Novel Insights into Concepts and Directionality of Maternal–Fetal Cholesterol Transfer across the Human Placenta
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Isolated Trophoblasts and Efficiency of In Vitro Differentiation
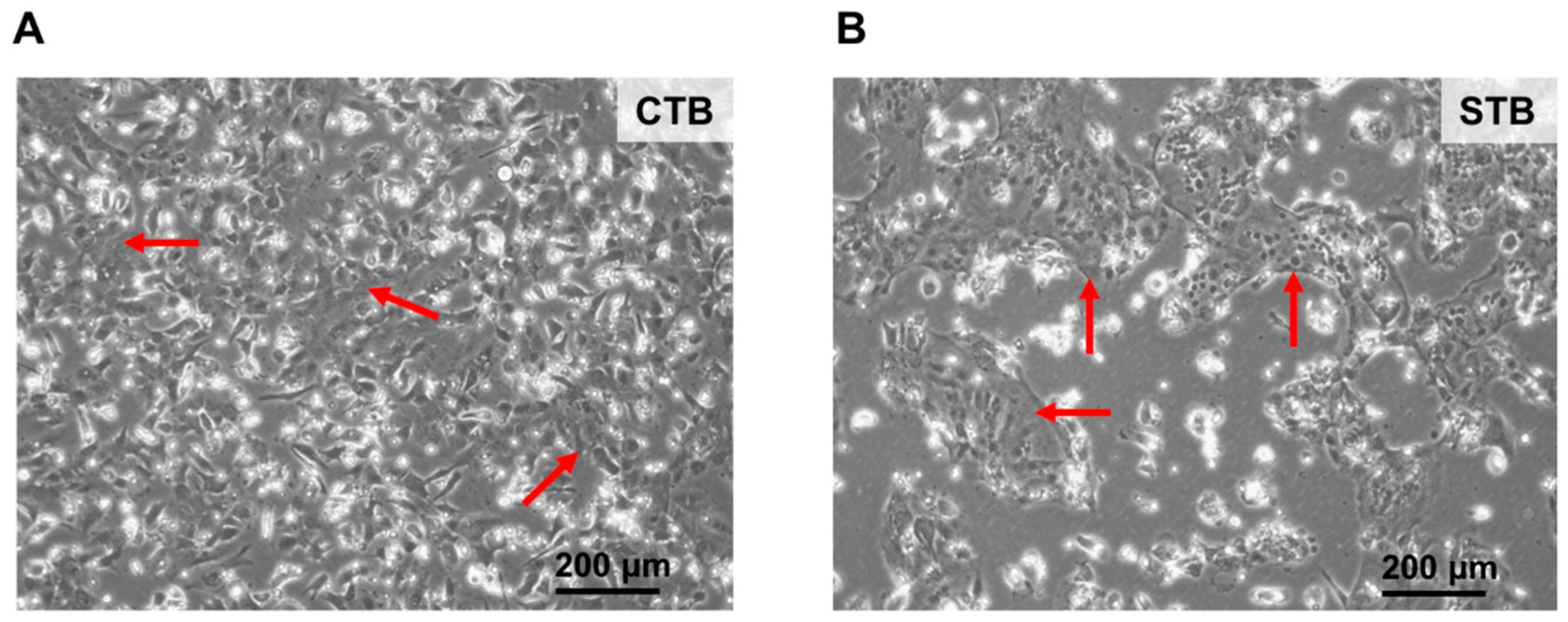
2.1.1. Cell Morphology
2.1.2. Protein Expression of Specific Cell Type Markers
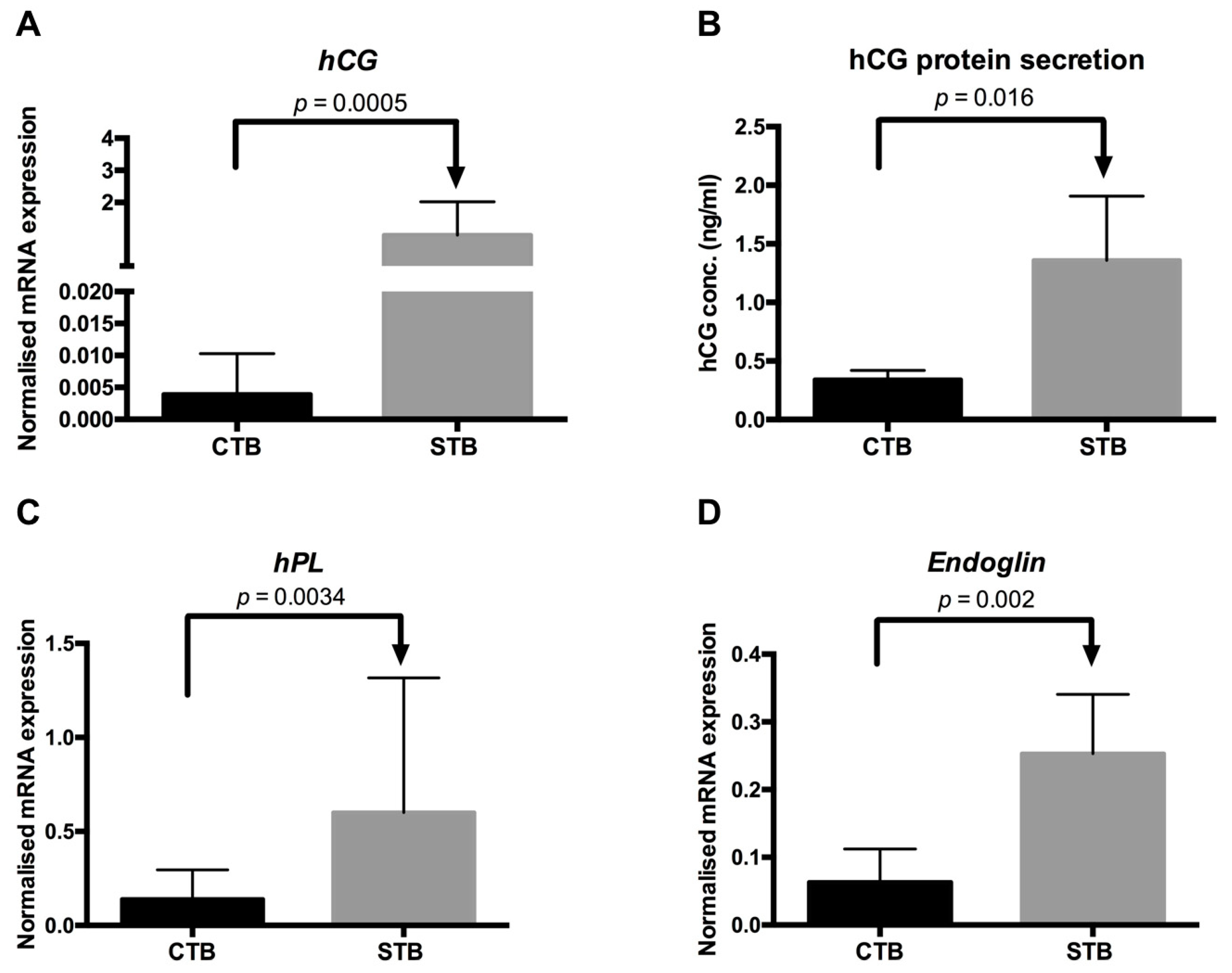
2.1.3. Secretion of Syncytial Factors
2.2. Cholesterol Transport in CTB and STB
2.2.1. Gene Expression Studies
2.2.2. Cholesterol Uptake: Inward Transport
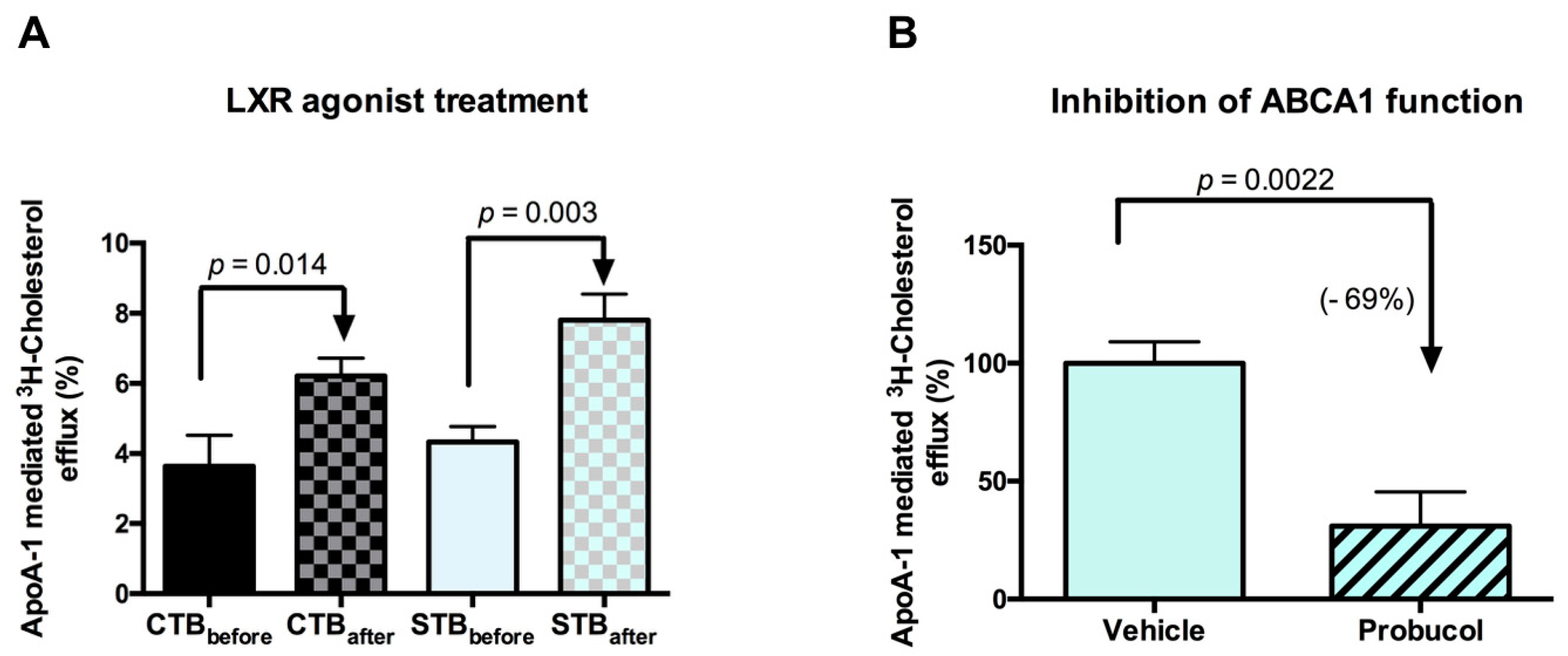
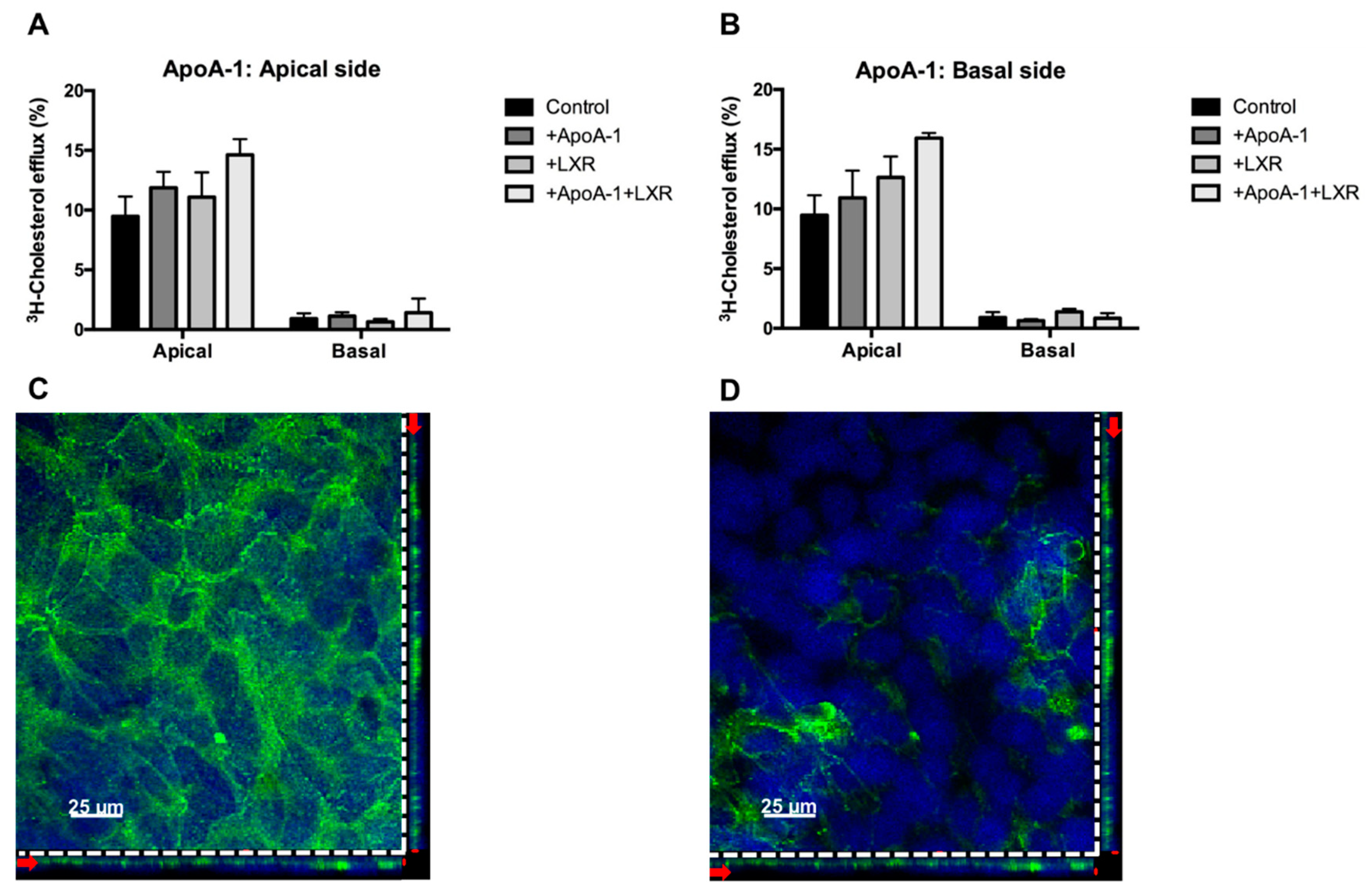
2.2.3. Cholesterol Efflux: Outward Transport
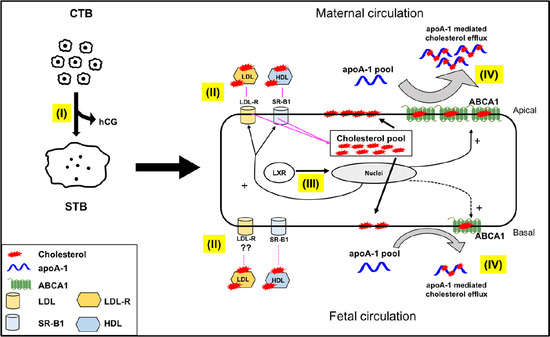
2.3. Directionality of the Cholesterol Transport and ABCA1 Localization
3. Materials and Methods
3.1. Human Placental Tissue Collection
3.2. Isolation of Primary Trophoblasts and Cell Culture
3.3. Cell Morphology
3.4. Flow Cytometry Analysis of Cell Purity
3.5. Quantitative RT-PCR
3.6. ELISA for hCG Secretion
3.7. Cholesterol Transport Studies
3.7.1. Cholesterol Transport in Conventional Plates
3.7.2. Cholesterol Transport in Transwell® System
3.8. Immunofluorescent Confocal Microscopy
3.9. Statistics
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cortes, V. Physiological and pathological implications of cholesterol. Front. Biosci. 2014, 19, 416. [Google Scholar] [CrossRef]
- Christenson, L.K.; Devoto, L. Cholesterol transport and steroidogenesis by the corpus luteum. Reprod. Biol. Endocrinol. 2003, 1, 90. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, M.; Tint, G.S.; Chen, J.; Xu, G.; Patel, S.B. Selective reconstitution of liver cholesterol biosynthesis promotes lung maturation but does not prevent neonatal lethality in Dhcr7 null mice. BMC Dev. Biol. 2007, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.W.; Newnham, J.P.; Challis, J.G.; Jobe, A.H.; Stock, S.J. The clinical use of corticosteroids in pregnancy. Hum. Reprod. Update 2016, 22, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.N.; Yang, M.L.; Hung, J.H.; Chou, P.; Shyn, S.K.; Ng, H.T. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci. 1995, 56, 2367–2375. [Google Scholar] [PubMed]
- Oram, F.J. HDL apolipoproteins and ABCA1 partners in the removal of excess cellular cholesterol. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Oram, F.J.; Vaughan, M.A. ABCA1-mediated transport of cellular cholesterol and phospholipids to HBL apolipoproteins. Curr. Opin. Lipidol. 2000, 11, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ontsouka, C.E.; Huang, X.; Aliyev, E.; Albrecht, C. In vitro characterization and endocrine regulation of cholesterol and phospholipid transport in the mammary gland. Mol. Cell. Endocrinol. 2017, 439, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Sadovsky, Y.; Jansson, T. Placenta and Placental Transport Function. In Knobil Neill’s Physiol Reproduction; Academic Press: Cambridge, MA, USA, 2014; pp. 1741–1782. [Google Scholar] [CrossRef]
- Stefulj, J.; Panzenboeck, U.; Becker, T.; Hirschmugl, B.; Schweinzer, C.; Lang, I.; Marsche, G.; Sadjak, A.; Lang, U.; Desoye, G.; et al. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ. Res. 2009, 104, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Ietta, F.; Bechi, N.; Romagnoli, R.; Paulesu, L. Localisation of ABCA1 in first trimester and term placental tissues—A reply. Placenta 2010, 31, 941. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Ietta, F.; Giacomello, E.; Bechi, N.; Romagnoli, R.; Fava, A.; Paulesu, L. Expression and localization of ATP binding cassette transporter A1 (ABCA1) in first trimester and term human placenta. Placenta 2010, 31, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, L.; Wenger, F.; Baumann, M.; Surbek, D.; Körner, M.; Albrecht, C. Expression and localization pattern of ABCA1 in diverse human placental primary cells and tissues. Placenta 2011, 32, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Körner, M.; Huang, X.; Wenger, F.; Surbek, D.; Albrecht, C. Placental ABCA1 and ABCG1 expression in gestational disease: Pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta 2013, 34, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Mistry, D.H.; Kurlak, O.L.; Mansour, T.Y.; Zurkinden, L.; Mohaupt, G.M.; Escher, G. Increased maternal and fetal cholesterol efflux capacity and placental CYP27A1 expression in preeclampsia. J. Lipid Res. 2017, 58, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindegaard, L.M.; Wassif, A.C.; Vaisman, B.; Amar, M.; Wasmuth, E.V.; Shamburek, R.; Nielsen, B.L.; Remaley, T.A.; Porter, D.F. Characterization of placental cholesterol transport: ABCA1 is a potential target for in utero therapy of Smith-Lemli-Opitz syndrome. Hum. Mol. Genet. 2008, 17, 3806–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanthaler, B.; Steichen-Gersdorf, E.; Kollerits, B.; Zschocke, J.; Witsch-Baumgartner, M. Maternal ABCA1 genotype is associated with severity of Smith-Lemli-Opitz syndrome and with viability of patients homozygous for null mutations. Eur. Hum. Genet. 2013, 21, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Plösch, T.; Van Straten, E.M.E.; Kuipers, F. Cholesterol Transport by the Placenta: Placental Liver X Receptor Activity as a Modulator of Fetal Cholesterol Metabolism? Placenta 2007, 28, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Costet, P.; Luo, Y.; Wang, N.; Tall, R.A. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000, 275, 28240–28245. [Google Scholar] [CrossRef] [PubMed]
- Chengmao, X.; Li, L.; Yan, L.; Jie, Y.; Xiaoju, W.; Xiaohui, C.; Huimin, G. ABCA1 affects placental function via trophoblast and macrophage. Life Sci. 2017, 191, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Plösch, T.; Gellhaus, A.; Van Straten, E.M.E.; Wolf, N.; Huijkman, N.C.A.; Schmidt, M.; Dunk, E.C.; Kuipers, F.; Winterhager, E. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: A reason for alterations in preeclampsia? Placenta 2010, 31, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Goldstein, L.J. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA 1999, 96, 11041–11048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Asthana, S.; Kraemer, F.; Azhar, S. Scavenger receptor B type 1: Expression, Molecular Regulation, and Cholesterol Transport Function. J. Lipid Res. 2018, 59, 1114–1131. [Google Scholar] [CrossRef] [PubMed]
- Ontsouka, C.E.; Albrecht, C. Cholesterol transport and regulation in the mammary gland. J. Mammary Gland Biol. Neoplasia 2014, 19, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Natesampillai, S.; Fernandez-Zapico, E.M.; Urrutia, R.; Veldhuis, D.J. A novel functional interaction between the Sp1-like protein KLF13 and SREBP-Sp1 activation complex underlies regulation of low density lipoprotein receptor promoter function. J. Biol. Chem. 2006, 281, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.; Phillips, T.; Ka, H.; Pace, J.; Hunt, J. Isolation and Culture of Term Human Trophoblast Cells. In Placenta Trophobl; SE-16; Humana Press: Totowa, NJ, USA, 2006; pp. 203–217. [Google Scholar] [CrossRef]
- Contractor, F.S.; Routledge, A.; Sooranna, R.S. Identification and estimation of cell types in mixed primary cell cultures of early and term human placenta. Placenta 1984, 5, 41–53. [Google Scholar] [CrossRef]
- Maldonado-Estrada, J.; Menu, E.; Roques, P.; Barré-Sinoussi, F.; Chaouat, G. Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J. Immunol. Methods 2004, 286, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Schust, J.D. Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod. Biol. Endocrinol. 2015, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, L.; Marcus, G.S.; Vasile, J.; Dobryansky, M.; Cohen, H.; Eng, K.; Shamamian, P.; Mignatti, P. Expression of von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: A potential method for objective assessment of tumor angiogenesis. Int. J. Cancer 2000, 85, 281–288. [Google Scholar] [CrossRef]
- Batzer, F. Hormonal evaluation of early pregnancy. Fertil. Steril. 1980, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gregory, L.A.; Xu, G.; Sotov, V.; Letarte, M. Review: The enigmatic role of endoglin in the placenta. Placenta 2014, 35, S93–S99. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Lei, Z.; Rao, C.; Lin, J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 1993, 132, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Gougos, A.; St Jacques, S.; Greaves, A.; O’Connell, P.J.; d’Apice, A.J.; Bühring, H.J.; Bernabeu, C.; van Mourik, J.A.; Letarte, M. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int. Immunol. 1992, 4, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.K.O.; Tsui, N.B.Y.; Lau, K.T.; Leung, N.T.; Chiu, R.W.K.; Panesar, S.N.; Lit, L.C.W.; Chan, K.-W.; Lo, Y.M.D. mRNA of placental origin is readily detectable in maternal plasma. Proc. Natl. Acad. Sci. USA 2003, 100, 4748–4753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, J.K.; Schmittgen, D.T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, X.; Xu, Y.; Lin, Y.; Zhu, N.; Liu, P.; Lu, D.; Si, S. Identification of a Novel Liver X Receptor Agonist that Regulates the Expression of Key Cholesterol Homeostasis Genes with Distinct Pharmacological Characteristics. Mol. Pharmacol. 2017, 91, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Rhainds, D.; Brissette, L. The role of scavenger receptor class B type I (SR-BI) in lipid trafficking: Defining the rules for lipid traders. Int. J. Biochem. Cell Biol. 2004, 36, 39–77. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, S.; Ma, W.W.; Cai, P.X.; Le, Y.Z.; Xiao, R.; Zhou, Q.; Yu, L.H. Modulation of cholesterol transport by maternal hypercholesterolemia in Human fullterm placenta. PLoS ONE 2017, 12, e0171934. [Google Scholar] [CrossRef]
- Ontsouka, E.C.; Burgener, A.I.; Mani, O.; Albrecht, C. Polyunsaturated fatty acid-enriched diets used for the treatment of canine chronic enteropathies decrease the abundance of selected genes of cholesterol homeostasis. Domest. Anim. Endocrinol. 2010, 38, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Malerød, L.; Juvet, K.L.; Hanssen-Bauer, A.; Eskild, W.; Berg, T. Oxysterol-activated LXRα/RXR induces hSR-BI-promoter activity in hepatoma cells and preadipocytes, Biochem. Biophys. Res. Commun. 2002, 299, 916–923. [Google Scholar] [CrossRef]
- Larkin, C.J.; Sears, B.S.; Sadovsky, Y. The influence of ligand-activated LXR on primary human trophoblasts. Placenta 2014, 35, 919–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Kopp, S.; Strutz, J.; Chakravarthi, C.; Zandl-lang, M.; Fanaee-danesh, E.; Kirsch, A.; Cvitic, S.; Sa, R. BBA—Molecular and Cell Biology of Lipids Gestational diabetes mellitus modulates cholesterol homeostasis in human fetoplacental endothelium. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Waddell, J.B.; Mark, J.P.; Keelan, A.J. Placental ABCA1 and ABCG1 transporters efflux cholesterol and protect trophoblasts from oxysterol induced toxicity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.C.; Tsujita, M.; Hayashi, M.; Yokoyama, S. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J. Biol. Chem. 2004, 279, 30168–30174. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lüthi, M.; Ontsouka, C.E.; Kallol, S.; Baumann, U.M.; Surbek, D.V.; Albrecht, C. Establishment of a confluent monolayer model with human primary trophoblast cells. Mol. Hum. Reprod. 2016, 22, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, A.D.; Paxton, W.J.; Keelan, A.J. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1357–R1365. [Google Scholar] [CrossRef] [PubMed]
- Woollett, A.L. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32 (Suppl. 2), S218–S221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, K.E.; Davidson, W.S.; Myatt, L.; Woollett, L.A. Transport of cholesterol across a BeWo cell monolayer: Implications for net transport of sterol from maternal to fetal circulation. J. Lipid Res. 2003, 44, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W. The fetal origins of atherosclerosis: Maternal hypercholesterolemia, and cholesterol-lowering or antioxidant treatment during pregnancy influence in utero programming and postnatal susceptibility to atherogenesis. FASEB J. 2002, 16, 1348–1360. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; D’Armiento, P.F.; Witztum, L.J.; De Nigris, F.; Casanada, F.; Condorelli, M.; Silvestre, M.; Napoli, C. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ. Res. 2001, 89, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Davis, P.E.; Shahbaba, B.; Pruessner, C.J.; Head, K.; Sandman, A.C. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. USA 2012, 109, E1312–E1319. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Anderle, P.; Hostettler, L.; Baumann, U.M.; Surbek, D.V.; Ontsouka, C.E.; Albrecht, C. Identification of placental nutrient transporters associated with intrauterine growth restriction and pre-eclampsia. BMC Genom. 2018, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Ontsouka, C.E.; Huang, X.; Stieger, B.; Albrecht, C. Characteristics and Functional Relevance of Apolipoprotein-A1 and Cholesterol Binding in Mammary Gland Tissues and Epithelial Cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Accession Number | Primer Pairs (5’ end to 3’ end) | Product Length (bp) |
|---|---|---|---|
| ABCA1 | XM_011518342.3 | For: CCACATTTTTGCCTGGGACG Rev: AGCGATTCTCCCCAAACCTT | 88 |
| ApoA-1 | NM_001318021.1 | For: AGCGGCAGAGACTATGTG Rev: CTGTCCCAGTTGTCAAGG | 83 |
| Endoglin | NM_001114753.2 | For: CAAGACCAGGAAGTCCATA Rev: CGTGTGCGAGTAGATGTA | 174 |
| LDL-R | XM_011528010.2 | For: GACGTGGCGTGAACATCTG Rev: CTGGCAGGCAATGCTTTGG | 111 |
| SR-BI | NM_005505.4 | For: CGGCTCGGAGAGCGACTAC Rev: GGGCTTATTCTCCATGATCACC | 76 |
| SREBP-2 | XM_017028922.2 | For: CCTTCCTCTGCCTCTCCTTT Rev: CACAAAGACGCTCAGGACAA | 200 |
| YWHAZ | XM_024447266.1 | For: CCGTTACTTGGCTGAGGTTG Rev: AGTTAAGGGCCAGACCCAGT | 143 |
| Steady State | LXR Agonist Stimulation | |||
|---|---|---|---|---|
| CTB | STB | CTB | STB | |
| mRNA Levels | ||||
| SR-B1 (×10−3) | 13.4 ± 5.90 | 15.0 ± 5.95 A | 16.4 ± 4.90 X | 21.2 ± 5.98 B,Y |
| LDL-R (×10−3) | 14.8 ± 8.7 a,* | 15.8 ± 10.9 A | 67.1 ± 54.6 b,* | 44.5 ± 25.9 B |
| ABCA1 (×10−3) | 13.7 ± 8.76 a | 21.0 ± 20.4 A | 132.8 ± 76.9 b | 117.8 ± 42.0 B |
| SREBP-2 (×10−2) | 3.6 ± 2.2 a | 4.7 ± 4.4 A | 8.6 ± 5.5 b | 7.4 ± 3.2 B |
| ApoA-1 (×10−5) | 6.9 ± 3.6 | 9.7 ± 12 | 6.6 ± 5 | 6.3± 2.7 |
| Cholesterol Uptake (CU) | ||||
| % CU | 13.5 ± 3.60 | 15.1 ± 3.98 A | 13.4 ± 3.75 X | 21.8 ± 3.49 B,Y |
| Correlation Coefficients | ||||
| CU vs. SR-B1 mRNA | r = −0.3; p = 0.302 | r = 0.5; p = 0.094 | r = 0.2; p = 0.478 | r = 0.2; p = 0.56 |
| CU vs. LDL-R | r = −0.52; p = 0.078 | r = −0.16; p = 0.60 | r = 0.59; p = 0.04 | r = −0.07; p = 0.80 |
| CU vs. SREBP-2 mRNA | r = 0.7; p = 0.010 | r = 0.6; p = 0.041 | r = 0.8; p = 0.004 | r = 0.2; p = 0.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallol, S.; Huang, X.; Müller, S.; Ontsouka, C.E.; Albrecht, C. Novel Insights into Concepts and Directionality of Maternal–Fetal Cholesterol Transfer across the Human Placenta. Int. J. Mol. Sci. 2018, 19, 2334. https://doi.org/10.3390/ijms19082334
Kallol S, Huang X, Müller S, Ontsouka CE, Albrecht C. Novel Insights into Concepts and Directionality of Maternal–Fetal Cholesterol Transfer across the Human Placenta. International Journal of Molecular Sciences. 2018; 19(8):2334. https://doi.org/10.3390/ijms19082334
Chicago/Turabian StyleKallol, Sampada, Xiao Huang, Stefan Müller, Corneille Edgar Ontsouka, and Christiane Albrecht. 2018. "Novel Insights into Concepts and Directionality of Maternal–Fetal Cholesterol Transfer across the Human Placenta" International Journal of Molecular Sciences 19, no. 8: 2334. https://doi.org/10.3390/ijms19082334