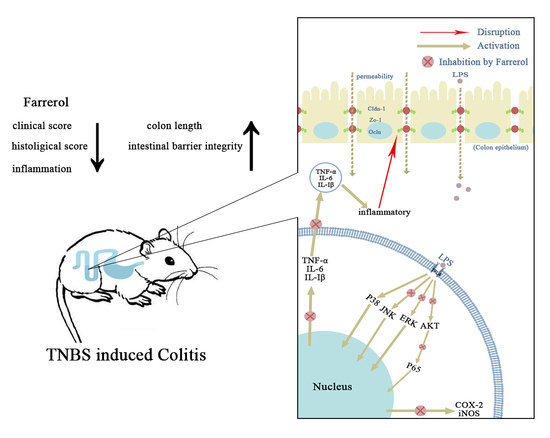
Farrerol Ameliorates TNBS-Induced Colonic Inflammation by Inhibiting ERK1/2, JNK1/2, and NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Results
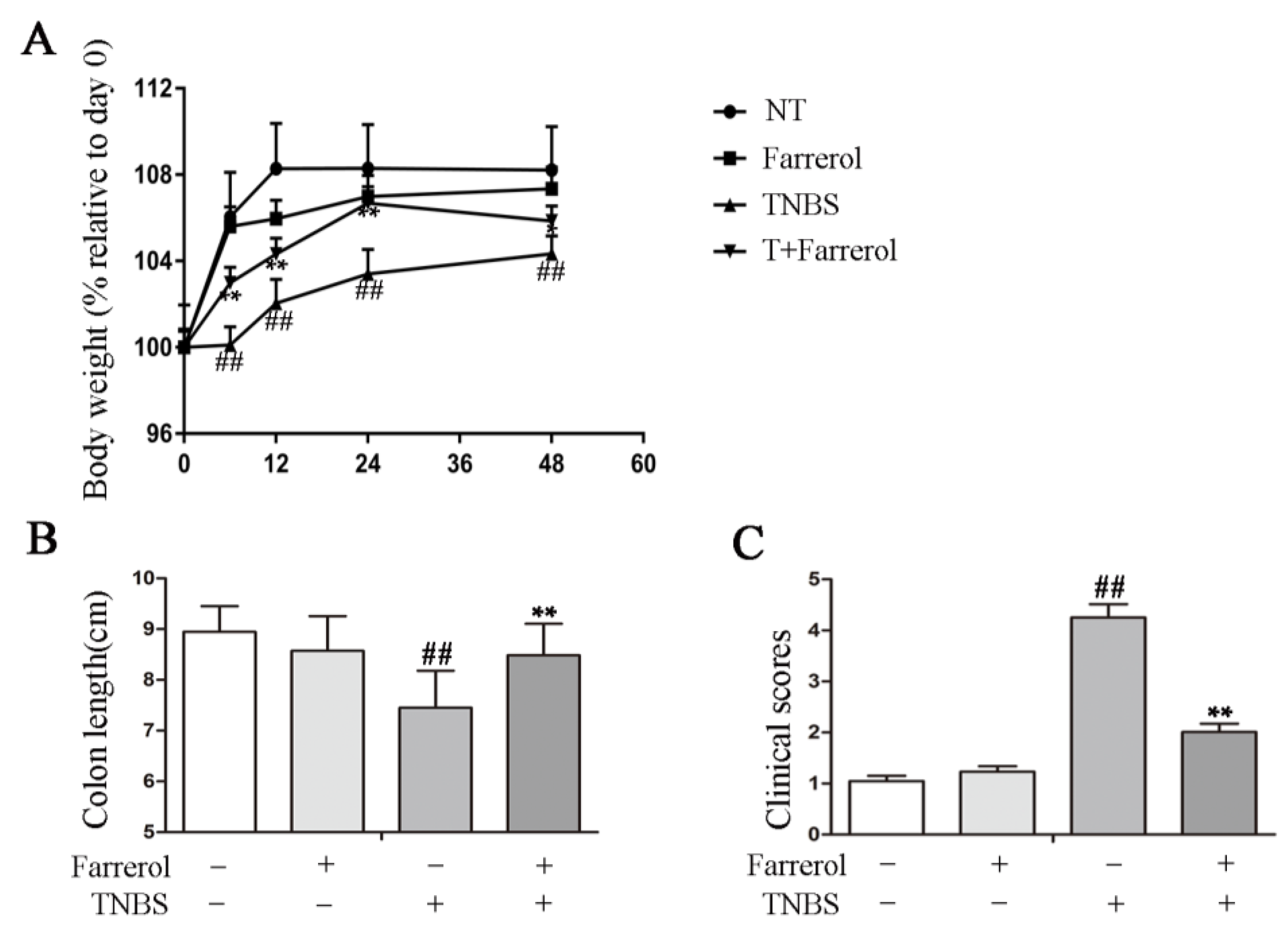
2.1. Farrerol Ameliorates TNBS-Induced Colonic Injury
2.2. Farrerol Inhibits Inflammatory Response in TNBS-Induced Mice
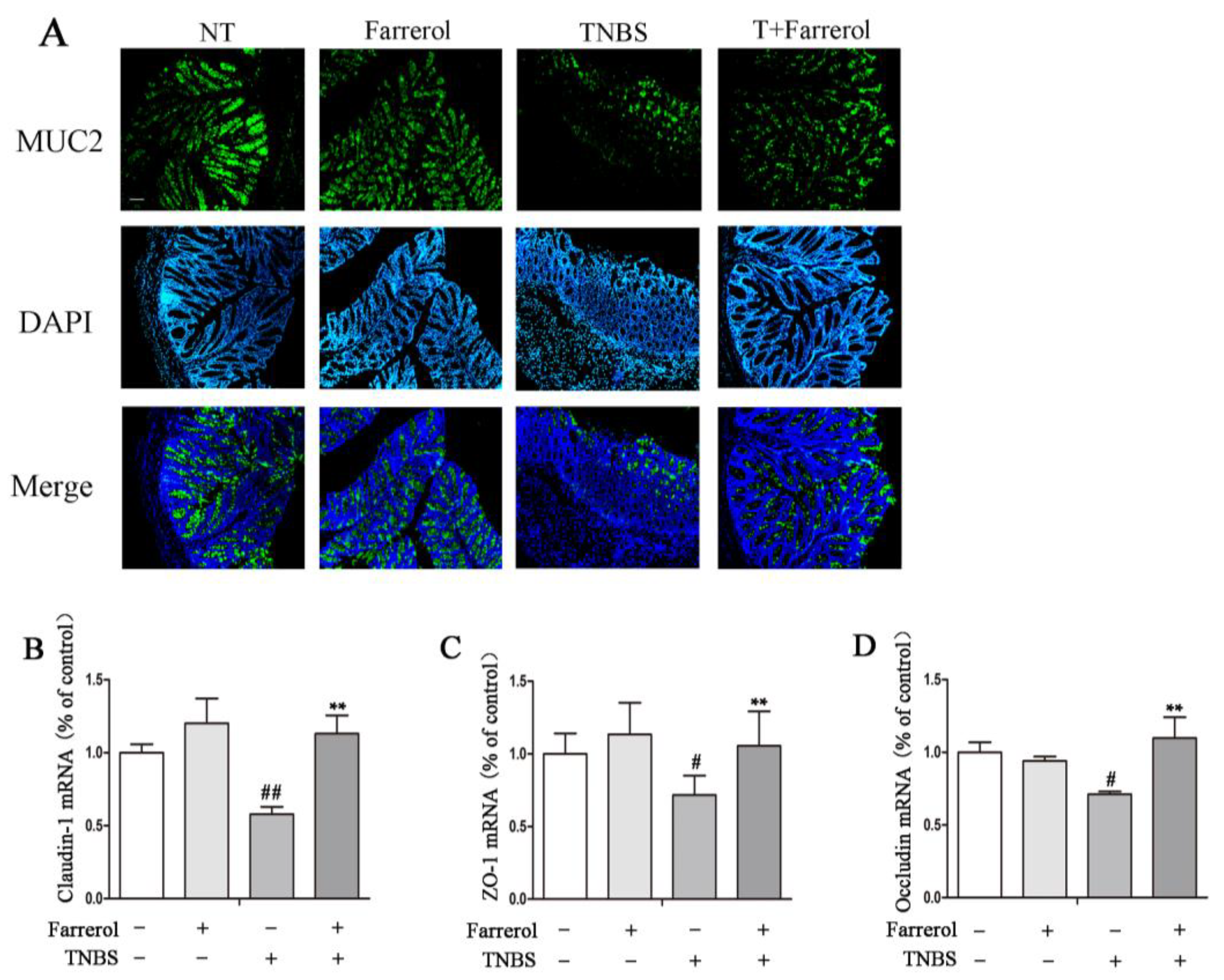
2.3. Farrerol Maintains Mucin2 Expression and Appropriate Tight Junctions in the Colon of TNBS-Induced Mice
2.4. Farrerol Inhibits Inflammatory Response in LPS-Stimulated RAW264.7 Cells
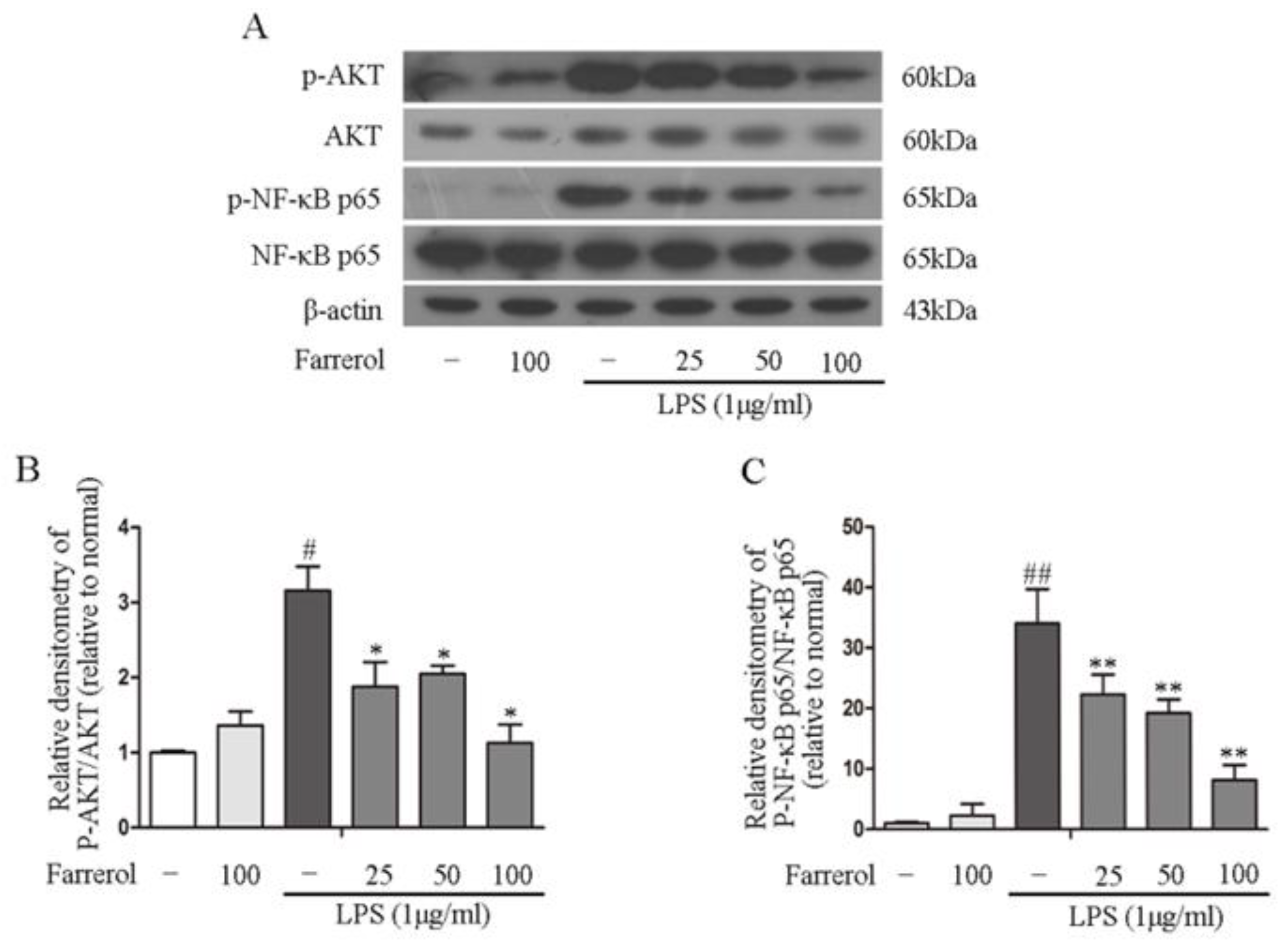
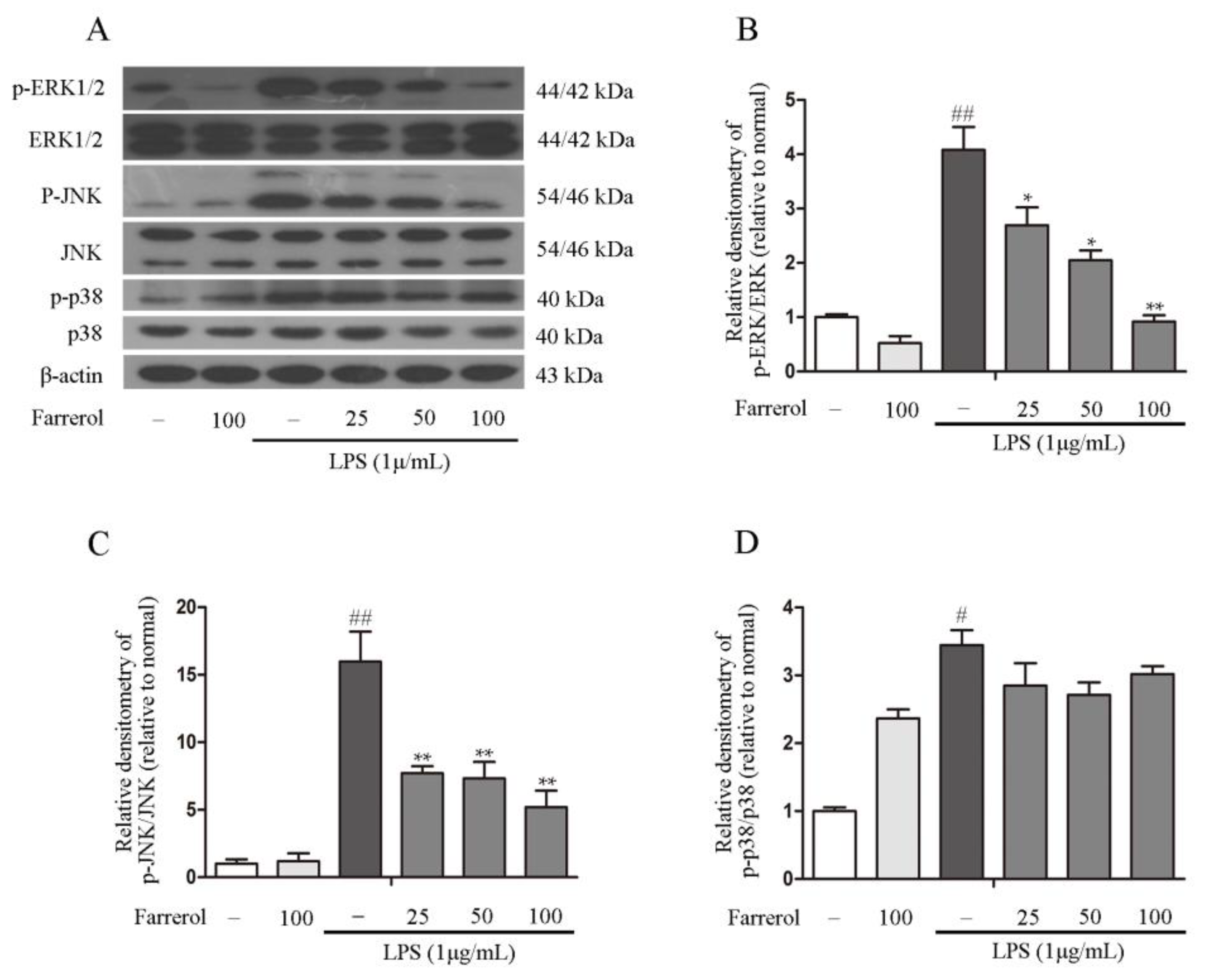
2.5. Farrerol Inhibits the Activation of NF-κB and MAPK Signaling Pathways in LPS-Stimulated RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Colitis Induction
4.2. Clinical Score
4.3. Hematoxylin and Eosin (H&E) Staining
4.4. Quantitative Real-Time PCR
4.5. ELISA
4.6. Immunofluorescence
4.7. Cell Culture and Treatment
4.8. MTT
4.9. Western Blot
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| TNBS | 2,4,6-Trinitrobenzene sulfonic acid |
| LPS | Lipopolysaccharide |
References
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Keighley, M.R.; Stockbrugger, R.W. Inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 18 (Suppl. 3), 66–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Fried, M.; Krabshuis, J.H.; Cohen, H.; Eliakim, R.; Fedail, S.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; et al. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm. Bowel Dis. 2010, 16, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Kawano, S.; Tsuji, S.; Kobayashi, I.; Takei, Y.; Nagano, K.; Fusamoto, H.; Kamada, T.; Ogihara, T.; Sato, N. Colonic mucosal hemodynamics and tissue oxygenation in patients with ulcerative colitis: Investigation by organ reflectance spectrophotometry. J. Gastroenterol. 1995, 30, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Matricon, J.; Barnich, N.; Ardid, D. Immunopathogenesis of inflammatory bowel disease. Self. Nonself. 2010, 1, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Bojic, D.; Basit, A.W. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl. Res. 2016, 176, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, F. Cytokine-based therapies for Crohn’s disease—New paradigms. N. Engl. J. Med. 2004, 351, 2045–2048. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Sono, M.; Yazumi, S. Usefulness of sulfasalazine for patients with refractory-ulcerative colits. BMJ Open Gastroenterol. 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Park, J.B.; Bae, K.H. Pharmacological effects of methanolic extract from the root of Scutellaria baicalensis and its flavonoids on human gingival fibroblast. Planta Med. 1995, 61, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chu, Q.; Ye, J. Chromatographic and electrophoretic methods for pharmaceutically active compounds in Rhododendron dauricum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 231–240. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, Y.; Liu, B.; Zhou, E.; Liu, Z.; Song, X.; Li, D.; Zhang, N. Farrerol regulates antimicrobial peptide expression and reduces staphylococcus aureus internalization into bovine mammary epithelial cells. Microb. Pathog. 2013, 65, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Chu, X.; Wei, M.; Yang, X.; Cai, Q.; Deng, X. Different effects of farrerol on an OVA-induced allergic asthma and LPS-induced acute lung injury. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.L.; Wallace, J.L. Cytokines in inflammatory bowel disease. Mediat. Inflamm. 1997, 6, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Giner, E.; Recio, M.C.; Rios, J.L.; Cerda-Nicolas, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Z.; Lv, Z.; Sun, Y.; Cao, W.; Zhang, R.; Liu, Z.; Li, C.; Cao, S.; Mei, Q. The beneficial effect of Rheum tanguticum polysaccharide on protecting against diarrhea, colonic inflammation and ulceration in rats with TNBS-induced colitis: The role of macrophage mannose receptor in inflammation and immune response. Int. Immunopharmacol. 2008, 8, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Grzywacz-Kisielewska, A.; Kala, K.; Gdula-Argasinska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, D.; Jin, J.; Wu, L. Binding analysis of farrerol to lysozyme by spectroscopic methods. Spectrochim. Acta Part A 2007, 68, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Luchini, A.C.; Hiruma-Lima, C.A.; Felisbino, S.L.; Garrido-Mesa, N.; Utrilla, P.; Galvez, J.; Di Stasi, L.C. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: Comparison with prednisolone and sulphasalazine. Chem. Biol. Interact. 2012, 195, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.D.; Butler, L.D. T cell responses induced by the parenteral injection of antigen-modified syngeneic cells. I. Induction, characterization, and regulation of antigen-specific T helper cells involved in delayed-type hypersensitivity responses. J. Immunol. 1983, 131, 77–85. [Google Scholar] [PubMed]
- Elson, C.O.; Beagley, K.W.; Sharmanov, A.T.; Fujihashi, K.; Kiyono, H.; Tennyson, G.S.; Cong, Y.; Black, C.A.; Ridwan, B.W.; McGhee, J.R. Hapten-induced model of murine inflammatory bowel disease: Mucosa immune responses and protection by tolerance. J. Immunol. 1996, 157, 2174–2185. [Google Scholar] [PubMed]
- Hardee, S.; Alper, A.; Pashankar, D.S.; Morotti, R.A. Histopathology of duodenal mucosal lesions in pediatric patients with inflammatory bowel disease: Statistical analysis to identify distinctive features. Pediatr. Dev. Pathol. 2014, 17, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, X.X.; Yin, Y.; Gong, F.Y.; Shen, Y.; Cai, T.T.; Zhou, X.B.; Wu, X.F.; Xu, Q. Novel immunomodulatory properties of cirsilineol through selective inhibition of IFN-gamma signaling in a murine model of inflammatory bowel disease. Biochem. Pharmacol. 2010, 79, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Luo, Q.; Xu, H.M.; Gong, F.Y.; Zhou, X.B.; Sun, Y.; Wu, X.F.; Liu, W.; Zeng, G.Z.; Tan, N.H.; et al. Mitochondria-dependent apoptosis of activated T lymphocytes induced by astin C, a plant cyclopeptide, for preventing murine experimental colitis. Biochem. Pharmacol. 2011, 82, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.P.; Sheu, M.J.; Ma, M.C.; Hu, J.T.; Sun, Y.Y.; Lee, C.C.; Chung, Y.C.; Tsai, Y.J.; Wang, J.Y.; Chen, C.L. Runx1-deficient afferents impair visceral nociception, exacerbating dextran sodium sulfate-induced colitis. Brain Behav. Immun. 2014, 35, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.N.; Cai, X.T.; Yan, J.; Luo, Y.; Shao, M.; Lu, Y.; Sun, Z.G.; Cao, P. In vivo and in vitro antinociceptive effect of fagopyrum cymosum (trev.) meisn extracts: A possible action by recovering intestinal barrier dysfunction. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Yamashita, K.; Ichikawa, N.; Fukai, M.; Suzuki, T.; Goto, R.; Oura, T.; Kobayashi, N.; Katsurada, T.; Ichihara, S.; et al. A novel NF-kappa B inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J. Crohns Colitis 2012, 6, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.R.; Rogatsky, I. Minireview: Glucocorticoids in autoimmunity: Unexpected targets and mechanisms. Mol. Endocrinol. 2011, 25, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Nemoto, M.; Nakata, T.; Kondo, S.; Takahashi, H.; Kimura, B.; Kuda, T. Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int. Immunopharmacol. 2015, 26, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Giner, E.; Andujar, I.; Recio, M.C.; Rios, J.L.; Cerda-Nicolas, J.M.; Giner, R.M. Oleuropein ameliorates acute colitis in mice. J. Agric. Food Chem. 2011, 59, 12882–12892. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lin, Y.; Lin, L.; Sun, Y.; Zheng, C. Carcinoembryonic antigen related cellular adhesion molecule 1 alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Life Sci. 2016, 149, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Chintanaboina, J. Allergic and immunologic perspectives of inflammatory bowel disease. Clin. Rev. Allergy Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.; Ye, J.; Zhai, X.; Song, J.; Jiang, C.; Wang, J.; Zhang, H.; Jia, X.; Zhu, F. Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-kappaB and MAPK signaling pathway in LPS-induced RAW264.7 macrophages. J. Ethnopharmacol. 2017, 196, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.; Li, Y.; Sarkar, F.H. Inactivation of akt and NF-kappaB play important roles during indole-3-carbinol-induced apoptosis in breast cancer cells. Nutr. Cancer 2004, 48, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Schwanke, R.C.; Marcon, R.; Meotti, F.C.; Bento, A.F.; Dutra, R.C.; Pizzollatti, M.G.; Calixto, J.B. Oral administration of the flavonoid myricitrin prevents dextran sulfate sodium-induced experimental colitis in mice through modulation of PI3K/Akt signaling pathway. Mol. Nutr. Food Res. 2013, 57, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Pawate, S.; Shen, Q.; Fan, F.; Bhat, N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 2004, 77, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Shen, P.; Cao, Y.; Lu, X.; Gao, X.; Fu, Y.; Liu, B.; Zhang, N. Zanthoxylum bungeanum pericarp extract prevents dextran sulfate sodium-induced experimental colitis in mice via the regulation of TLR4 and TLR4-related signaling pathways. Int. Immunopharmacol. 2016, 41, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Murano, M.; Maemura, K.; Hirata, I.; Toshina, K.; Nishikawa, T.; Hamamoto, N.; Sasaki, S.; Saitoh, O.; Katsu, K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin. Exp. Immunol. 2000, 120, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Avila-Roman, J.; Talero, E.; Rodriguez-Luna, A.; Garcia-Maurino, S.; Motilva, V. Anti-inflammatory effects of an oxylipin-containing lyophilised biomass from a microalga in a murine recurrent colitis model. Br. J. Nutr. 2016, 116, 2044–2052. [Google Scholar] [CrossRef] [PubMed]



| Score | Roachback or Emaciation | Colon Thickening | Pellet Morphology |
|---|---|---|---|
| 0 | None | None | Normal |
| 1 | Yes | Slight | Soft stool |
| 2 | Moderate | Diarrhea | |
| 3 | Severe | Bloody stool |
| Gene | Sequence | Length (bp) |
|---|---|---|
| β-actin | F: 5′-GTCAGGTCATCACTATCGGCAAT-3′ | 147 |
| R: 5′-AGAGGTCTTTACGGATGTCAACGT-3′ | ||
| TNF-α | F: 5′-CCACGCTCTTCTGTCTACTG-3′ | 136 |
| R: 5′-CCACGCTCTTCTGTCTACTG-3′ | ||
| IL-1β | F: 5′-TGTGATGTTCCCATTAGAC-3′ | 139 |
| R: 5′-AATACCACTTGTTGGCTTA-3′ | ||
| IL-6 | F: 5′-AGCCACTGCCTTCCCTAC-3′ | 138 |
| R: 5′-TTGCCATTGCACAACTCTT-3′ | ||
| iNOS | F: 5′-CACCCAGAAGAGTTACAGC-3′ | 158 |
| R: 5′-GGAGGGAAGGGAGAATAG-3′ | ||
| COX-2 | F: 5′-GGAGGGAAGGGAGAATAG-3′ | 127 |
| R: 5′-CTTGTAGTAGGCTTAAACATAG-3′ | ||
| Claudin-1 | F: 5′-AGGTCTGGCGACATTAGTGG-3′ | 204 |
| R: 5′-CGTGGTGTTGGGTAAGAGGT-3′ | ||
| Occludin | F: 5′-GACCTTGATTTGCATGACGA-3′ | 197 |
| R: 5′-AGGACCGTGTAATGGCAGAC-3′ | ||
| ZO-1 | F: 5′-ACACTTGCTTGGGACAGAGG-3′ | 199 |
| R: 5′-AAGGAAGCGATGAAGCAGAA-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, X.; Li, Y.; Chen, G.; Fu, S.; He, D.; Huang, B.; Wei, L.; Lin, Y.; Guo, Y.; Hu, G. Farrerol Ameliorates TNBS-Induced Colonic Inflammation by Inhibiting ERK1/2, JNK1/2, and NF-κB Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2037. https://doi.org/10.3390/ijms19072037
Ran X, Li Y, Chen G, Fu S, He D, Huang B, Wei L, Lin Y, Guo Y, Hu G. Farrerol Ameliorates TNBS-Induced Colonic Inflammation by Inhibiting ERK1/2, JNK1/2, and NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2018; 19(7):2037. https://doi.org/10.3390/ijms19072037
Chicago/Turabian StyleRan, Xin, Yuhang Li, Guangxin Chen, Shoupeng Fu, Dewei He, Bingxu Huang, Libin Wei, Yuanqing Lin, Yingcheng Guo, and Guiqiu Hu. 2018. "Farrerol Ameliorates TNBS-Induced Colonic Inflammation by Inhibiting ERK1/2, JNK1/2, and NF-κB Signaling Pathway" International Journal of Molecular Sciences 19, no. 7: 2037. https://doi.org/10.3390/ijms19072037