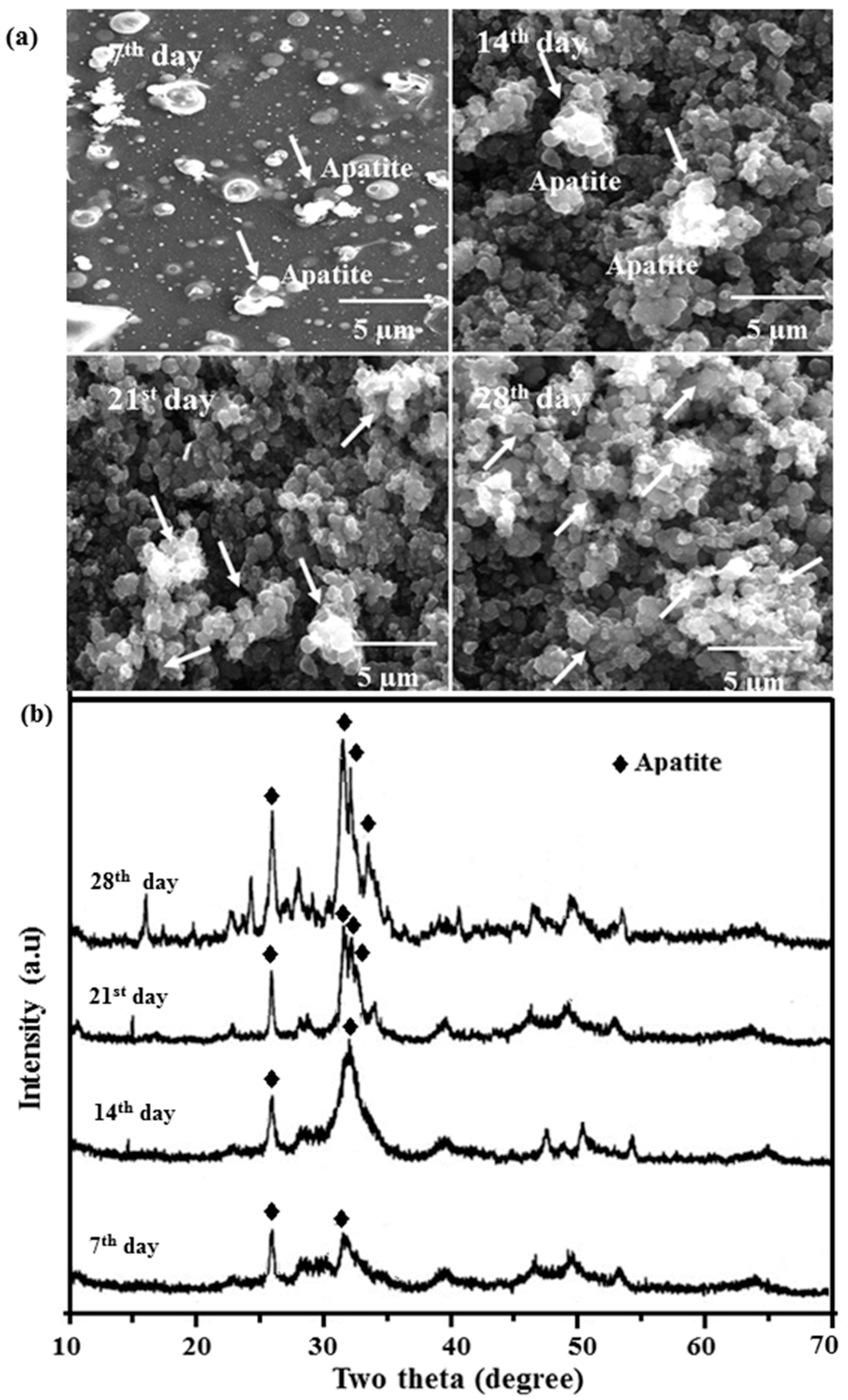
2.3. X-ray Diffraction (XRD) Analysis
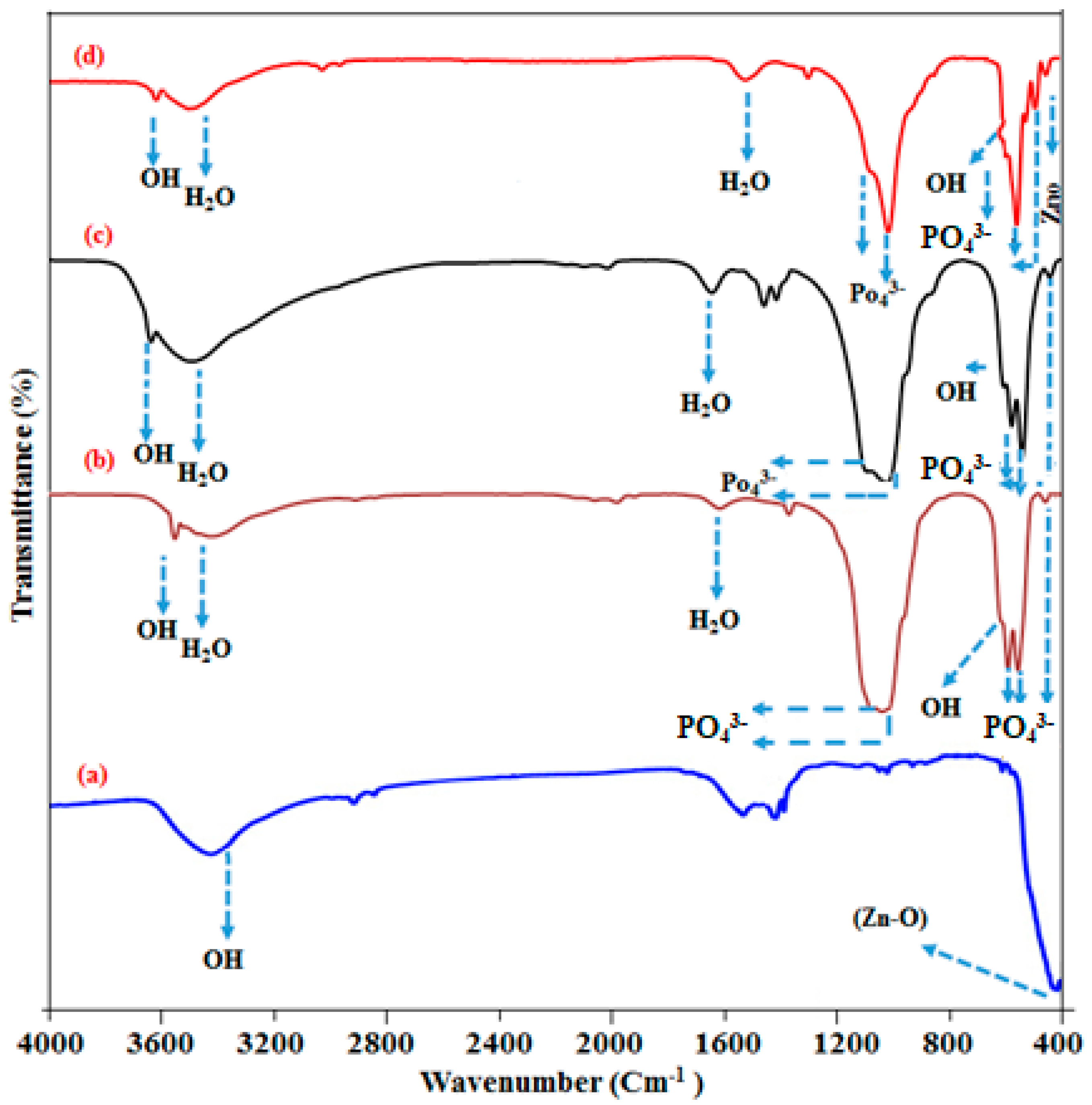
2.4. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
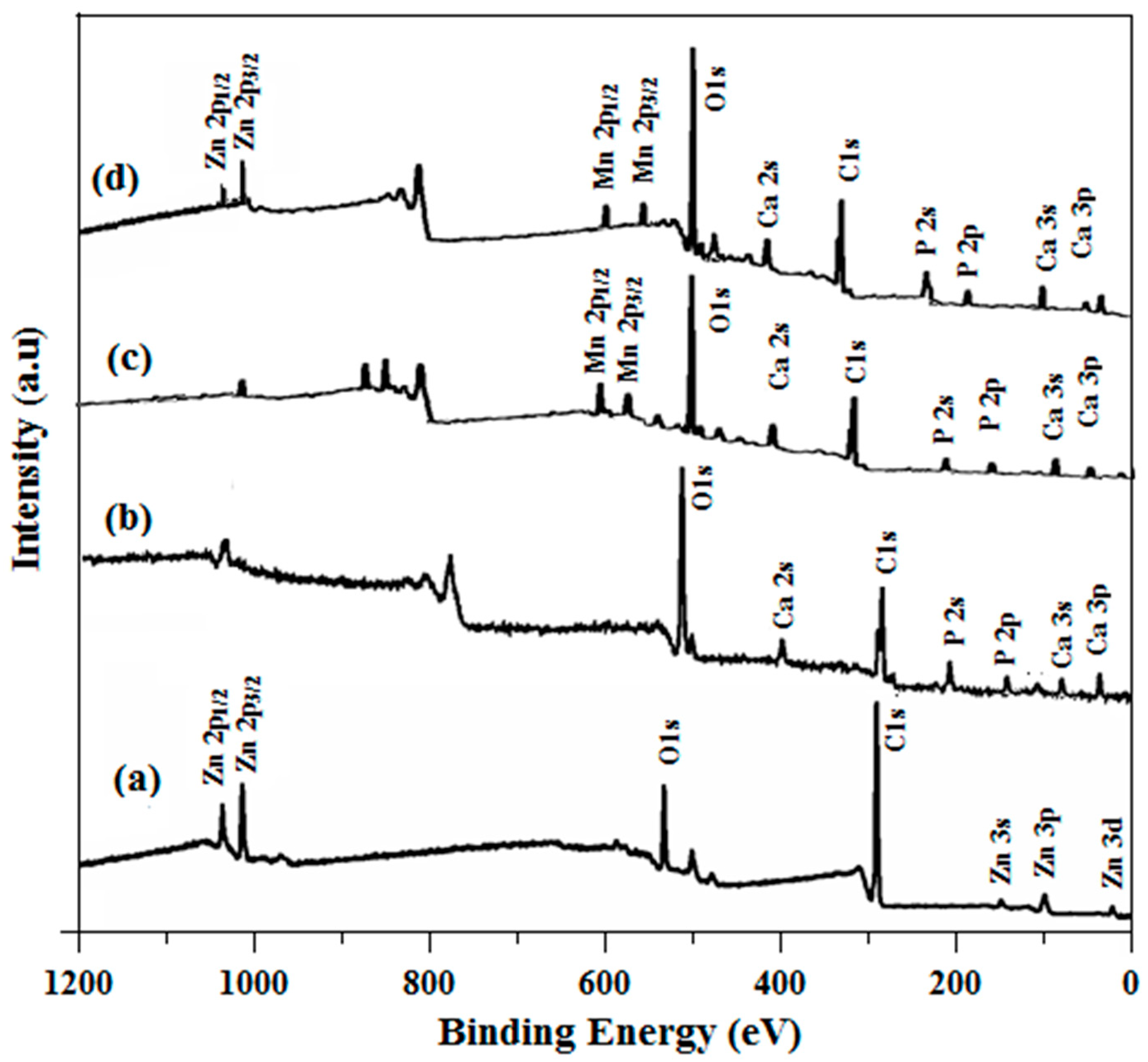
2.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.6. Mechanical Characterization
2.7. Electrochemical Characterization of Coating
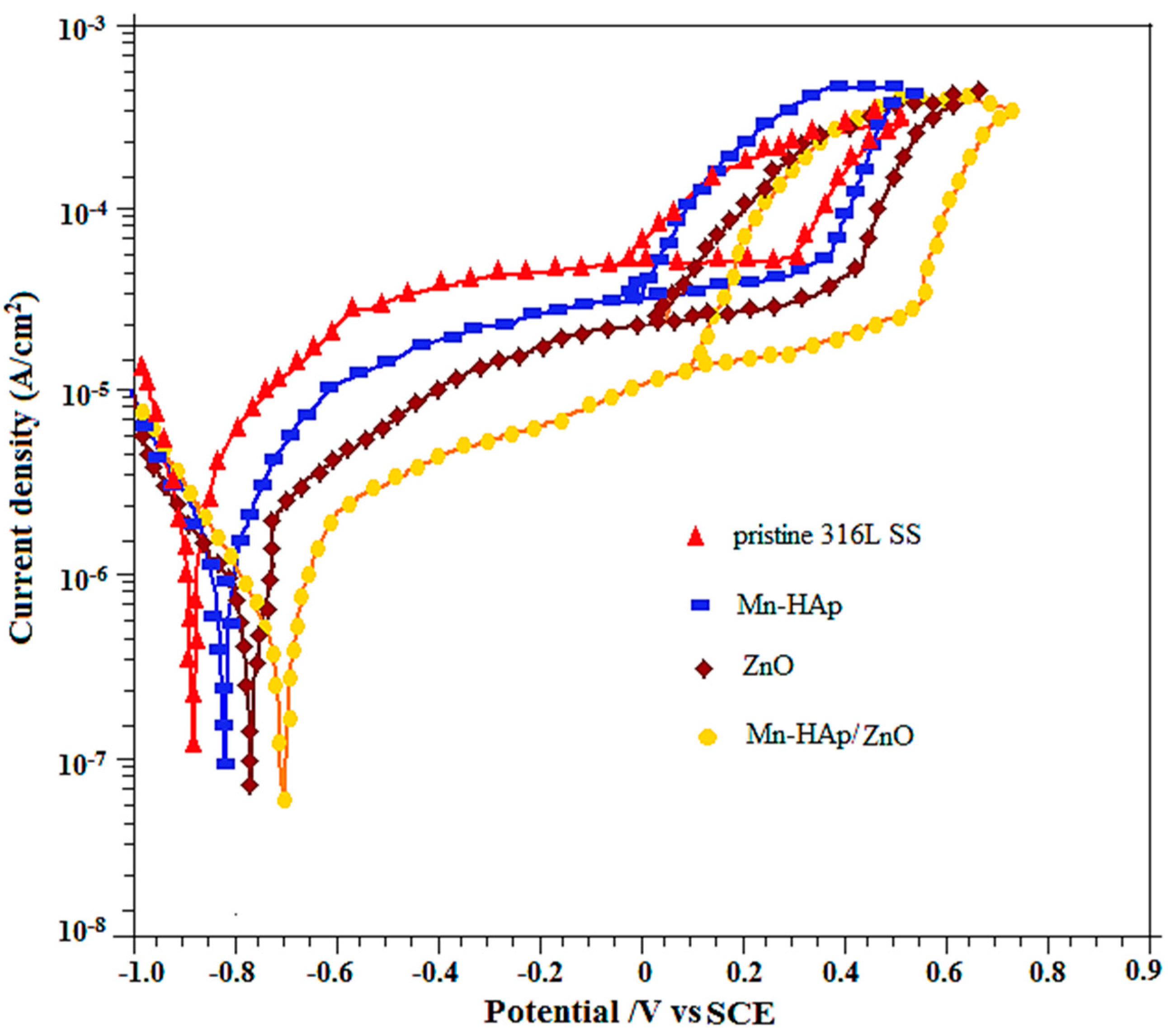
2.7.1. Potentiodynamic Polarization Measurements
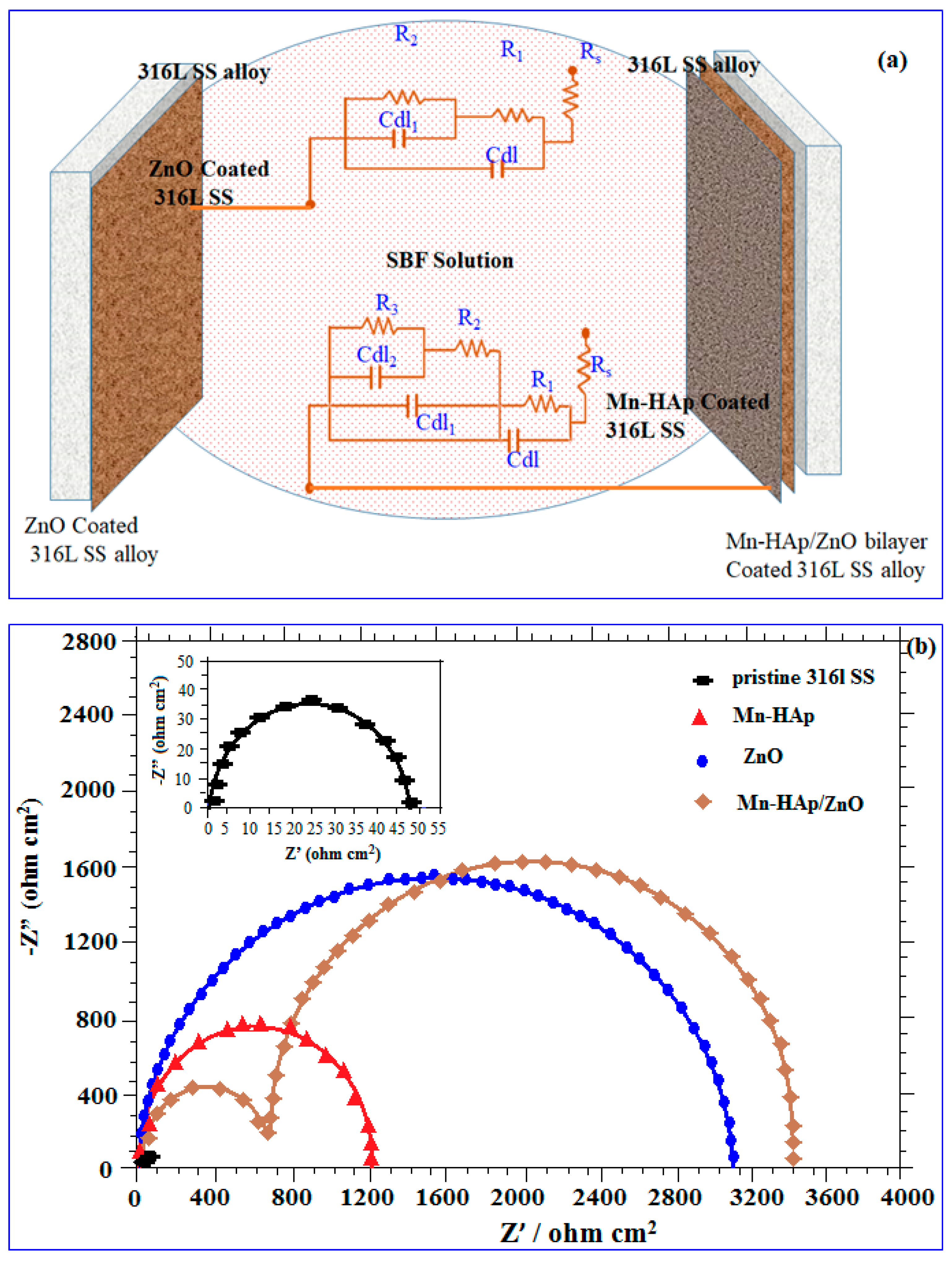
2.7.2. Electrochemical Impedance Spectroscopy (EIS) Analysis
2.8. In Vitro Bioactivity
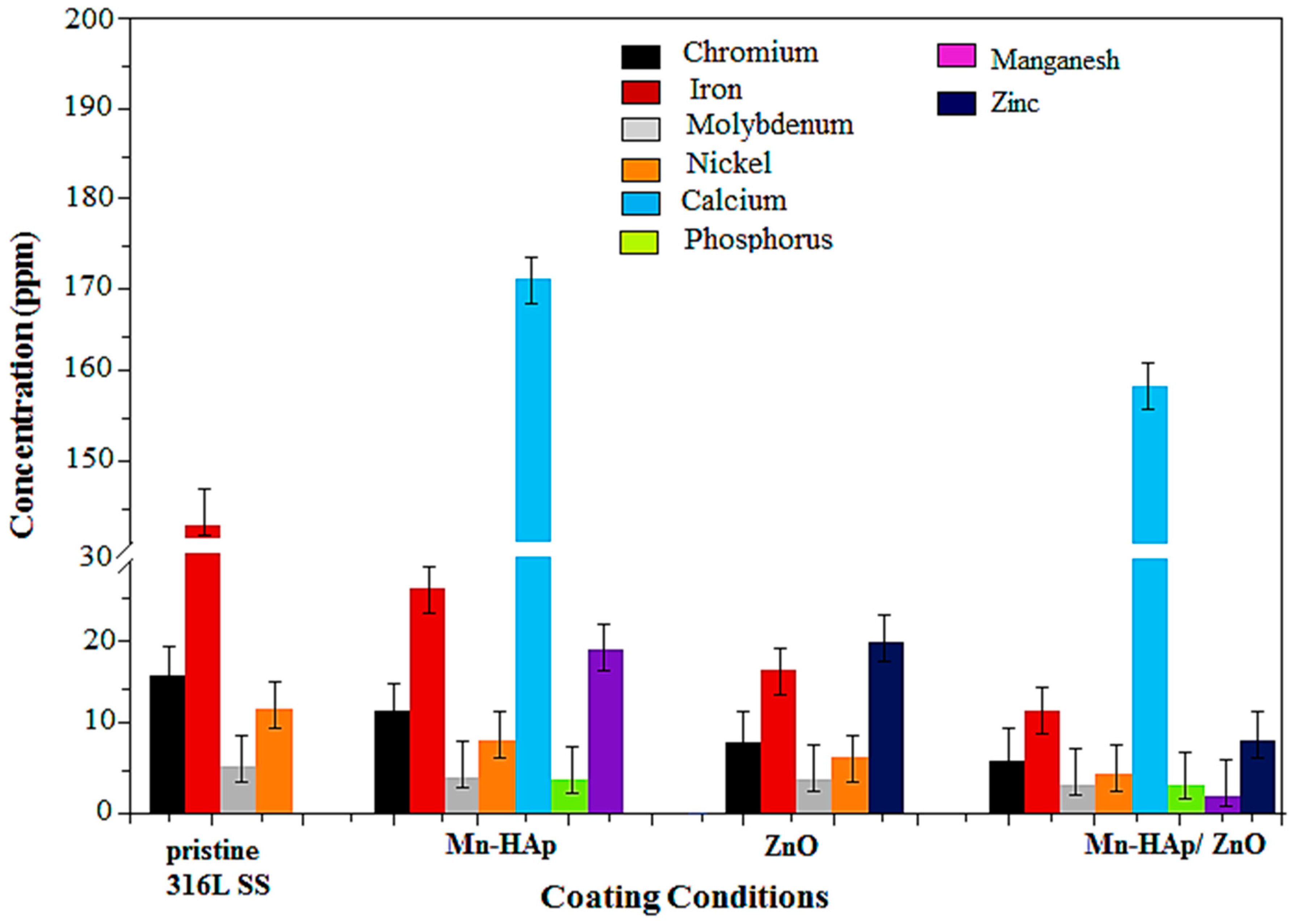
2.9. Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) Analysis
2.10. In Vitro Biocompatibility Cell Culture Studies
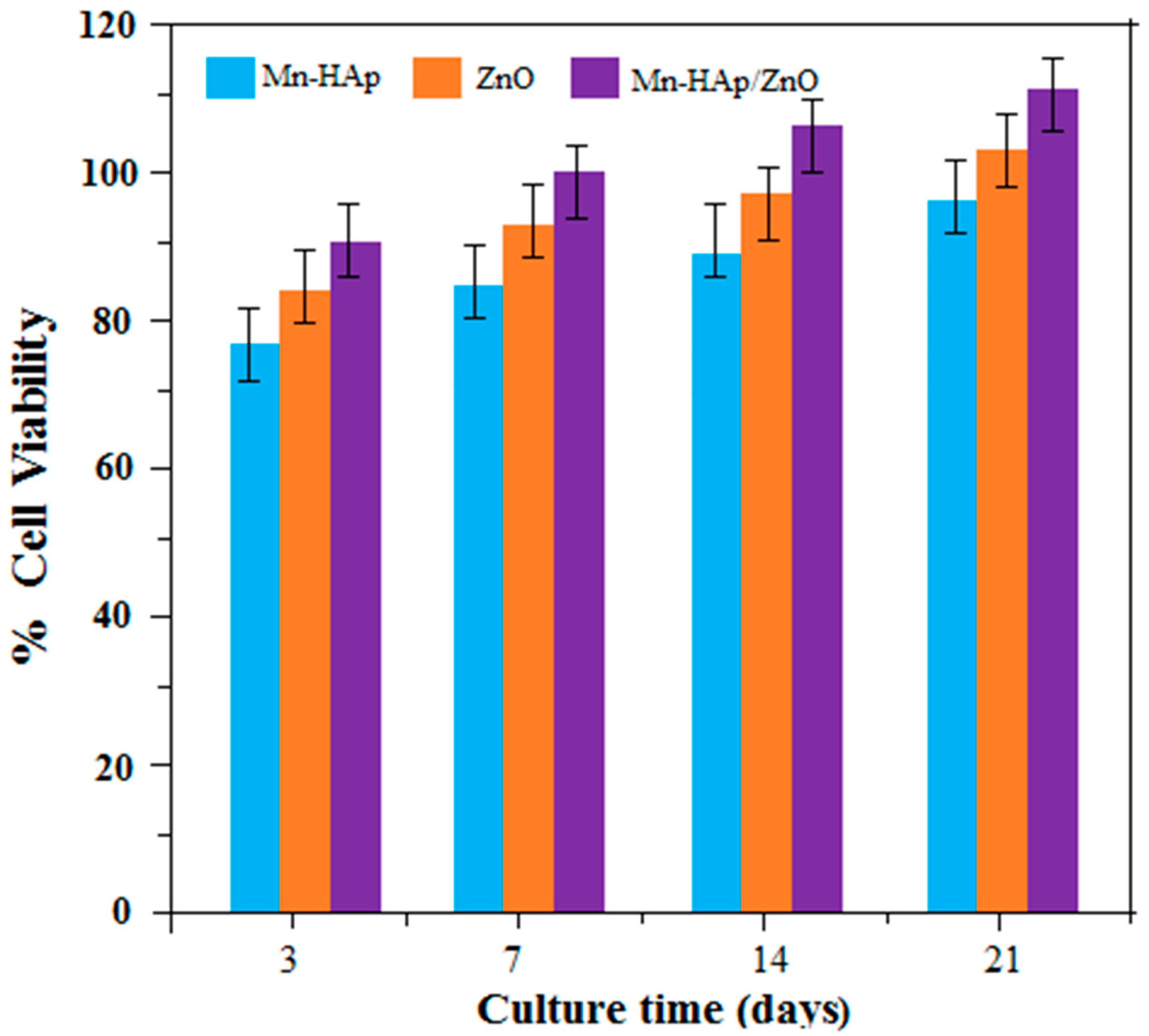
2.10.1. Cell Viability
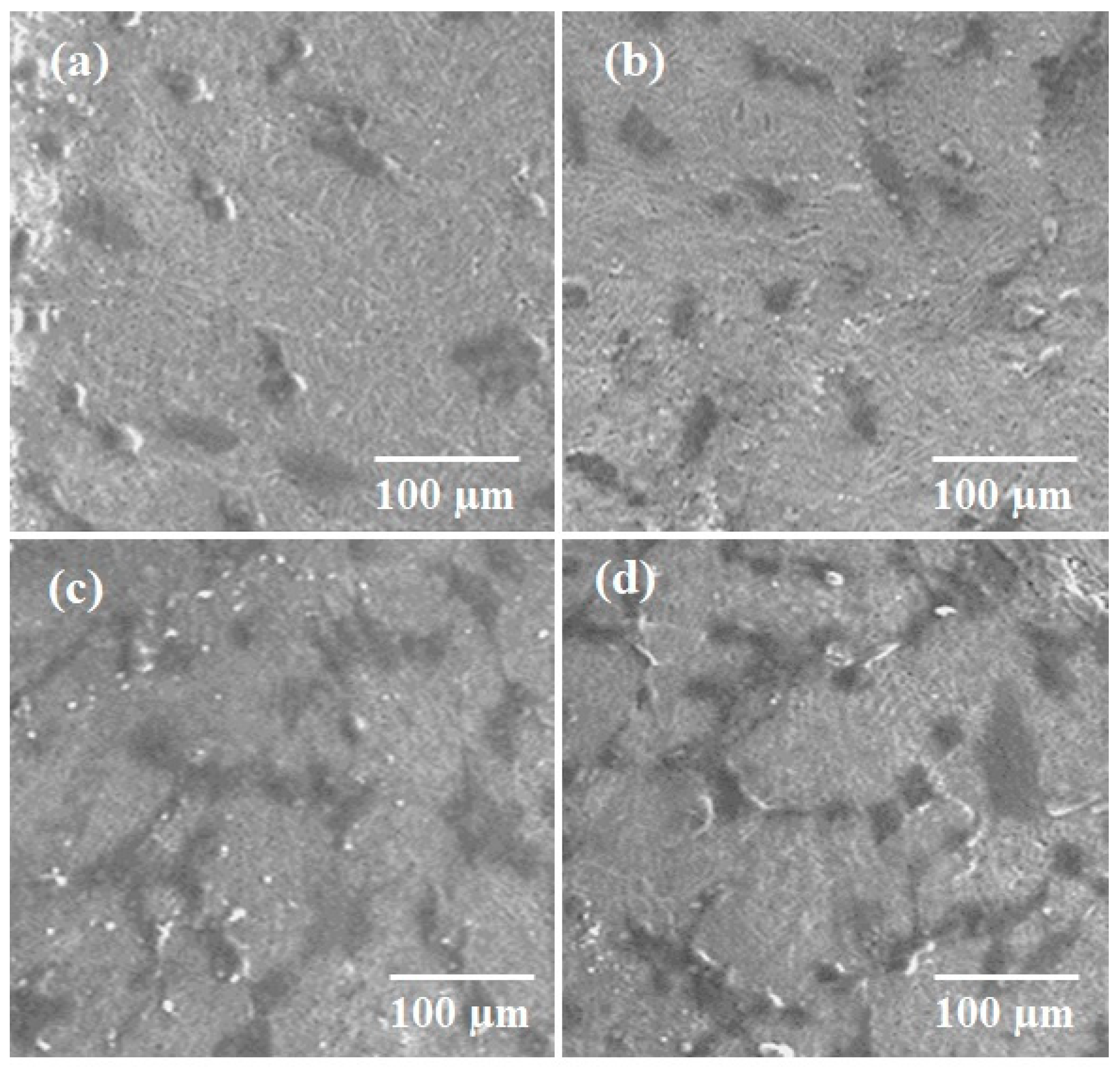
2.10.2. Cell Adhesion
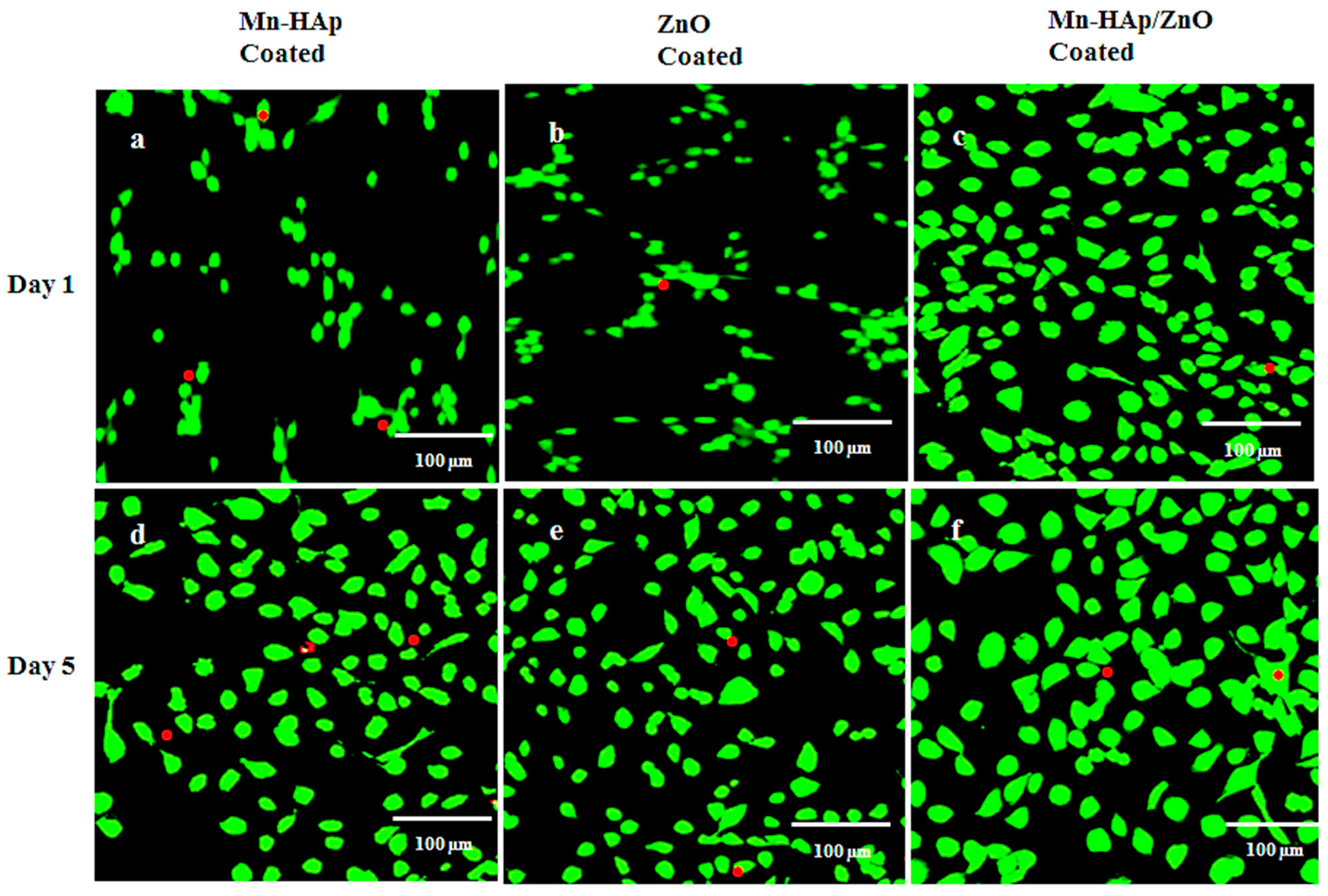
2.10.3. Live/Dead Assay
3. Materials and Methods
3.1. Materials
3.2. ZnO Coating on 316L SS
3.3. Mn-HAp Coating on 316L SS
3.4. Mn-HAp/ZnO Bilayer Coating on 316L SS
3.5. Surface Characterization of Coating
3.6. Mechanical Properties of Coatings
3.7. Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
3.8. Electrochemical Investigation of Coatings
3.9. Simulated Body Fluid (SBF) Solution Preparation
3.10. In Vitro Biocompatibility Analysis
3.10.1. Cell Cultures
3.10.2. Cell Viability
3.10.3. Cell Adhesion Test
3.10.4. Live/Dead Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Le, X.T.; Zeb, G.; Jegou, P.; Berthelot, T. Electrografting of stainless steel by the diazonium salt of 4-aminobenzylphosphonic acid. Electrochim. Acta 2012, 71, 66–72. [Google Scholar] [CrossRef]
- Balaceanu, M.; Petreus, T.; Braic, V.; Zoita, C.N.; Vladescu, A.; Cotrutz, C.E.; Braic, M. Characterization of Zr-based hard coatings for medical implant applications. Surf. Coat. Technol. 2010, 204, 2046–2050. [Google Scholar] [CrossRef]
- Sharifnabi, A.; Fathi, M.H.; Yekta, B.E.; Hossainalipour, M. The structural and bio-corrosion barrier performance of Mg-substituted fluorapatite coating on 316L stainless steel human body implant. Appl. Surf. Sci. 2014, 288, 331–340. [Google Scholar] [CrossRef]
- Azar, B.; Hashemi, B.; Mahboobeh, R. The effect of shot peening on fatigue and corrosion behavior of 316L stainless steel in Ringer’s solution. Surf. Coat. Technol. 2010, 204, 3546–3551. [Google Scholar] [CrossRef]
- Meth, S.; Savchenko, N.; Koltypin, M.; Starosvetsky, D.; Viva, F.A.; Groysman, A.; Sukenik, C.N. Corrosion studies of stainless steel protected by a TiO2 thin film deposited on a sulfonate-functionalized self-assembled monolayer. Corros. Sci. 2010, 52, 125–129. [Google Scholar] [CrossRef]
- Sanctis, D.; Gomez, L.; Pellegri, N.C.; Parodi, N.; Marajofsky, A.; Duran, A. Protective glass coatings on metallic substrates. J. Non-Cryst. Solids 1990, 121, 338–343. [Google Scholar] [CrossRef]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg–Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Guan, S.K.; Chen, J.; Wang, L.G.; Zhu, S.J.; Hu, J.H. Fabrication and characterization of rod-like nano-hydroxyapatite on MAO coating supported on Mg–Zn–Ca alloy. Appl. Surf. Sci. 2011, 257, 2231–2237. [Google Scholar] [CrossRef]
- Gopi, D.; Murugan, N.; Ramya, S.; Kavitha, L. Electrodeposition of a porous strontium substituted hydroxyapatite/zinc oxide duplex layer on AZ91 magnesium alloy for orthopedic applications. J. Mater. Chem. B 2014, 2, 5531–5540. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.D.; Macmanus-Driscoll, J.L. ZnO–nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, F.C.; Han, E.H. Effects of P/B on the properties of anticorrosive coatings with different particle size. Prog. Org. Coat. 2005, 53, 91–98. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A.S.; Sinha, T.J. Effect of nano-ZnO particles on the corrosion behavior of alkyd-based waterborne coatings. Prog. Org. Coat. 2009, 64, 371–382. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Han, E.; Wei, Y. Effects of Nano pigments on the corrosion resistance of alkyd coating. J. Mater. Sci. Technol. 2007, 23, 551–558. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef] [Green Version]
- Meurice, E.; Leriche, A.; Hornez, J.C.; Bouchart, F.; Rguti, E.; Boilet, L.; Descampsa, M.; Cambier, F. Functionalisation of porous hydroxyapatite for bone substitutes. J. Eur. Ceram. Soc. 2012, 32, 673–2678. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Dietrich, D.; Steinhaeuser, S.; Wielage, B. Electrocrystallization of nanocrystallite calcium phosphate coatings on titanium substrate at different current densities. Surf. Coat. Technol. 2008, 202, 5895–5900. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherland, 1994. [Google Scholar]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Ripamonti, A.; Roveri, N.; Romanello, M.; Noris, S.K.; Moro, L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J. Inorg. Biochem. 1997, 68, 45–51. [Google Scholar] [CrossRef]
- Gaines, R.V.; Skinner, H.C.V.; Foord, E.F.; Mason, B.; Rosenzweig, A. Dana’s New Mineralogy; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Mayer, I.; Cuisinier, F.J.G.; Gdalya, S.; Popov, I. TEM study of the morphology of Mn doped calcium hydroxyapatite and b-tri-calcium phosphate. J. Inorg. Biochem. 2008, 102, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Sopyan, I.; Ramesh, S.; Nawaw, N.A.; Tampieri, A.; Sprio, S. Effects of manganese doping on properties of sol–gel derived biphasic calcium phosphate ceramics. Ceram. Int. 2011, 37, 3703–3715. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Jang, J.H. Surface characteristics and in vitro biocompatibility of a manganese-containing titanium oxide surface. Appl. Surf. Sci. 2011, 258, 977–985. [Google Scholar] [CrossRef]
- Singh, G.; Singh, H.; Sidhu, B.S. In vitro, corrosion investigations of plasma-sprayed hydroxyapatite and hydroxyapatite-calcium phosphate coatings on 316L SS. Bull. Mater. Sci. 2014, 37, 1519–1528. [Google Scholar] [CrossRef]
- Marvis, B.C.; Tas, A.C. Dip Coating of calcium hydroxyapatite on Ti-6Al-4V substrates. J. Am. Ceram. Soc. 2000, 83, 989–991. [Google Scholar] [CrossRef]
- Subramanian, B.; Ananthakumar, R. Surface modification of 316L stainless steel with magnetron sputtered TiN/VN nanoscale multilayers for bio implant applications. J. Mater. Sci. Mater. Med. 2012, 23, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Ni, Y.J.; Huang, J.C. Fabrication and characterization of HAp/Al2O3 composite cating on titanium substrate. J. Biomed. Sci. Eng. 2008, 1, 190–194. [Google Scholar] [CrossRef]
- Wei, M.; Ruys, A.J.; Milthorpe, B.K.; Sorrell, C.C.; Evans, J.H. Electrophoretic deposition of hydroxyapatite coatings on metal substrates: A nanoparticulate dual-coating approach. J. Sol-Gel Sci. Technol. 2001, 21, 39–48. [Google Scholar] [CrossRef]
- Xiao, X.F.; Liu, R.F.; Zheng, Y.Z. Haracterization of hydroxyapatite/Ti6Al4V composite powder under various sintering temperature. Surf. Coat. Technol. 2006, 200, 4406–4413. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Li, J.; Zhao, S.; Zhang, X. Electrodeposition of Ca–P coatings on biodegradable Mg alloy: In vitro biomineralization behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Djosic, M.S.; Panic, V.; Stojanovic, J.; Mitric, M.; MiskovicStankovic, V.B. The effect of applied current density on the surface morphology of deposited calcium phosphate coatings on titanium. Colloids. Surf. A Physicochem. Eng. Asp. 2012, 400, 36–43. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, A.; Jiang, J.; Song, D.; Chen, J.; Yang, D. Anti-corrosion performance of waterborne Zn-rich coating with modified silicon-based vehicle and lamellar Zn (Al) pigments. Prog. Nat. Sci. Mater. Int. 2012, 22, 326–333. [Google Scholar] [CrossRef]
- Hong, R.Y.; Li, J.H.; Chen, L.L.; Liu, D.Q.; Li, H.Z.; Zheng, Y.; Ding, J. Synthesis, surface modification and photocatalytic property of ZnO nanoparticles. Powder Technol. 2009, 189, 426–432. [Google Scholar] [CrossRef]
- Kumar, R.; Prakash, K.H.; Cheang, P.; Khor, K.A. Temperature driven morphological changes of chemically precipitated hydroxyapatite nanoparticles. Langmuir 2004, 20, 5196–5200. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, R.N.; Nambiar, R.; Zaffar, H.S.; Malshe, V.C. Predictive power for life and residual life of the zinc rich primer coatings with electrical measurement. Prog Org Coat. 2007, 58, 253–258. [Google Scholar] [CrossRef]
- Rajendran, S.P.; Sengodan, K. Synthesis and characterization of zinc oxide and iron oxide nanoparticles using sesbania grandiflora leaf extract as reducing agent. J. Nanoscience 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Ananth, K.P.; Nathanael, A.J.; Jose, S.P.; Oh, T.H.; Mangalaraj, D. A novel silica nanotube reinforced ionic incorporated hydroxyapatite composite coating on polypyrrole coated 316L SS for implant application. Mater. Sci. Eng. C 2016, 59, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional au-zno plasmonic nanostructures for enhanced uv photodetector and room temperature no sensing devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [PubMed]
- Hongbo, B.L.; Campbell, T.C.; Graham, T.J.; Ratner, B.D. Surface characterization of hydroxyapatite and related calcium phosphates by xps and tof-sims. Anal. Chem. 2000, 72, 2886–2894. [Google Scholar]
- Vijayalakshmi, U.; Rajeswari, R. Influence of process parameters on the sol–gel synthesis of nano hydroxyapatite using various phosphorus precursors. J. Sol-Gel Sci. Technol. 2012, 63, 45–55. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Malathy, M.; Rajavel, R. Poly(ophenylenediaminecoaniline)/ZnO coated on passivated low nickel stainless steel. J. Sci. Adv. Mater. Device 2016, 2, 86–92. [Google Scholar] [CrossRef]
- Lopez, D.Z.; Simison, S.N.; de Sánchez, S.R. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochem. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Motalebi, A.; Nasr-Esfahani, M.; Ali, R.; Pourriahi, M. Improvement of corrosion performance of 316l stainless steel via pvtms/henna thin film. Prog. Nat. Sci. Mater. Int. 2012, 22, 392–400. [Google Scholar] [CrossRef]
- Savaloni, H.; Agha-Taheri, E.; Abdi, F. On the corrosion resistance of AISI 316L-type stainless steel coated with manganese and annealed with flow of oxygen. J. Theor. Appl. Phys. 2016, 10, 149–156. [Google Scholar] [CrossRef]
- Ostomel, T.A.; Shi, Q.; Tsung, C.K. Spherical bioactive glass with enhanced rates of hydroxyapatite deposition and hemostatic activity. Small 2006, 2, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhaoa, Q.; Guo, X.; Dangb, X.; Hao, J.; Lai, J.; Wang, K. Preparation and properties of composite MAO/ECD coatings on magnesium alloy. Colloids Surf. B Biointerface 2013, 102, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, G.E. Extracellular calcium as an integrator of tissue function. Int. J. Biochem. Cell Biol. 2008, 40, 1467–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondo, T.; Puckett, S.; Webster, T.J. Greater osteoblast and endothelial cell adhesion on nanostructured polyethylene and titanium. Int. J. Nanomed. 2010, 5, 647–652. [Google Scholar]
- Liu, G.; Li, G.; Qiu, X.; Li, L. Synthesis of ZnO/titanate nanocomposites with highly photocatalytic activity under visible light irradiation. J. Alloys Compd. 2009, 481, 492–497. [Google Scholar] [CrossRef]
- Lin, D.Y.; Wang, X.X. Preparation of hydroxyapatite coating on smooth implant surface by electrodeposition. Ceram. Int. 2011, 37, 403–406. [Google Scholar] [CrossRef]
- ASTM Standard F 1044-05; ASTM International: West Conshohocken, PA, USA, 2005.
- Kokubo, T.; Takadama, T. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]

| Polarization Parameter | ||||
|---|---|---|---|---|
| Sample Condition | Ecorr (mV vs. SCE) | Eb (mV) | Epp (mV) | Rb (Ω/cm2) |
| Pristine 316L SS | −874 | 348 | −75 | 48 |
| Mn-HAp | −832 | 410 | −48 | 1200 |
| ZnO | −781 | 486 | −26 | 2980 |
| Mn-HAp/ZnO | −696 | 574 | −92 | 3400 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ananth, K.P.; Sun, J.; Bai, J. An Innovative Approach to Manganese-Substituted Hydroxyapatite Coating on Zinc Oxide–Coated 316L SS for Implant Application. Int. J. Mol. Sci. 2018, 19, 2340. https://doi.org/10.3390/ijms19082340
Ananth KP, Sun J, Bai J. An Innovative Approach to Manganese-Substituted Hydroxyapatite Coating on Zinc Oxide–Coated 316L SS for Implant Application. International Journal of Molecular Sciences. 2018; 19(8):2340. https://doi.org/10.3390/ijms19082340
Chicago/Turabian StyleAnanth, Karuppasamy Prem, Jinxing Sun, and Jiaming Bai. 2018. "An Innovative Approach to Manganese-Substituted Hydroxyapatite Coating on Zinc Oxide–Coated 316L SS for Implant Application" International Journal of Molecular Sciences 19, no. 8: 2340. https://doi.org/10.3390/ijms19082340




