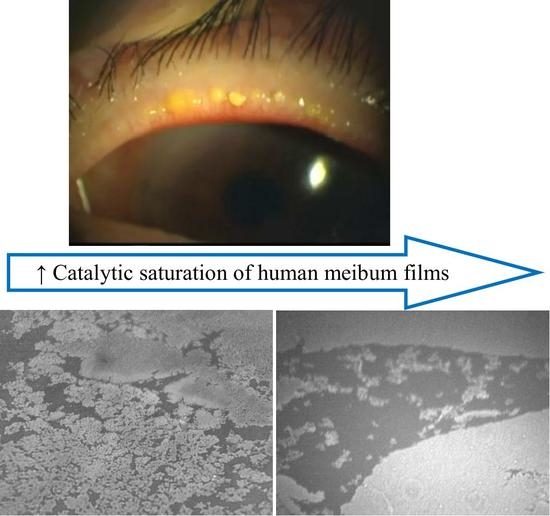
Effects of Lipid Saturation on the Surface Properties of Human Meibum Films
Abstract
:1. Introduction
2. Results
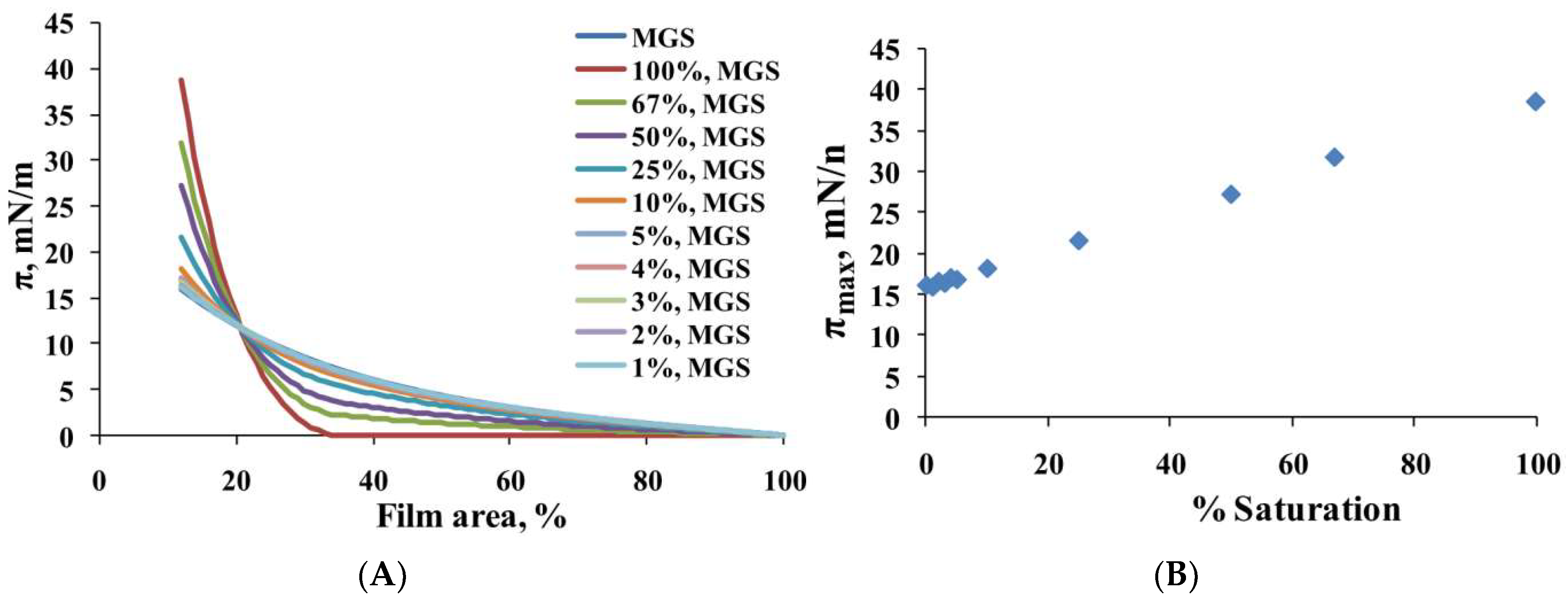
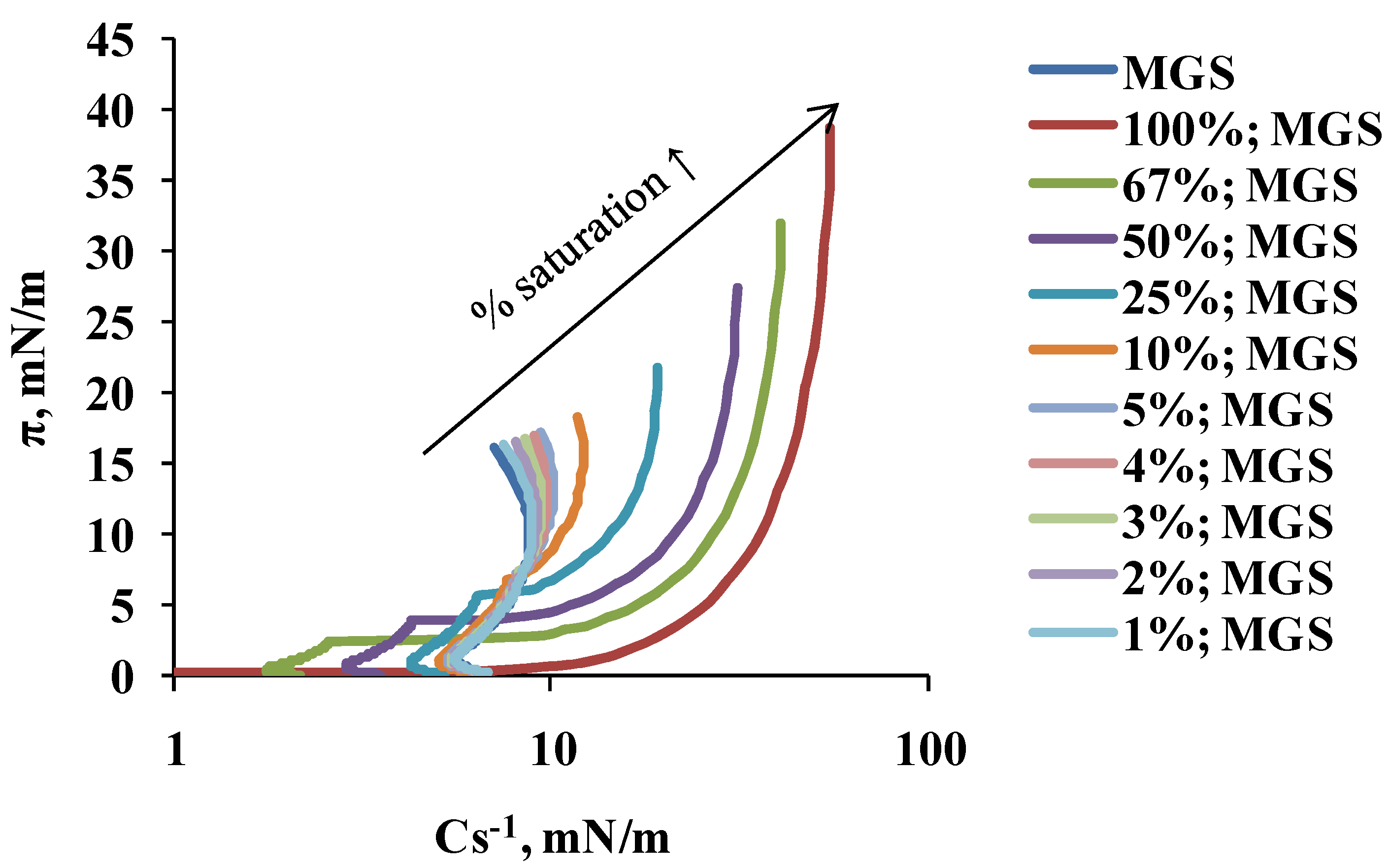
2.1. Surface Pressure-Area Isotherms
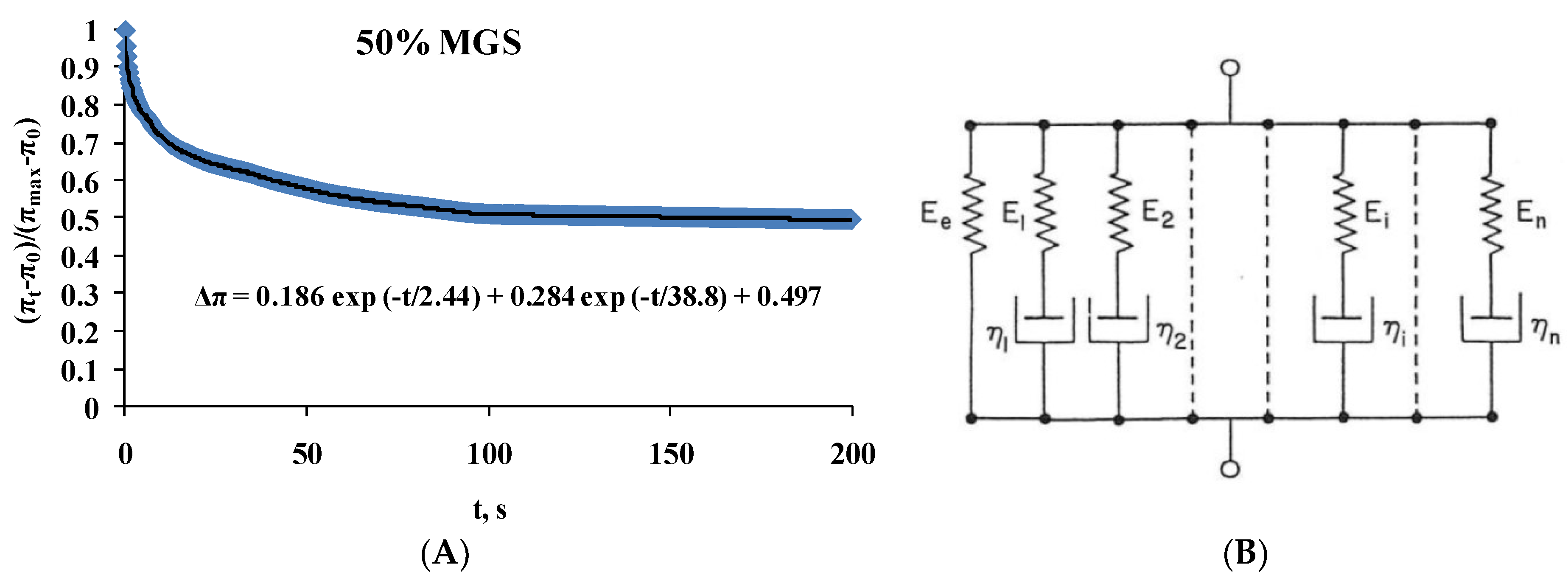
2.2. Dilatational Rheology
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Langmuir Trough Studies
4.2.1. Compression Isotherms
4.2.2. Stress-Relaxation Studies via the Small Deformations Method
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TF | Tear film |
| TFLL | Tear film lipid layer |
| MGS | Meibomian gland secretion (or simply meibum) |
| MGD | Meibomian gland disease |
| π | Surface pressure |
| BAM | Brewster angle microscopy |
References
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A. On the lipid composition of human meibum and tears: Comparative analysis of nonpolar lipids. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3779–3789. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.K.; Borchman, D.; Foulks, G.N.; Yappert, M.C.; Milliner, S.E. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults, and adults with meibomian gland dysfunction using (1)H-NMR spectroscopy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7350–7358. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Duan, X.; Petznick, A.; Wenk, M.R.; Shui, G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J. Lipid Res. 2014, 55, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Crews, L.A.; Bron, A.J.; Foulks, G.N.; Sullivan, B.D. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012, 31, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Tonchev, V.; Nencheva, Y.; Krastev, R. Surface relaxations as a tool to distinguish the dynamic interfacial properties of films formed by normal and diseased meibomian lipids. Soft Matter 2014, 10, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- King-Smith, P.E.; Bailey, M.D.; Braun, R.J. Four characteristics and a model of an effective tear film lipid layer (TFLL). Ocul. Surf. 2013, 11, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Millar, T.J. A mechanism to explain the behavior of spread films of meibomian lipids. Curr. Eye Res. 2013, 38, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, L.; Cerretani, C.; Leiske, D.L.; Toney, M.F.; Radke, C.J.; Fuller, G.G. Structural and rheological properties of meibomian lipid. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2720–2732. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017, 163, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C. Lipids and the Ocular Lens. J. Lipid Res. 2010, 51, 2473–2488. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D. From the Bench to the Bedside: Infrared Spectroscopy and the Diagnosis and Treatment of Dry Eye and Cataracts. Spectroscopy 2014, 29, 38–52. [Google Scholar]
- Mudgil, P.; Borchman, D.; Yappert, M.C.; Duran, D.; Cox, G.W.; Smith, R.J.; Bhola, R.; Dennis, G.R.; Whitehall, J.S. Lipid order, saturation and surface property relationships: A study of human meibum saturation. Exp. Eye Res. 2013, 116, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sledge, S.; Henry, C.; Borchman, D.; Yappert, M.C.; Bhola, R.; Ramasubramanian, A.; Blackburn, R.; Austin, J.; Massey, K.; Sayied, S.; et al. Human Meibum Age, Lipid-Lipid Interactions and Lipid Saturation in Meibum from Infants. Int. J. Mol. Sci. 2017, 18, E1862. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Bell, J.; Wells, E.; Neravetla, S.; Greenstone, V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Foulks, G.N.; Yappert, M.C.; Kakar, S.; Podoll, N.; Rychwalski, P.; Schwietz, E. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010, 44, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.; Ramasubramanian, A.; Borchman, D.; Sledge, S.; Yeo, H.; Yappert, M.C.; Schikler, K.N.; Mehta, S.; Mehta, A. Meibum Structural Differences in Adolescents and Adults with and without Dry Eye Induced by Graft-versus-Host Disease. JAMA 2018. submitted. [Google Scholar]
- Borchman, D.; Tang, D.; Yappert, M.C. Lipid composition, membrane structure relationships in lens and muscle sarcoplasmic reticulum. Biospectroscopy 1999, 5, 151–167. [Google Scholar] [CrossRef]
- Isenberg, S.J.; Del Signore, M.; Chen, A.; Wei, J.; Guillon, J.P. The lipid layer and stability of the preocular tear film in newborns and infants. Ophthalmology 2003, 110, 1408–1411. [Google Scholar] [CrossRef]
- Jones, S.M.; Nischal, K.K. The non-invasive tear film break-up time in normal children. Br. J. Ophthalmol. 2013, 97, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Montés-Micó, R.; Alió, J.L.; Muñoz, G.; Charman, W.N. Temporal Changes in Optical Quality of Air–Tear Film Interface at Anterior Cornea after Blink. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1752–1757. [Google Scholar] [CrossRef]
- Németh, J.; Erdélyi, B.; Csákány, B.; Gáspár, P.; Soumelidis, A.; Kahlesz, F.; Lang, Z. High-Speed Videotopographic Measurement of Tear Film Build-up Time. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1783–1790. [Google Scholar]
- Joffre, C.; Souchier, M.; Gregoire, S.; Viau, S.; Bretillon, L.; Acar, N.; Bron, A.M.; Creuzot-Garcher, C. Differences in meibomian fatty acid composition inpatients with meibomian gland dysfunction and aqueous-deficient dry eye. Br. J. Ophthalmol. 2008, 92, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D.; Yappert, M.C.; Foulks, G.N. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 2010, 91, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulks, G.N.; Borchman, D. Meibomian gland dysfunction: The past, present, and future. Eye Contact Lens 2010, 36, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Green-Church, K.B.; Butovich, I.; Willcox, M.; Borchman, D.; Paulsen, F.; Barabino, S.; Glasgow, B.J. The international workshop on meibomian gland dysfunction: Report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N. The correlation between the tear film lipid layer and dry eye disease. Surv. Ophthalmol. 2007, 52, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Foulks, G.N.; Borchman, D.; Yappert, M.; Kakar, S. Topical azithromycin andoral doxycycline therapy of meibomian gland dysfunction: A comparativeclinical and spectroscopic pilot study. Cornea 2013, 32, 44–53. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; Lu, H.; Butovich, I.A. The spectrophotometric sulfo-phospho-vanillin assessment of total lipids in human meibomian gland secretions. Lipids 2013, 48, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Lu, H.; McMahon, A.; Ketelson, H.; Senchyna, M.; Meadows, D.; Campbell, E.; Molai, M.; Linsenbardt, E. Biophysical and morphological evaluation of human normal and dry eye meibum using hot stage polarizedlight microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelpour, M.; Watts, P.O.; Boulton, M.E.; Cai, J.; Murphy, P.J. Tear Film Volume and Protein Analysis in Full-term Newborn Infants. Cornea 2011, 30, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiske, D.; Leiske, C.; Leiske, D.; Toney, M.; Senchyna, M.; Ketelson, H.; Meadows, D.; Fuller, G.G. Temperature-induced transitions in the structure and interfacial rheology of human meibum. Biophys. J. 2012, 102, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Tragoulias, S.T.; Anderton, P.J.; Dennis, G.R.; Miano, F.; Millar, T.J. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea 2005, 24, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Tonchev, V.; Yokoi, N.; Yappert, M.C.; Borchman, D.; Georgiev, G.A. Surface Properties of Squalene/Meibum Films and NMR Confirmation of Squalene in Tears. Int. J. Mol. Sci. 2015, 16, 21813–21831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudgil, P.; Borchman, D.; Gerlach, D.; Yappert, M.C. Sebum/Meibum Surface Film Interactions and Phase Transitional Differences. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of pharmaceutical ingredients with human meibum films. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Nencheva, Y.; Peev, N.; Daull, P. Surface Chemistry Interactions of Cationorm with Films by Human Meibum and Tear Film Compounds. Int. J. Mol. Sci. 2017, 18, 1558. [Google Scholar] [CrossRef] [PubMed]
- Skrzypieca, M.; Georgiev, G.A.; Rojewska, M.; Prochaska, K. Interaction of polyhedral oligomericsilsesquioxanes and dipalmitoylphosphatidylcholine at the air/water interface: Thermodynamic and rheological study. Biochim. Biophys. Acta Binmembr. 2017, 1859, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Kaercher, T.; Hönig, D.; Möbius, D. Brewster angle microscopy. A new method of visualizing the spreading of Meibomian lipids. Int. Ophthalmol. 1993, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H.J.; Ransnas, L.A. Fitting curves to data using nonlinear regression: A practical and nonmathematical review. FASEB J. 1987, 1, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics, I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Cárdenas-Valera, A.E.; Bailey, A.I. The interfacial rheological behaviour of monolayers of PEO/PMMA graft copolymers spread at the air/water and oil/water interfaces. Colloids Surf. A 1993, 19, 115–127. [Google Scholar] [CrossRef]
- Tschoegl, N. The Modelling of Multimodal Distributions of Respondance Times. In The Phenomenological Theory of Linear Viscoelastic Behavior; Springer: Berlin/Heidelberg, Germany, 1989; pp. 489–507. ISBN 978-3-642-73602-5. [Google Scholar]
- Ferry, J. Viscoelastic Properties of Polymers; Wiley and Sons: Hoboken, NJ, USA, 1981; ISBN 978-0-471-04894-7. [Google Scholar]
- Murata, H. Rheology—Theory and Application to Biomaterials. In Polymerization- Theory and Application to Biomaterials; De Souza Gomes, A., Ed.; In Tech Open: Rijeka, Croatia, 2012; pp. 404–424. [Google Scholar]
- White, S.H.; King, G.I. Molecular packing and area compressibility of lipid bilayers. Proc. Natl. Acad. Sci. USA 1985, 82, 6532–6536. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.Y.; Lai, Y.C.; Chiang, Y.W.; Chen, Y.F. Coupling of lipid membrane elasticity and in-plane dynamics. Phys. Rev. E 2017, 96, 012410. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaul, A.; Szleifer, L.; Gelbart, W.M. Molecular theory for amphiphile packing and elastic properties of monolayers and bilayers. In Physics of Amphiphilic Layers; Meunier, J., Langevin, D., Boccara, N., Eds.; Springer-Verlag: New York, NY, USA, 1987; pp. 2–8. ISBN 978-0-38-718255-1. [Google Scholar]
- Kilp, H.; Schmid, E.; Kirchner, L.; Pohl, A. Tear Film Observation by Reflecting Microscopy and Differential Interference Contrast Microscopy. In The Preocular Tear Film in Health, Disease and Contact Lens Wear; Holly, F.J., Ed.; The Dry Eye Institute: Lubbock, TX, USA, 1986; pp. 564–569. ISBN 978-0-96-169380-0. [Google Scholar]
- Georgiev, G.A.; Kutsarova, E.; Jordanova, A.; Krastev, R.; Lalchev, Z. Interactions of Meibomian gland secretion with polar lipids in Langmuir monolayers. Colloids Surf. B Biointerfaces 2010, 78, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena, 2nd ed.; Academic Press: New York, NY, USA, 1963; Volume 265, ISBN 978-0-32-316166-4. [Google Scholar]
- Loglio, G.; Tesei, U.; Cini, R. Viscoelastic dilatation processes of fluid/fluid interfaces: Time-domain representation. Colloid Polym. Sci. 1986, 264, 712–718. [Google Scholar] [CrossRef]
- Monroy, F.; Ortega, F.; Rubio, R.G. Dilatational rheology of insoluble polymer monolayers: Poly(vinylacetate). Phys. Rev. E 1998, 58, 7629–7641. [Google Scholar] [CrossRef]
- Flannery, B.P.; Teukolsky, S.; Press, W.H.; Vetterling, W.T. Fast Fourier Transform. In Numerical Recipes in C++; Cambridge University Press: Cambridge, UK, 2007; pp. 501–537. ISBN 978-0-521-88068-8. [Google Scholar]



| Composition | Maxwell Rheological Model Equation |
|---|---|
| MGS | Δπ = 0.267exp(−t/3.3) + 0.42exp(−t/42.6) + 0.29 |
| 100%; MGS | Δπ = 0.144exp(−t/0.8) + 0.145exp(−t/32.7) + 0.699 |
| 67%; MGS | Δπ = 0.17exp(−t/2.5) + 0.229exp(−t/42.5) + 0.56 |
| 50%; MGS | Δπ = 0.186exp(−t/2.4) + 0.284exp(−t/38.5) + 0.497 |
| 25%; MGS | Δπ = 0.22exp(−t/2.8) + 0.353exp(−t/39.8) + 0.396 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nencheva, Y.; Ramasubramanian, A.; Eftimov, P.; Yokoi, N.; Borchman, D.; Georgiev, G.A. Effects of Lipid Saturation on the Surface Properties of Human Meibum Films. Int. J. Mol. Sci. 2018, 19, 2209. https://doi.org/10.3390/ijms19082209
Nencheva Y, Ramasubramanian A, Eftimov P, Yokoi N, Borchman D, Georgiev GA. Effects of Lipid Saturation on the Surface Properties of Human Meibum Films. International Journal of Molecular Sciences. 2018; 19(8):2209. https://doi.org/10.3390/ijms19082209
Chicago/Turabian StyleNencheva, Yana, Aparna Ramasubramanian, Petar Eftimov, Norihiko Yokoi, Douglas Borchman, and Georgi As. Georgiev. 2018. "Effects of Lipid Saturation on the Surface Properties of Human Meibum Films" International Journal of Molecular Sciences 19, no. 8: 2209. https://doi.org/10.3390/ijms19082209