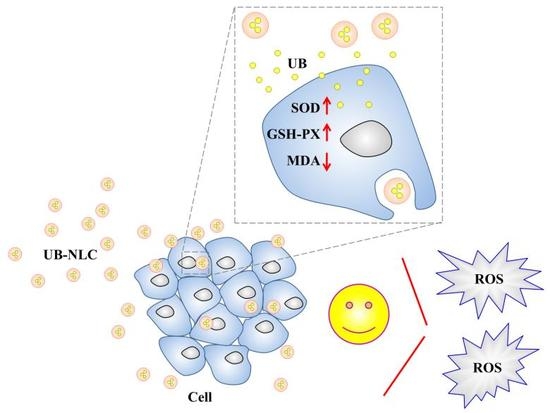
Ubidecarenone-Loaded Nanostructured Lipid Carrier (UB-NLC): Percutaneous Penetration and Protective Effects Against Hydrogen Peroxide-Induced Oxidative Stress on HaCaT Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructure Observations and Size Characterization
2.2. Percutaneous Penetration
2.3. Antioxidant Activity of UB-NLC against Oxidative Damage
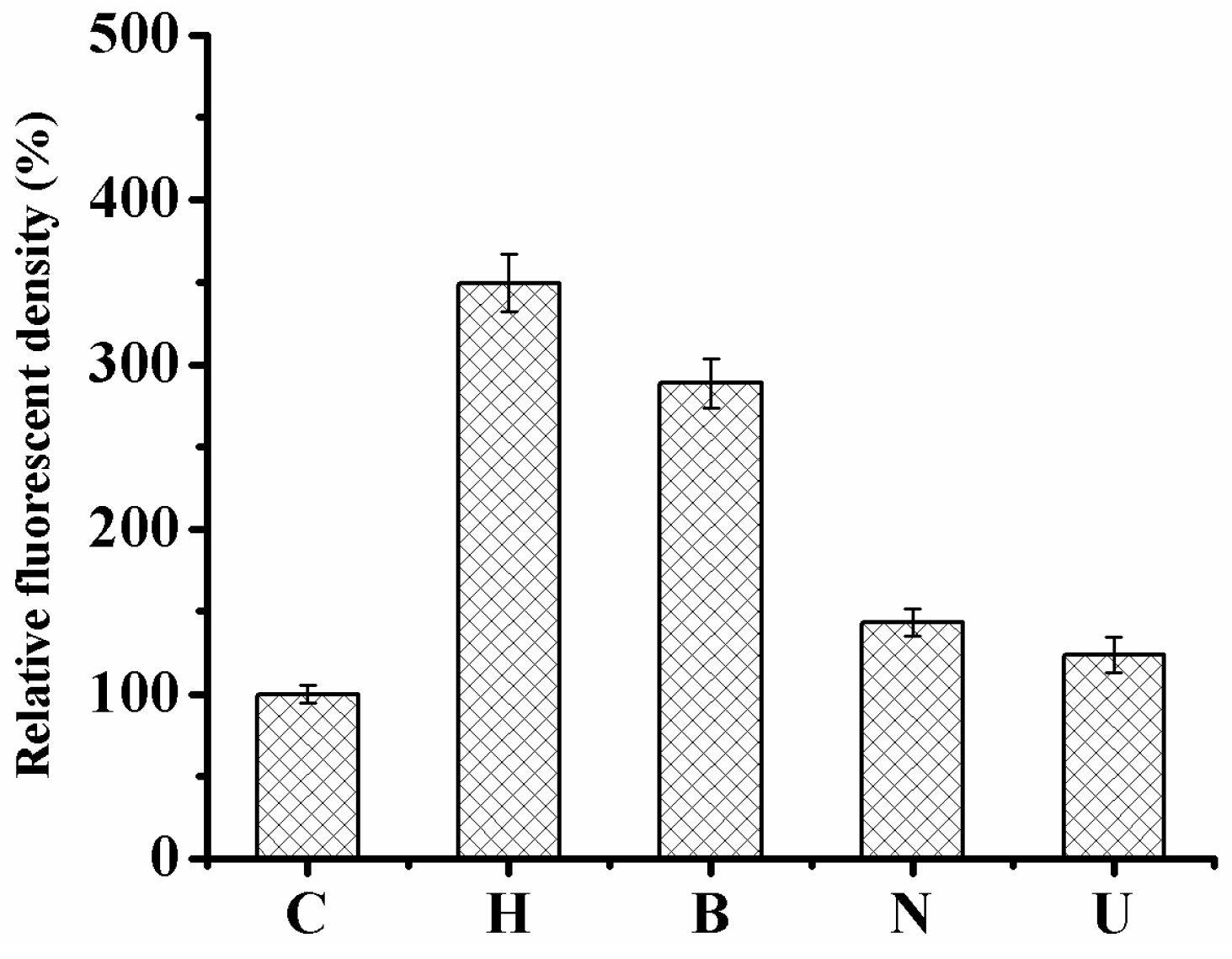
2.4. Monitoring the Level of Reactive Oxygen Species in HaCaT Cells
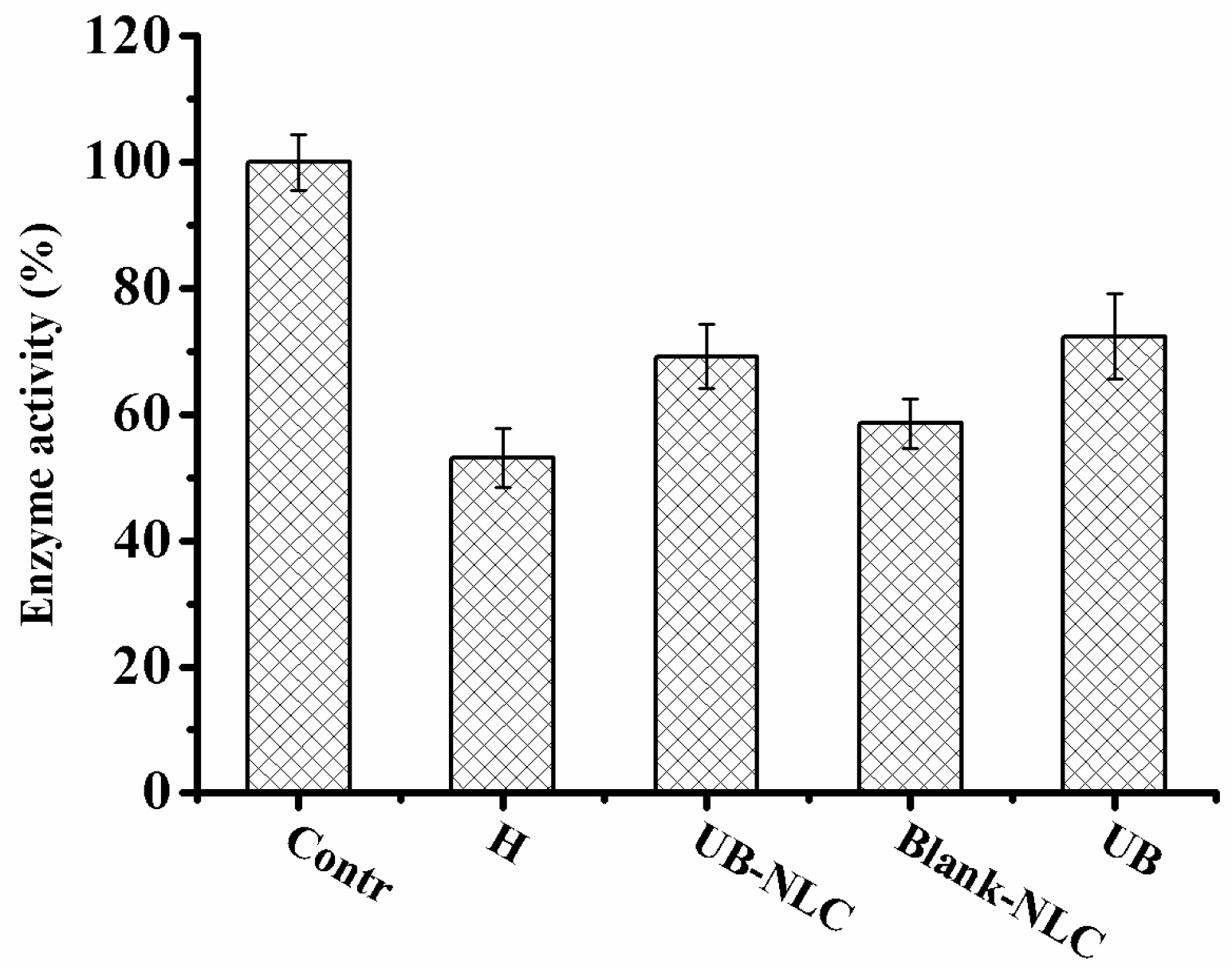
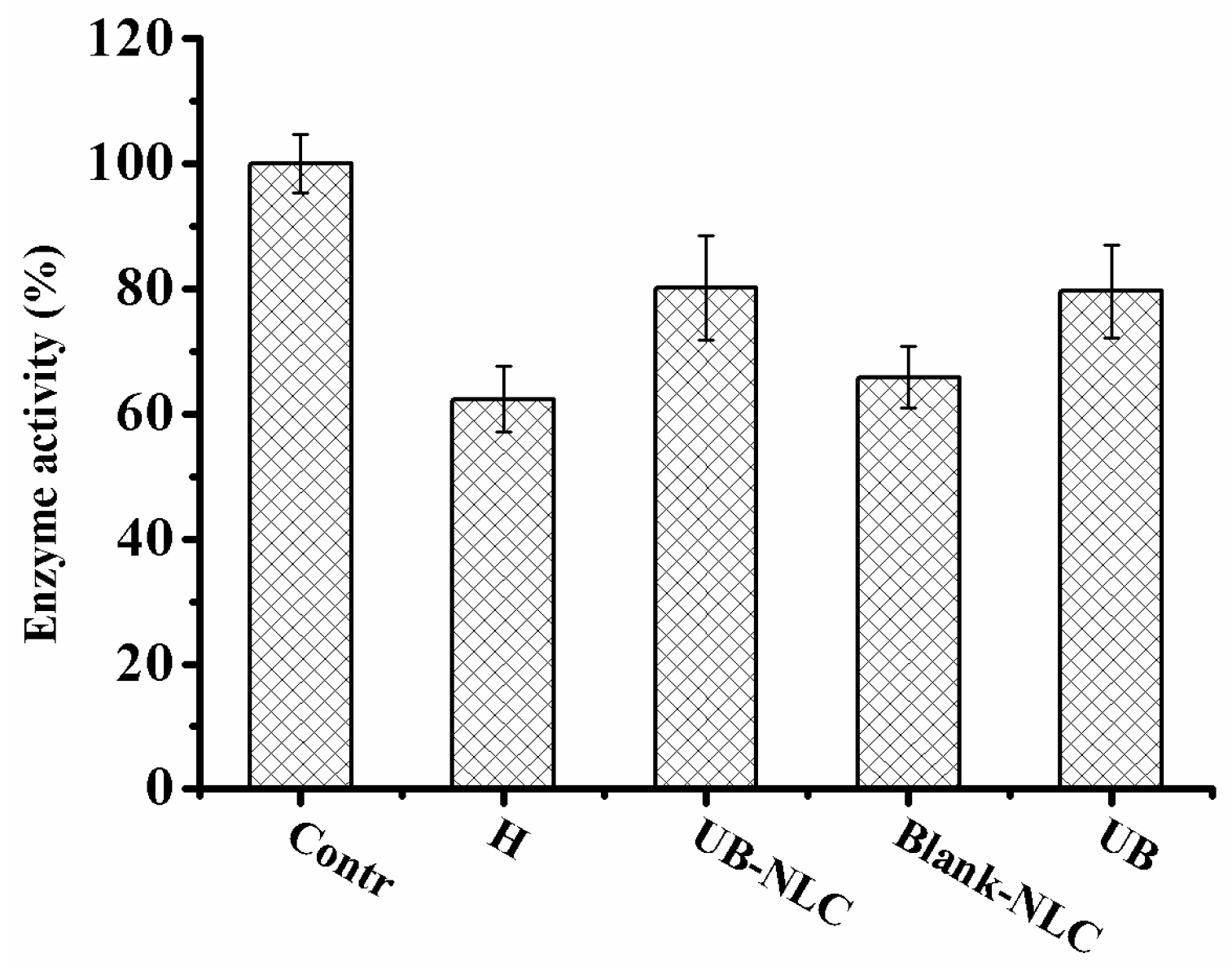
2.5. Effect of UB-NLC on the Activity of Superoxide Dismutase (SOD) and Glutathione Peroxidase (GSH-PX)
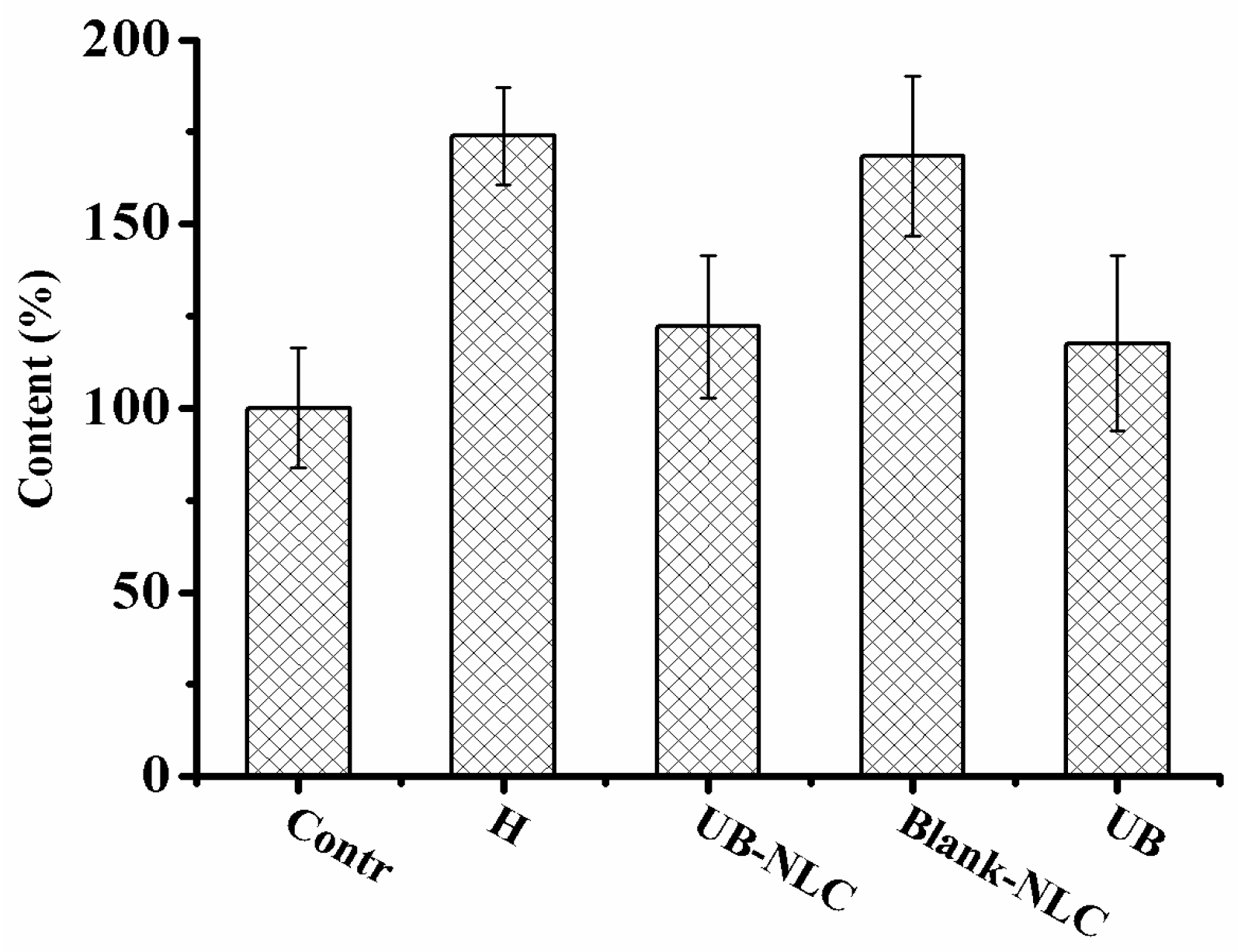
2.6. Effect of UB-NLC on Lipid Peroxidation
3. Materials and Methods
3.1. Materials
3.2. Preparation of UB-NLC
3.3. Size Analysis
3.4. Freeze-Fracture Transmission Electron Microscopy (FF-TEM) Observations
3.5. High Performance Liquid Chromatography (HPLC) Analysis
3.6. Skin Deposition Study
3.7. Cell Culture
3.8. Cell Viability Assay
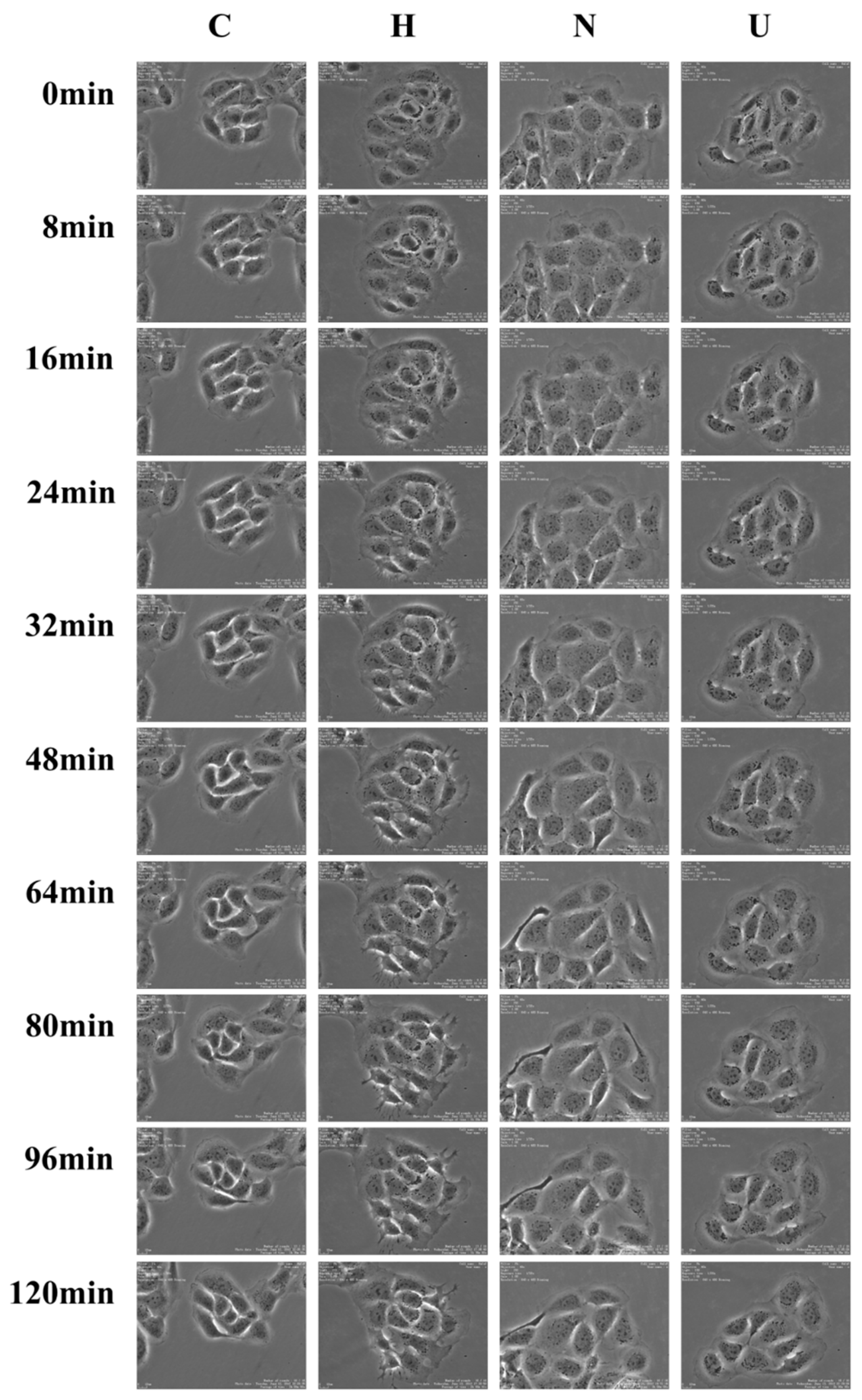
3.9. Time-Lapse Imaging Assays
3.10. Measurement of Reactive Oxygen Species (ROS)
3.11. Assay of Superoxide Dismutase (SOD) Activity
3.12. Glutathione Peroxidase (GSH-PX) Activity Assay
3.13. Determination of Malondialdehyde (MDA)
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhong, X.L.; Yi, X.; Sa, R.; Zhang, Y.J.; Liu, K.H.; Xiao, F.; Zhong, C.G. CoQ10 deficiency may indicate mitochondrial dysfunction in Cr(VI) toxicity. Int. J. Mol. Sci. 2017, 18, 816. [Google Scholar] [CrossRef] [PubMed]
- Mirmalek, S.A.; Boushehrinejad, A.G.; Yavari, H.; Kardeh, B.; Parsa, Y.; Salimi-Tabatabaee, S.A.; Yadollah-Damavandi, S.; Parsa, T.; Shahverdi, E.; Jangholi, E. Antioxidant and anti-inflammatory effects of coenzyme Q10 on l-arginine-induced acute pancreatitis in rat. Oxidative Med. Cell. Longev. 2016, 2016, 5818479. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Noto, C.; Rizzo, L.B.; Rios, A.C.; Nunes, S.O.V.; Barbosa, D.S.; Sethi, S.; Zeni, M.; Mansur, R.B.; Maes, M.; et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 65, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ma, H.Y.; Qiu, Y.Y.; Liu, B.; Qi, H.; Li, Z.Y.; Kong, H.; Kong, L. The downregulation of thioredoxin accelerated Neuro2a cell apoptosis induced by advanced glycation end product via activating several pathways. Neurochem. Int. 2015, 87, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, E.; Zavareh, S.; Lashkarbolouki, T. Antioxidant properties of coenzyme Q10-pretreated mouse pre-antral follicles derived from vitrified ovaries. J. Obstet. Gynaecol. Res. 2017, 43, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, J.K.; Lian, Y.R.; Zhang, Y.; Li, X.R.; Liu, Y.; Zou, L.Y.; Wu, T. Protective effects of coenzyme Q10 against hydrogen peroxide-induced oxidative stress in PC12 cell: The role of nrf2 and antioxidant enzymes. Cell. Mol. Neurobiol. 2016, 36, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.H.; He, W.N.; Xu, L.D.; Wang, J.; Shi, J.; Sheng, M.X. Effects of astragaloside IV against the TGF-beta 1-induced epithelial-to-mesenchymal transition in peritoneal mesothelial cells by promoting Smad 7 expression. Cell. Physiol. Biochem. 2015, 37, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Kashka, R.H.; Zavareh, S.; Lashkarbolouki, T. Augmenting effect of vitrification on lipid peroxidation in mouse preantral follicle during cultivation: Modulation by coenzyme Q(10). Syst. Biol. Reprod. Med. 2016, 62, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cui, C.; Chen, C.Q.; Hu, X.L.; Liu, Y.H.; Fan, Y.H.; Meng, W.H.; Zhao, Q.C. Anti-proliferative and pro-apoptotic activities of alpinia oxyphylla on HepG2 cells through ROS-mediated signaling pathway. J. Ethnopharmacol. 2015, 169, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Gupta, R.K.; Paitandi, R.P.; Rana, N.K.; Sharma, G.; Koch, B.; Rana, L.K.; Hundal, M.S.; Pandey, D.S. Synthesis, Structure, DNA/Protein binding, and anticancer activity of some half-sandwich cyclometalated Rh(III) and Ir(III) complexes. Organometallics 2015, 34, 4491–4506. [Google Scholar] [CrossRef]
- Chen, M.H.; Hanagata, N.; Ikoma, T.; Huang, J.Y.; Li, K.Y.; Lin, C.P.; Lin, F.H. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016, 37, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lone, Y.; Bhide, M.; Koiri, R.K. Amelioratory effect of coenzyme Q10 on potential human carcinogen microcystin-LR induced toxicity in mice. Food Chem. Toxicol. 2017, 102, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.S.; Shahin, M.I.; Kelada, N.A. Hepatoprotective effect of taurine and coenzyme Q10 and their combination against acrylamide-induced oxidative stress in rats. Trop. J. Pharm. Res. 2017, 16, 1849–1855. [Google Scholar] [CrossRef]
- Motawi, T.K.; Darwish, H.A.; Hamed, M.A.; El-Rigal, N.S.; Naser, A.F.A. A therapeutic insight of niacin and coenzyme Q10 against diabetic encephalopathy in rats. Mol. Neurobiol. 2017, 54, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Ziosi, M.; Di Meo, I.; Kleiner, G.; Gao, X.H.; Barca, E.; Sanchez-Quintero, M.J.; Tadesse, S.; Jiang, H.F.; Qiao, C.H.; Rodenburg, R.J.; et al. Coenzyme Q deficiency causes impairment of the sulfide oxidation pathway. EMBO Mol. Med. 2017, 9, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M.; Elkhoely, A.A. Targeting proinflammatory cytokines, oxidative stress, TGF-beta 1 and STAT-3 by rosuvastatin and ubiquinone to ameliorate trastuzumab cardiotoxicity. Biomed. Pharmacother. 2017, 93, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.K.; Nanda, S.; Sharma, G.; Katare, O.P. Systematically optimized coenzyme q10-loaded novel proniosomal formulation for treatment of photo-induced aging in mice: Characterization, biocompatibility studies, biochemical estimations and anti-aging evaluation. J. Drug Target. 2016, 24, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Balaraman, R.; Sen, A.K.; Shukla, D.; Seth, A. Effect of concomitant administration of coenzyme Q10 with sitagliptin on experimentally induced diabetic nephropathy in rats. Ren. Fail. 2017, 39, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Khodir, A.E.; Atef, H.; Said, E.; ElKashef, H.A.; Salem, H.A. Implication of Nrf2/HO-1 pathway in the coloprotective effect of coenzyme Q10 against experimentally induced ulcerative colitis. Inflammopharmacology 2017, 25, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, H.N.; Hegazy, G.A.; El Awdan, S.A.; AbdelBaset, M. Protective role of CoQ10 or l-carnitine on the integrity of the myocardium in doxorubicin induced toxicity. Tissue Cell 2017, 49, 410–426. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Boschi, A.; Ravani, L.; Cortesi, R.; Drechsler, M.; Mariani, P.; Moscatelli, S.; Contado, C.; Domenico, G.; Nastruzzi, C.; et al. Biodistribution of nanostructured lipid carriers: A tomographic study. Eur. J. Pharm. Biopharm. 2015, 89, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Ravani, L.; Drechsler, M.; Mariani, P.; Contado, C.; Ruokolainen, J.; Ratano, P.; Campolongo, P.; Trezza, V.; Nastruzzi, C.; et al. Cannabinoid antagonist in nanostructured lipid carriers (NLCs): Design, characterization and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Ferulic acid-loaded nanostructured lipid carriers: A promising nanoformulation against the ischemic neural injuries. Life Sci. 2018, 193, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Simoes, S.; Araujo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Boakye, C.H.A.; Patel, K.; Doddapaneni, R.; Bagde, A.; Behl, G.; Chowdhury, N.; Safe, S.; Singh, M. Ultra-flexible nanocarriers for enhanced topical delivery of a highly lipophilic antioxidative molecule for skin cancer chemoprevention. Colloids Surf. B Biointerfaces 2016, 143, 156–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.C.; Chiang, B.H.; Huang, D.W.; Li, P.H. Skin permeation of D-limonene-based nanoemulsions as a transdermal carrier prepared by ultrasonic emulsification. Ultrason. Sonochem. 2014, 21, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Firooz, A.; Nafisi, S.; Maibach, H.I. Novel drug delivery strategies for improving econazole antifungal action. Int. J. Pharm. 2015, 495, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Raza, K.; Katare, O.P. Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv. 2016, 23, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Chen, Y.; Ding, N.; Li, N.; Jiang, H.; Zhao, C.; Kang, F.; Cao, Z.; Quan, H.; Luo, F.; et al. Xanthotoxin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis. Osteoporos. Int. 2016, 27, 2335–2344. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.A.; Liu, W.T.; Peng, Y.H.; Roberts, W.; Whitelaw, D.; Graham, A.M. The role of maternal gestational diabetes in inducing fetal endothelial dysfunction. J. Cell. Physiol. 2015, 230, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.P.; Liu, Y.C.; Wang, H.L.; Qin, J.L.; Cheng, F.J.; Tang, S.F.; Liang, H. Synthesis and antitumor mechanisms of a copper(II) complex of anthracene-9-imidazoline hydrazone (9-AIH). Metallomics 2015, 7, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, V.; Loreto, C.; Ledda, C.; Musumeci, G.; Bracci, M.; Santarelli, L.; Renis, M.; Ferrante, M.; Cardile, V. Cytotoxicity, oxidative stress and genotoxicity induced by glass fibers on human alveolar epithelial cell line A549. Toxicol. In Vitro 2015, 29, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, S.S.; Kim, J.Y.; Tchah, H. The antioxidant N-acetylcysteine inhibits inflammatory and apoptotic processes in human conjunctival epithelial cells in a high-glucose environment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5614–5621. [Google Scholar] [CrossRef] [PubMed]
- Kung, M.L.; Hsieh, S.L.; Wu, C.C.; Chu, T.H.; Lin, Y.C.; Yeh, B.W.; Hsieh, S.C. Enhanced reactive oxygen species overexpression by CuO nanoparticles in poorly differentiated hepatocellular carcinoma cells. Nanoscale 2015, 7, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Wang, H.X.; Zhou, X.F.; Tang, Z.M.; Liu, G.Q.; Liu, G.Y.; Xia, Q. Physicochemical characterization, photo-stability and cytotoxicity of coenzyme Q10-loading nanostructured lipid carrier. J. Nanosci. Nanotechnol. 2012, 12, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.; Ilari, S.; Giancotti, L.A.; Ventura, C.A.; Morabito, C.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Paolino, D.; Mollace, V.; et al. Pharmacological effect of a new idebenone formulation in a model of carrageenan-induced inflammatory pain. Pharmacol. Res. 2016, 111, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, G.Y.; Song, A.X.; Wang, L.; Lin, M.Q.; Dong, Z.X.; Yang, Z.H. Faceted fatty acid vesicles formed from single-tailed perfluorinated surfactants. Soft Matter 2015, 11, 7143–7150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Hao, J.C. Ionogels of sugar surfactant in ethylammonium nitrate: Phase transition from closely packed bilayers to right-handed twisted ribbons. J. Phys. Chem. B 2015, 119, 13321–13329. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Cui, W.L.; Yi, S.J. pH-responsive vesicles from supra-amphiphiles based on dynamic imine bond. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 28–36. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.G.; Chai, J.L.; Chen, M.J.; Yang, Q.; Hao, J.C. Self-assembly and rheological properties of a pseudogemini surfactant formed in a salt-free catanionic surfactant mixture in water. Langmuir 2015, 31, 11209–11219. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.L.; Pang, J.Y.; Xu, G.Y.; Xin, X.; Yang, Y.J.; Luan, X.Y.; Yuan, S.L. Smart stimuli-responsive fluorescent vesicular sensor based on inclusion complexation of cyclodextrins with Tyloxapol. RSC Adv. 2016, 6, 11683–11690. [Google Scholar] [CrossRef]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; De Palma, G.; Bovenzi, M.; et al. Titanium dioxide nanoparticle penetration into the skin and effects on HaCaT cells. Int. J. Environ. Res. Public Health 2015, 12, 9282–9297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Louhivuori, L.M.; Turunen, P.M.; Louhivuori, V.; Yellapragada, V.; Nordstrom, T.; Uhlen, P.; Akerman, K.E. Regulation of radial glial process growth by glutamate via mGluR5/TRPC3 and neuregulin/ErbB4. Glia 2018, 66, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Jeknic, S.; Macklin, D.N.; Akhter, S.; Hughey, J.J.; Regot, S.; Covert, M.W. Live-cell measurements of kinase activity in single cells using translocation reporters. Nat. Protoc. 2018, 13, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, H.; Wang, J.B. Echinacoside inhibits amyloid fibrillization of HEWL and protects against a beta-induced neurotoxicity. Int. J. Biol. Macromol. 2015, 72, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Zhang, Y.P.; Wu, Q.N.; Chen, B. Uric acid induces oxidative stress via an activation of the renin-angiotensin system in 3T3-L1 adipocytes. Endocrine 2015, 48, 135–142. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, H.; Xia, Q. Ubidecarenone-Loaded Nanostructured Lipid Carrier (UB-NLC): Percutaneous Penetration and Protective Effects Against Hydrogen Peroxide-Induced Oxidative Stress on HaCaT Cells. Int. J. Mol. Sci. 2018, 19, 1865. https://doi.org/10.3390/ijms19071865
Wang J, Wang H, Xia Q. Ubidecarenone-Loaded Nanostructured Lipid Carrier (UB-NLC): Percutaneous Penetration and Protective Effects Against Hydrogen Peroxide-Induced Oxidative Stress on HaCaT Cells. International Journal of Molecular Sciences. 2018; 19(7):1865. https://doi.org/10.3390/ijms19071865
Chicago/Turabian StyleWang, Jianmin, Huiyun Wang, and Qiang Xia. 2018. "Ubidecarenone-Loaded Nanostructured Lipid Carrier (UB-NLC): Percutaneous Penetration and Protective Effects Against Hydrogen Peroxide-Induced Oxidative Stress on HaCaT Cells" International Journal of Molecular Sciences 19, no. 7: 1865. https://doi.org/10.3390/ijms19071865