Diosgenin Glucoside Protects against Spinal Cord Injury by Regulating Autophagy and Alleviating Apoptosis
Abstract
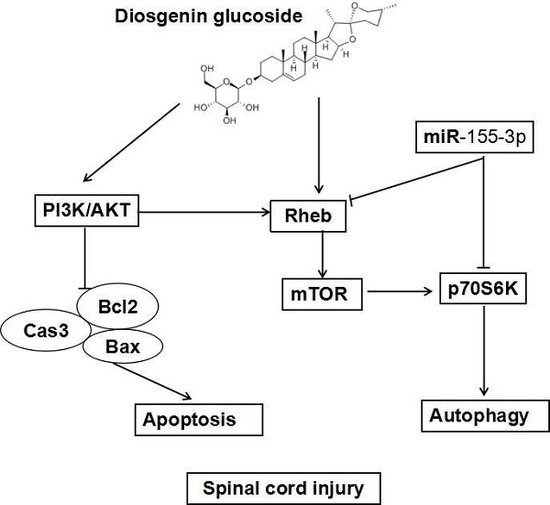
:1. Introduction
2. Results
2.1. DG Decreased the Structural Damage of Spinal Cord Tissue and Promoted Functional Recovery after SCI
2.2. DG Attenuated Apoptosis Caused by SCI
2.3. DG Down-Regulated miR-155-3p in Spinal Cord Tissue after SCI
2.4. DG-Activated Rheb/mTOR Signal Pathway after SCI
2.5. DG Rescued Abnormal Autophagy in Spinal Cord Tissues of SCI Rats
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Animal Grouping and Treatments
4.3. Functional Evaluation
4.4. Sampling
4.5. Hematoxylin-Eosin (HE) and Nissl Staining
4.6. Immunochemistry
4.7. Transmission Electron Microscopy Evaluation
4.8. Terminal-Deoxynucleotidyl Transferase (TdT) Mediated Nick End Labeling (TUNEL) Assay
4.9. Protein Extraction from Spinal Cord Tissue and Western Blot
4.10. Real-Time PCR of miR-155-3p
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cripps, R.A.; Lee, B.B.; Wing, P.; Weerts, E.; Mackay, J.; Brown, D. A global map for traumatic spinal cord injury epidemiology: Towards a living data repository for injury prevention. Spinal Cord 2011, 49, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, X.; Zhu, X.; Cao, J.; Xu, D.; Ni, Y.; Liu, Y.; Yan, S.; Cheng, X.; Liu, Y. Expression of SGTA correlates with neuronal apoptosis and reactive gliosis after spinal cord injury. Cell Tissue Res. 2014, 358, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, C.; Xiao, J.R.; Wei, H.F.; Zhou, X.H.; Mao, X.X.; Zhang, W.D.; Qian, R.; Chen, X.L.; He, M.Q. An Up-regulation of IRF-1 After a Spinal Cord Injury: Implications for Neuronal Apoptosis. J. Mol. Neurosci. 2015, 57, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.B.; Li, S.X.; Chen, X.P.; Yang, L.; Zhang, Y.G.; Liu, R.; Tao, L.Y. Autophagy is activated and might protect neurons from degeneration after traumatic brain injury. Neurosci. Bull. 2008, 24, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Kanno, H.; Ozawa, H.; Yamaya, S.; Itoi, E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J. Neurotrauma 2012, 29, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Fong, T.H.; Hsu, P.W.; Chiu, W.T. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J. Surg. Res. 2013, 179, e203–e210. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, H.; Bai, X.; Lu, Y.; Dong, H.; Xiong, L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 2011, 1402, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Ozawa, H.; Sekiguchi, A.; Yamaya, S.; Itoi, E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine 2011, 36, e1427–e1434. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.L.; Walker, M.J.; Liu, N.K.; Risberg, E.C.; Gao, X.; Chen, J.; Xu, X.M. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS ONE 2012, 7, e30012. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.H.; Wang, L.; Guo, Z.J.; Bai, L.; Zhang, R.P.; Shuang, W.B.; Jia, Y.J.; Wang, J.; Li, X.Y.; Liu, Q. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neurosci. Bull. 2013, 29, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andorfer, C.A.; Necela, B.M.; Thompson, E.A.; Perez, E.A. MicroRNA signatures: Clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.H.; Davis, A.J.; Lee, T.L.; Pang, A.L.; Nagrani, S.; Rennert, O.M.; Chan, W.Y. Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene 2011, 30, 3404–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellago, H.; Preschitz-Kammerhofer, B.; Terlecki-Zaniewicz, L.; Schreiner, C.; Fortschegger, K.; Chang, M.W.; Hackl, M.; Monteforte, R.; Kühnel, H.; Schosserer, M.; et al. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell 2013, 12, 446–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovicic, A.; Jolissaint, J.F.Z.; Moser, R.; Silva, Santos M. de F.; Luthi-Carter, R. MicroRNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific Huntington’s disease-related mechanisms. PLoS ONE 2013, 8, e54222. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. miR-155 gene: A typical multifunctional microRNA. Biochim. Biophys. Acta 2009, 1792, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Lu, C.; Diao, N.; Zhang, S.; Wang, S.; Wang, F.; Gao, Y.; Chen, J.; Shao, L.; Lu, J.; et al. Analysis of microRNA expression profiling identifies miR-155 and miR-155 as potential diagnostic markers for active tuberculosis: A preliminary study. Hum. Immunol. 2012, 73, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, M.; Li, M.; Li, D.; Peng, A.; Nie, X.; Sun, M.; Wang, J.; Wu, Y.; Deng, Q.; et al. miR-155 suppresses bacterial clearance in Pseudomonas aeruginosa-induced keratitis by targeting Rheb. J. Infect. Dis. 2014, 210, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, K.; Zhou, L.; Wu, M.; Wu, Y.; Zhu, M.; Lai, X.; Chen, T.; Feng, L.; Li, M.; et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013, 9, e1003697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.R.; Cao, Y.C.; Tian, J.L.; Zhen, D.; Luo, X.F.; Wang, X.M.; Tian, J.H.; Gao, J.M. Mammalian target of rapamycin/eukaryotic initiation factor 4F pathway regulates follicle growth and development of theca cells in mice. Reprod. Fertil. Dev. 2017, 29, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Corradetti, M.N.; Inoki, K.; Guan, K.L. TSC2: Filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 2004, 29, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, M.; Yanai, Y.; Ikeda, T.; Okawa, M.; Nohara, T. Steroids from the underground parts of Trillium kamtschaticum. Chem. Pharm. Bull. (Tokyo) 2003, 51, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Sugita, F.; Shigematsu, S.; Takamura, C.; Yoshimitsu, H.; Miyashita, H.; Ikeda, T.; Nohara, T. Three new steroid glycosides from the underground parts of Trillium kamtschaticum. Chem. Pharm. Bull. (Tokyo) 2007, 55, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qiu, Y.; Shu, Z.; Zhang, X.; Li, R.; Liu, S.; Chen, L.; Liu, H.; Chen, N. Protective effect of Trillium tschonoskii saponin on CCl4-induced acute liver injury of rats through apoptosis inhibition. Can. J. Physiol. Pharmacol. 2016, 94, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yu, X.; Xu, K. Analysing on aging model rats by injecting d-gal into abdominal cavity and Subcutaneous. Chin. J. Gerontol. 2013, 33, 1101–1103. [Google Scholar]
- Chen, X.; Zhu, M.; Qin, F. Effect of extract of Trillium tschonoskii Maxim on ciliary neurotropic factor and its receptor α in rats suffering from spinal cord injury. Med. J. Chin. People’s Lib. Army 2015, 40, 622–626. [Google Scholar]
- Wang, L.; Du, J.; Zhao, F.; Chen, Z.; Chang, J.; Qin, F.; Wang, Z.; Wang, F.; Chen, X.; Chen, N. Trillium tschonoskii maxim saponin mitigates D-galactose-induced brain aging of rats through rescuing dysfunctional autophagy mediated by Rheb-mTOR signal pathway. Biomed. Pharmacother. 2018, 98, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Esposito, E.; Ahmad, A.; Di Paola, R.; Wallace, J.L.; Cuzzocrea, S. A hydrogen sulfide-releasing cyclooxygenase inhibitor markedly accelerates recovery from experimental spinal cord injury. FASEB J. 2013, 27, 4489–4499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.M.; Lu, Y.; Zhang, L.X.; Zhang, G.P.; Zhang, Z.Q. Bone marrow stromal cells transplantation combined with ultrashortwave therapy promotes functional recovery on spinal cord injury in rats. Synapse 2015, 69, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Mei, X.; Shen, Z.; Bi, Y.; Yuan, Y.; Guo, Z.; Wang, H.; Zhao, H.; Zhou, Z.; Wang, C. Netrin-1 Improves Functional Recovery through Autophagy Regulation by Activating the AMPK/mTOR Signaling Pathway in Rats with Spinal Cord Injury. Sci. Rep. 2017, 7, 42288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Cui, Z.; Li, W.; Shen, A.; Xu, G.; Bao, G.; Sun, Y.; Wang, L.; Fan, J.; Zhang, J.; et al. MCM7 expression is altered in rat after spinal cord injury. J. Mol. Neurosci. 2013, 51, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Z.; Huang, J.H.; Zeng, L.; Wang, G.; Cao, M.; Lu, H.B. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J. Neurotrauma 2013, 30, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Filip, A. MiRNA—New mechanisms of gene expression control. Postepy Biochem. 2007, 53, 413–419. [Google Scholar] [PubMed]
- Liu, D.Z.; Tian, Y.; Ander, B.P.; Xu, H.; Stamova, B.S.; Zhan, X.; Turner, R.J.; Jickling, G.; Sharp, F.R. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J. Cereb. Blood Flow Metab. 2010, 30, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Blandini, F.; Amantea, D.; Tassorelli, C. Endothelial nitric oxide synthase inhibition triggers inflammatory responses in the brain of male rats exposed to ischemia-reperfusion injury. J. Neurosci. Res. 2018, 96, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Leng, H.; Shi, X.; Ji, J.; Fu, J.; Leng, H. MiR-155 promotes cell proliferation and inhibits apoptosis by PTEN signaling pathway in the psoriasis. Biomed. Pharmarcother. 2017, 90, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Q.; Zhao, Y.; He, C.; Bi, K.; Chen, Y.; Zhao, B.; Chen, Y.; Ma, X. MicroRNA-155 Regulates ROS Production, NO Generation, Apoptosis and Multiple Functions of Human Brain Microvessel Endothelial Cells Under Physiological and Pathological Conditions. J. Cell Biochem. 2015, 116, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Xie, W.; Liu, Z.; Xu, W.; Lao, Y.; Huang, N.; Cui, K.; Liao, M.; He, J.; Jiang, Y.; et al. Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy 2014, 10, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L.; Kaun, K.R.; Edgar, B.A. The small GTPase Rheb affects central brain neuronal morphology and memory formation in Drosophila. PLoS ONE 2012, 7, e44888. [Google Scholar] [CrossRef] [PubMed]
- Gracias, N.G.; Shirkey-Son, N.J.; Hengst, U. Local translation of TC10 is required for membrane expansion during axon outgrowth. Nat. Commun. 2014, 5, 3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, T.; Zhao, T.; Hewes, R.S. Insulin signaling regulates neurite growth during metamorphic neuronal remodeling. Biol. Open 2014, 3, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Gregory, E.N.; Codeluppi, S.; Gregory, J.A.; Steinauer, J.; Svensson, C.I. Mammalian target of rapamycin in spinal cord neurons mediates hypersensitivity induced by peripheral inflammation. Neuroscience 2010, 169, 1392–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Zhou, L.; Du, X.X.; Ji, Y.; Xu, J.; Tian, J.; Jiang, W.; Zou, Y.; Yu, S.; Gan, L.; et al. Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev. Cell 2011, 20, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Tan, X.; Jin, W.; Zheng, H.; Xu, W.; Rui, Y.; Li, L.; Cao, J.; Wu, X.; Cui, G.; et al. Upregulation of Ras homolog enriched in the brain (Rheb) in lipopolysaccharide-induced neuroinflammation. Neurochem. Int. 2013, 62, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Hartman, N.W.; Lin, T.V.; Zhang, L.; Paquelet, G.E.; Feliciano, D.M.; Bordey, A. mTORC1 targets the translational repressor 4E-BP2, but not S6 kinase 1/2, to regulate neural stem cell self-renewal in vivo. Cell Rep. 2013, 5, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.J.; Yang, Y. Function of SOD1, SOD2, and PI3K/AKT signaling pathways in the protection of propofol on spinal cord ischemic reperfusion injury in a rabbit model. Life Sci. 2016, 148, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, D.Y.; Yune, T.Y.; Shin, D.H.; Baek, S.B.; Kim, C.J. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp. Ther. Med. 2014, 7, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Don, A.S.; Tsang, C.K.; Kazdoba, T.M.; D’Arcangelo, G.; Young, W.; Zheng, X.F. Targeting mTOR as a novel therapeutic strategy for traumatic CNS injuries. Drug Discov. Today 2012, 17, 861–868. [Google Scholar] [PubMed] [Green Version]
- Shu, Q.; Xu, Y.; Zhuang, H.; Fan, J.; Sun, Z.; Zhang, M.; Xu, G. Ras homolog enriched in the brain is linked to retinal ganglion cell apoptosis after light injury in rats. J. Mol. Neurosci. 2014, 54, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Gruner, J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 1992, 9, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.E.; Takizawa, B.T.; Strittmatter, S.M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003, 23, 1416–1423. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-B.; Wang, Z.-L.; Yang, Q.-Y.; Zhao, F.-Y.; Qin, X.-L.; Tang, X.-E.; Du, J.-L.; Chen, Z.-H.; Zhang, K.; Huang, F.-J. Diosgenin Glucoside Protects against Spinal Cord Injury by Regulating Autophagy and Alleviating Apoptosis. Int. J. Mol. Sci. 2018, 19, 2274. https://doi.org/10.3390/ijms19082274
Chen X-B, Wang Z-L, Yang Q-Y, Zhao F-Y, Qin X-L, Tang X-E, Du J-L, Chen Z-H, Zhang K, Huang F-J. Diosgenin Glucoside Protects against Spinal Cord Injury by Regulating Autophagy and Alleviating Apoptosis. International Journal of Molecular Sciences. 2018; 19(8):2274. https://doi.org/10.3390/ijms19082274
Chicago/Turabian StyleChen, Xian-Bing, Zi-Li Wang, Qing-Yu Yang, Fang-Yu Zhao, Xiao-Li Qin, Xian-E Tang, Jun-Long Du, Zong-Hai Chen, Kui Zhang, and Fei-Jun Huang. 2018. "Diosgenin Glucoside Protects against Spinal Cord Injury by Regulating Autophagy and Alleviating Apoptosis" International Journal of Molecular Sciences 19, no. 8: 2274. https://doi.org/10.3390/ijms19082274