A Crude 1-DNJ Extract from Home Made Bombyx Batryticatus Inhibits Diabetic Cardiomyopathy-Associated Fibrosis in db/db Mice and Reduces Protein N-Glycosylation Levels
Abstract
:1. Introduction
2. Results
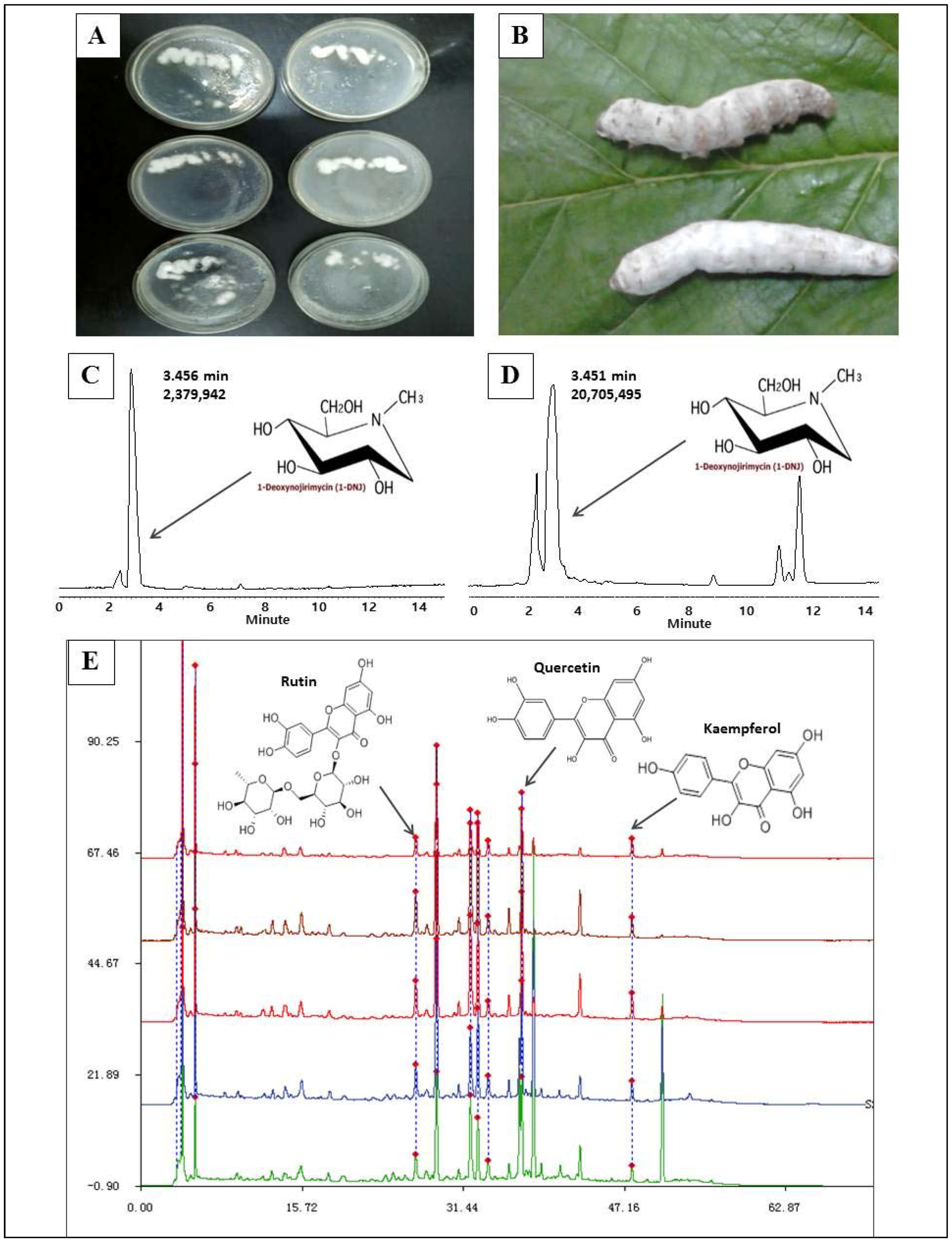
2.1. 1-Deoxynojirimycin Enrichment and Purity Test
2.2. Effects of 1-Deoxynojirimycin on Clinical Biochemistry Indicators in db/db Mice
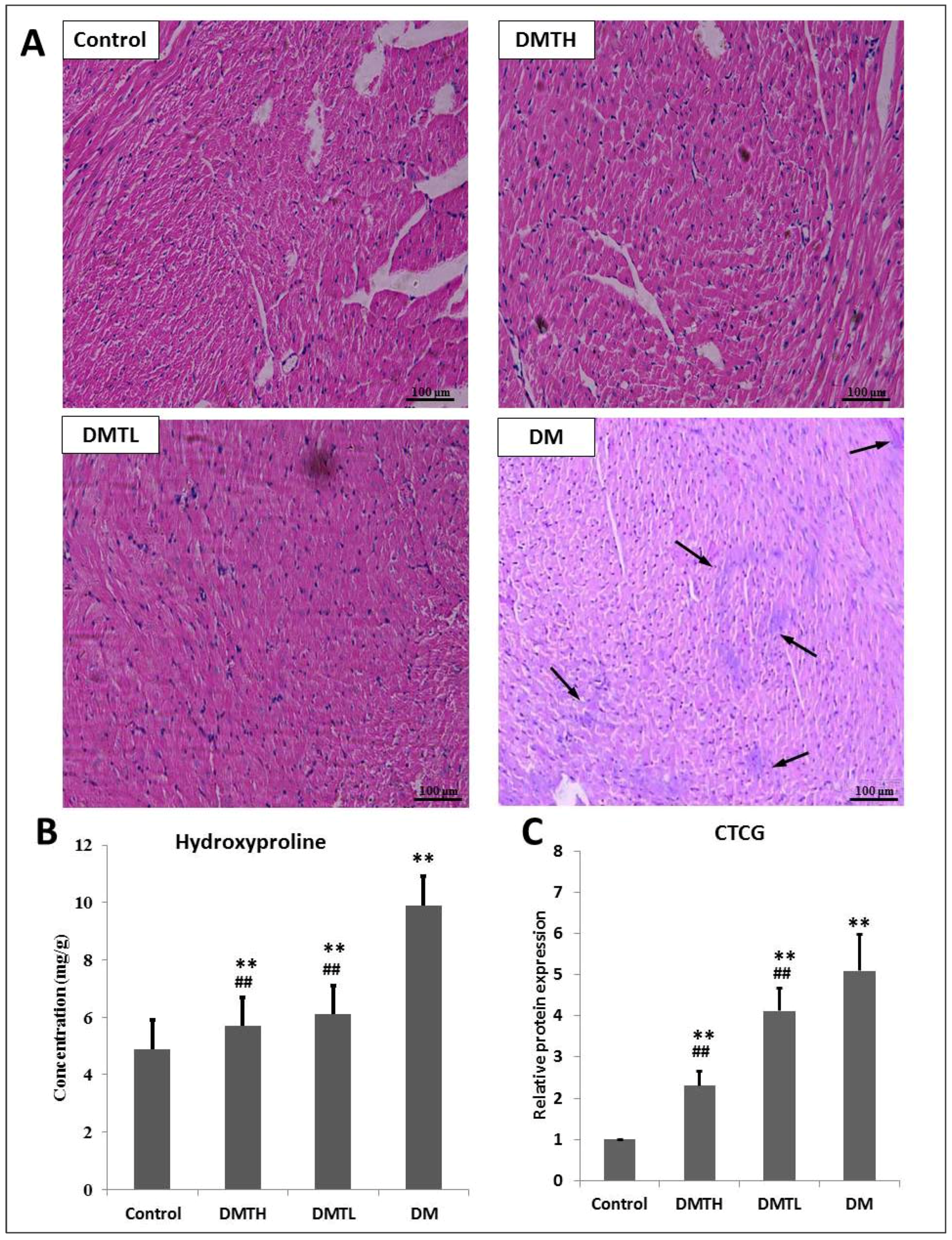
2.3. Myocardial Histomorphometry
2.4. Regulatory Effect on the Expression Levels of Connective Tissue Growth Factor and Hydroxyproline in Myocardial Tissues
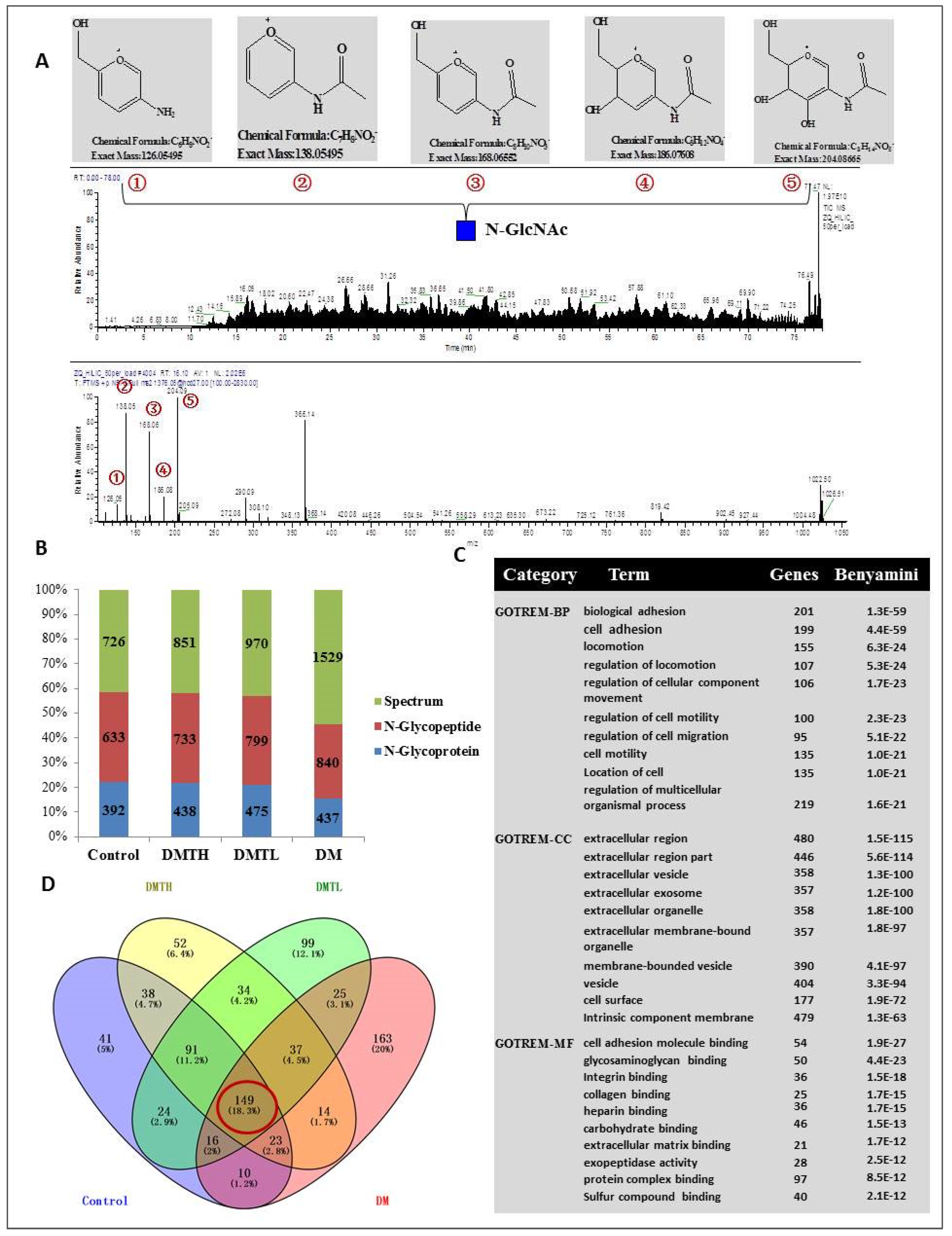
2.5. Site-Specific N-Glycosylated Proteome Identification and Motif Analysis
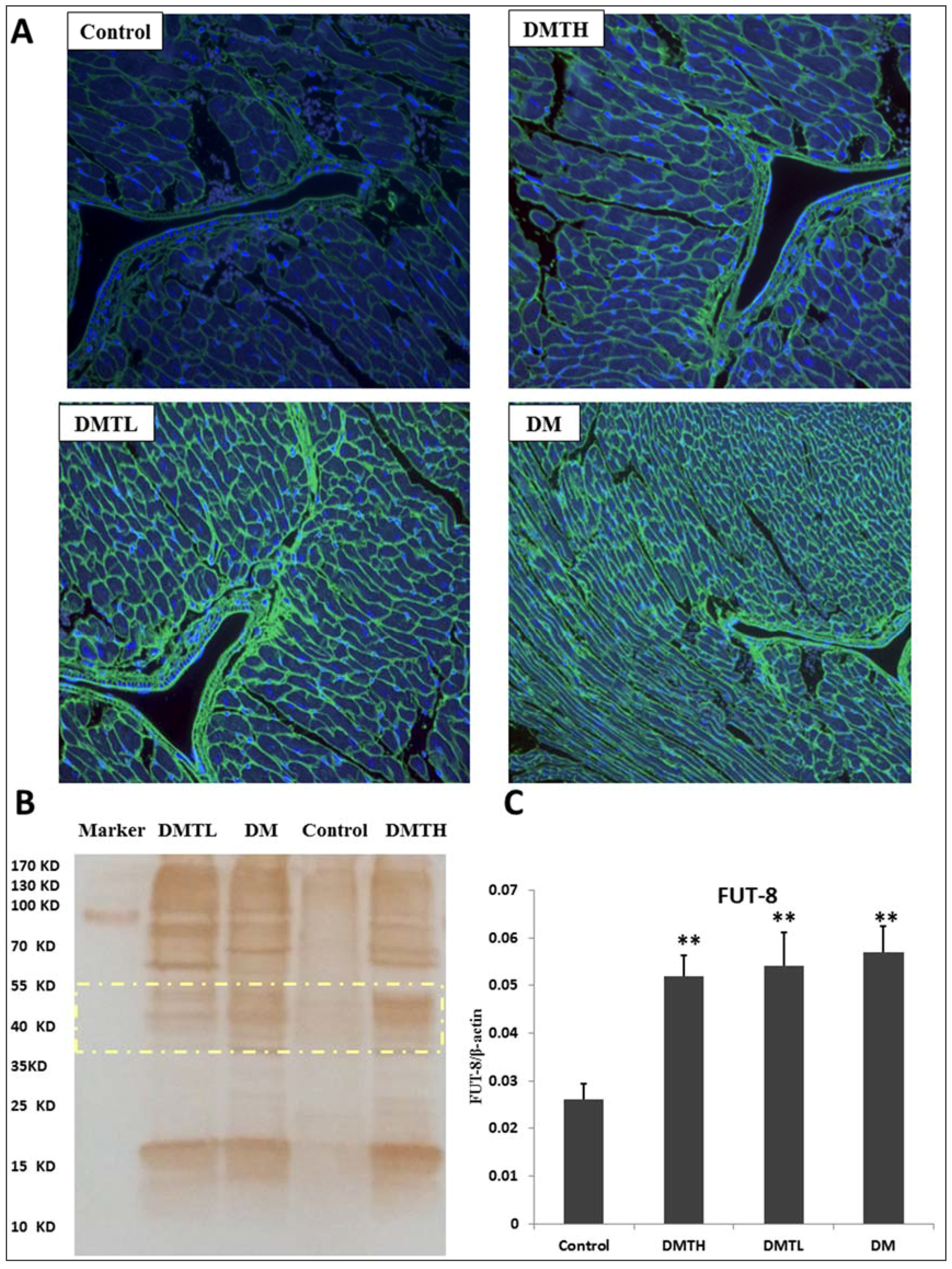
2.6. FITC-Labelled Lectin Affinity Histochemistry
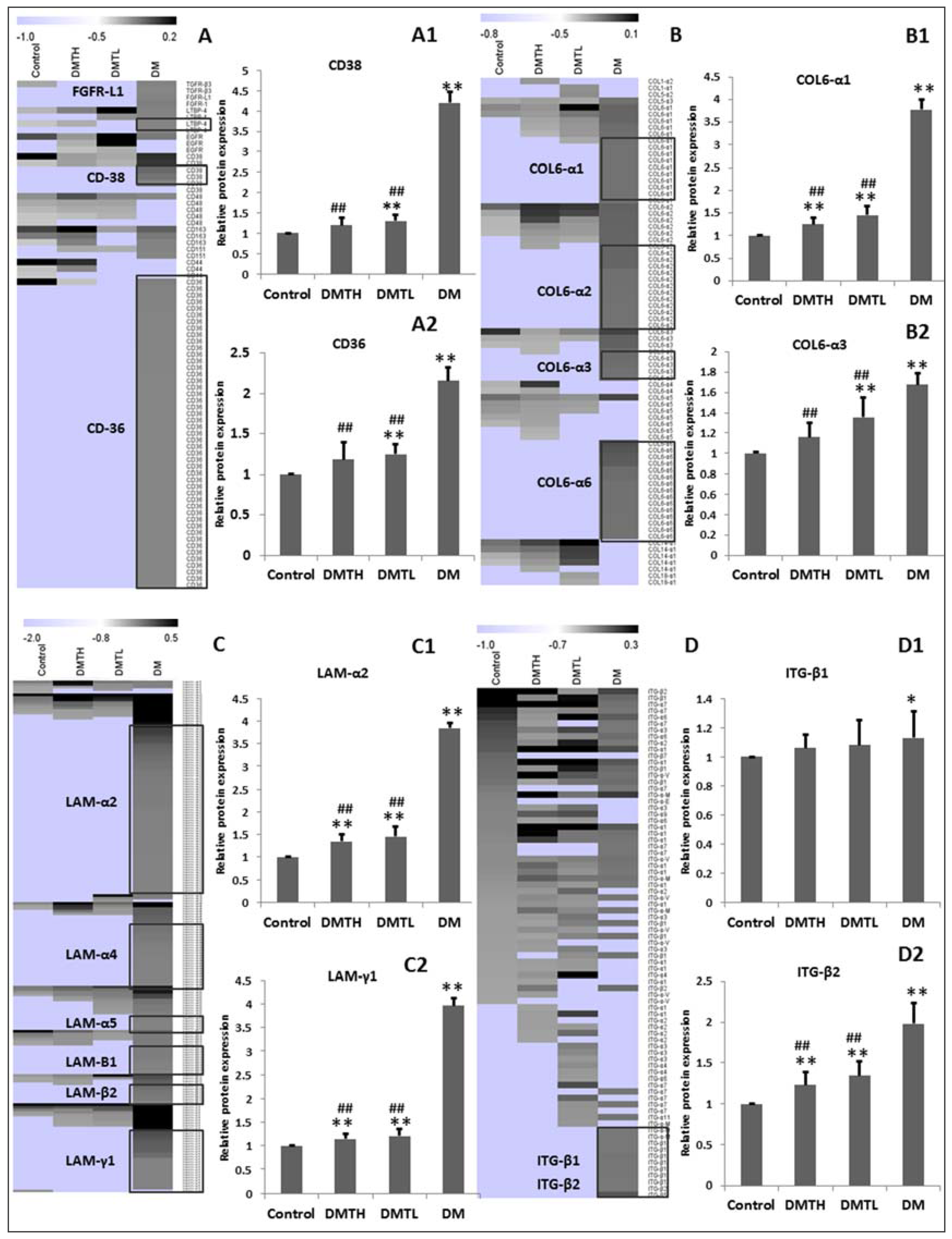
2.7. Lectin Blot Analysis and α-1,6-Fucosyltransferase mRNA Expression Quantification
2.8. Transforming Growth Factor-β/Smad2/3 Pathway Protein Profiling with Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents and Drugs
4.2. 1-Deoxynojirimycin Extract Preparation and Purity Detection
4.3. Animals and Administration
4.4. Clinical Biochemistry Indicator Determination and Histopathology Analysis
4.5. Protein Extract and Digestion
4.6. Glycosylated Peptides Enrichment and N-Glycan Resection
4.7. Proteomic Analysis with LC-MS/MS
4.8. Database Retrieval Conditions and Statistical Analysis Toolkits
4.9. Lectin Blot Analysis
4.10. Western-Blot Analysis
4.11. Real Time PCR Analysis of FUT8
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, T.A.; Kjeldsen, S.E.; Rostrup, M.; Omvik, P.; Hua, T.A.; Julius, S. Impact of new-onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial population. Hypertension 2007, 50, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Sharma, S.D.; Goyal, A.K.; Sarna, A. Coexistence of nephrotic syndrome, celiac disease, and insulin-dependent diabetes mellitus. Indian J. Gastroenterol. 2014, 33, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.M.; Nesto, R.W. Acute myocardial infarction in the diabetic patient: Pathophysiology, clinical course and prognosis. J. Am. Coll. Cardiol. 1992, 20, 736–744. [Google Scholar] [CrossRef]
- Asbun, J.; Villarreal, F.J. The Pathogenesis of Myocardial Fibrosis in the Setting of Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Umeda, K.; Sugihara, N.; Yoshio, H.; Ino, H.; Takeda, R.; Okada, Y.; Nakanishi, I. Collagen remodelling in myocardia of patients with diabetes. J. Clin. Pathol. 1993, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, L.; Tasca, C.; Havriliuc, C.; Vasilescu, C. A morphological quantitative study of small vessels in diabetic cardiomyopathy. Morphol. Embryol. 1985, 31, 191–195. [Google Scholar]
- Nunoda, S.; Genda, A.; Sugihara, N.; Nakayama, A.; Mizuno, S.; Takeda, R. Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus. Heart Vessels 1985, 1, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, D.; Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Mellor, K.M.; Brimble, M.A.; Delbridge, L.M.D. Glucose as an agent of post-translational modification in diabetes-New cardiac epigenetic insights. Life Sci. 2015, 129, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Links, T.; Zeebregts, C.; Tio, R.; Hillebrands, J.L.; Smit, A. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc. Diabetol. 2008, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.L.; Forbes, J.M.; Cooper, M.E. AGE, RAGE, and ROS indiabetic nephropathy. Semin. Nephrol. 2007, 27, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Fu, G.S.; Chen, F.M.; Wang, H. The effect of valsartan and fluvastatin on the connective tissue growth factor expression in experimental diabetic. Cardiomyopathy 2009, 48, 660–665. [Google Scholar]
- Jun, J.I.; Lau, L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 945–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall-Glenn, F.; Lyons, K.M. Roles for CCN2 in normal physiological processes. Cell. Mol. Life Sci. 2011, 68, 3209–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanwar, Y.S.; Sun, L.; Xie, P.; Liu, F.Y.; Chen, S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 395–423. [Google Scholar] [CrossRef] [PubMed]
- Keembiyehetty, C.; Love, D.C.; Harwood, K.R.; Gavrilova, O.; Comly, M.E.; Hanover, J.A. Conditional knock-out reveals a requirement for O-linked N-Acetylglucosaminase (O-GlcNAcase) in metabolic homeostasis. J. BioChem. 2015, 290, 7097–7113. [Google Scholar]
- Chandler, K.B.; Pompach, P.; Goldman, R.; Edwards, N. Exploring Site-Specific N-Glycosylation microheterogeneity of haptoglobin using glycopeptide CID tandem mass spectra and glycan database search. J. Proteome Res. 2013, 12, 3652–3666. [Google Scholar] [CrossRef] [PubMed]
- Poland, D.C.; Schalkwijk, C.G.; Stehouwer, C.D.; Koeleman, C.A.; van het Hof, B.; van Dijk, W. Increased α3-fucosylation of αl-acid glycoprotein in Type I diabetic patients is related to vascular function. Glycoconj. J. 2001, 18, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Higai, K.; Azuma, Y.; Aoki, Y.; Matsumoto, K. Altered glycosylation of αl-acid glycoprotein in patients with inflammation and diabetes mellitus. Clin. Chim. Acta 2003, 329, 117–125. [Google Scholar] [CrossRef]
- Orestes, P.; Osuru, H.P.; McIntire, W.E.; Jacus, M.O.; Salajegheh, R.; Jagodic, M.M.; Choe, W.; Lee, J.; Lee, S.S.; Rose, K.E.; et al. Reversal of neuropathic pain in diabetes by targeting glycosylation of Cav3.2 T-type calcium channels. Diabetes 2013, 621, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Sakaue, S.; Nakagawa, H.; Kurogochi, M.; Ohira, H.; Deguchi, K.; Nishimura, S.I.; Nishimura, M. Analysis of N-glycan inserum glycoproteins from db/db mice and humans with type 2diabetes. Am. J. Physiol. Endocrinol. Metabol. 2007, 293, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Lin, H.; Wu, T.; Wang, D.; Wang, W.; Xie, H.; Zhang, J.; Feng, Z. Inhibition of TGF-β1-receptor posttranslational core fucosylation attenuates rat renal interstitial fibrosis. Kidney Int. 2013, 84, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Meng, K.; Pu, Y.; Zhang, X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 2017, 133, 124–130. [Google Scholar] [CrossRef] [PubMed]
- AlJaroudi, W.A.; Refaat, M.M.; Habib, R.H.; Al-Shaar, L.; Singh, M.; Gutmann, R.; Bloom, H.L.; Dudley, S.C.; Ellinor, P.T.; Saba, S.F.; et al. Effect of Angiotensin-Converting Enzyme Inhibitors and Receptor Blockers on Appropriate Implantable Cardiac Defibrillator Shock in Patients with Severe Systolic Heart Failure (from the GRADE Multicenter Study). Am. J. Cardiol. 2015, 115, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Lee, M.H.; Song, H.K.; Hyun, Y.Y.; Cha, J.J.; Ko, G.J.; Kim, S.H.; Lee, J.E.; Han, J.Y.; Cha, D.R. Aliskiren improves insulin resistance and ameliorates diabetic vascular complications in db/db mice. Nephrol. Dial. Transplant. 2011, 26, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Schwebe, M.; Ameling, S.; Hammer, E.; Monzel, J.V.; Bonitz, K.; Budde, S.; Schult, K.; Oswald, S.; Scheuch, E.; Grube, M.; et al. Protective effects of endothelin receptor A and B inhibitors against doxorubicin-induced cardiomyopathy. Bioch. Pharmacol. 2015, 94, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Nojima, H.; Kimura, I.; Chen, F.J.; Sugihara, Y.; Haruno, M. Antihyperglycemic effects of N-Containing sugars from Xanthocercis zambesiaca, Morus bombycis, Aglaonema treubii, and Castanospermum australe in streptozotocin-diabetic Mice. J. Nat. Prod. 1998, 61, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Shi, X.Q.; Sun, B.; Ye, J.J.; Duan, Z.A.; Zhou, X.L.; Cui, W.Z.; Wu, X.F. Accumulation of 1-deoxynojirimycin in silkworm, Bombyx mori L. J. Zhejiang Univ. Sci. B 2010, 11, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Yatsunami, K.; Murata, K.; Kamei, T. 1-Deoxynojirimycin Content and α-Glucosidase Inhibitory Activity and Heat Stability of 1-Deoxynojirimycin in Silkworm Powder. Food Nutr. Sci. 2011, 2, 87–89. [Google Scholar] [CrossRef]
- Kikuchi, H.; Takahashi, N.; Oshima, Y. Novel aromatics bearing 4-O-methylglucose unit isolated from the oriental crude drug Bombyx Batryticatus. Tetrahedron Lett. 2004, 45, 367–370. [Google Scholar] [CrossRef]
- Liu, C.; Xiang, W.; Yu, Y.; Shi, Z.Q.; Huang, X.Z.; Xu, L. Comparative analysis of 1-deoxynojirimycin contribution degree to α-glucosidase inhibitory activity and physiological distribution in Morus alba L. Ind. Crops Prod. 2015, 70, 309–315. [Google Scholar] [CrossRef]
- Saunier, B.; Killer, R.D., Jr.; Tkacz, J.S.; Quaroni, A.; Herscovics, A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J. Biol. Chem. 1982, 257, 14155–14161. [Google Scholar] [PubMed]
- Lee, D.S.; Yu, I.S.; Jung, K.; Kim, Y.S. Incidence and levels of Aflatoxins contamination in Medical Plants in Korea. Mycobiology 2014, 42, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gai, Y.; Liu, F.; Gao, W.; Zhang, Y.; Xu, M.; Li, Z. Trimetazidine inhibits pressure overload-induced cardiac fibrosis through NADPH oxidase–ROS–CTGF pathway. Cardiovasc. Res. 2010, 88, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, J.; Bächinger, H.P. Structure, stability and folding of the collagen triple helix. Top. Curr. Chem. 2005, 247, 7–33. [Google Scholar]
- Nicolaou, N.; Margadant, C.; Kevelam, S.H.; Lilien, M.R.; Oosterveld, M.J.; Kreft, M.; van Eerde, A.M.; Pfundt, R.; Terhal, P.A.; van der Zwaag, B.; et al. Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J. Clin. Investig. 2012, 122, 4375–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitsiou, P.V.; Tzinia, A.K.; Stetler-Stevenson, W.G.; Michael, A.F.; Fan, W.W.; Zhou, B.; Tsilibary, E.C. Glucoseinduced changes in integrins and matrix-related functions in cultured human glomerular epithelial cells. Am. J. Physiol. Renal Physiol. 2003, 284, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.K.; Thanigaraj, S.; Schechtman, K.B.; Perez, J.E. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am. J. Cardiol. 2004, 93, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Andre, S.; Kožár, T.; Kojima, S.; Unverzagt, C.; Gabius, H.J. From structural to functional glycomics: Core substitutions as molecular switches for shape and lectin affinity of N-glycans. J. Biol. Chem. 2007, 390, 557–565. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Kožár, T.; Schuberth, R.; Unverzagt, C.; Kojima, S.; Gabius, H.J. substitutions in the N-glycan core as regulator of biorecognition: The case of core-fucose and bisecting GlcNAc moieties. Biochemistry 2009, 46, 6984–6995. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Nakamura-Tsuruta, S.; Hirabayashi, J. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 2009, 19, 5527–5536. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, V.; Thaysen-Andersen, M.; Chen, S.C.; Nevalainen, H.; Packer, N.H. Cystic fibrosis and bacterial colonization define the sputum N-glycosylation phenotype. Glycobiology 2015, 25, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Michalski, J.C.; Morelle, W. Modifications of human total serum N-glycome during liver fibrosis–cirrhosis, is it all about immunoglobulins. Proteom. Clin. 2010, 4, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Medford, H.M.; Chatham, J.C.; Marsh, S.A. Chronic ingestion of a Western diet increases O-linked-β-N-acetylglucosamine (O-GlcNEc) protein modification in the rat heart. Life Sci. 2012, 90, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.; Van Bilsen, M.; Goldschmeding, R.; Van Der Vusse, G.J.; van Nieuwenhoven, F.A. Connective tissue growth factor and cardiac fibrosis. Acta Physiol. 2009, 3, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.A.; Weinberg, J.M. New wrinkles in old receptors: Core fucosylation is yet another target to inhibit TGF-β signaling. Kidney Int. 2013, 84, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Blobel, G. Intracellular protein topogenesis. Proc. Natl. Acad. Sci. USA 1980, 77, 1496–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liu, Y.; Ma, C.; Qu, J.; Calderon, A.D.; Wu, B.; Wei, N.; Wang, X.; Guo, Y.; Xiao, Z.; et al. Efficient chemoenzymatic synthesis of an N-glycan isomer library. Chem. Sci. 2015, 6, 5652–5661. [Google Scholar] [CrossRef] [PubMed]
- Morling, J.R.; Yeoh, S.E.; Kolbach, D.N. Rutosides for treatment of post-thrombotic syndrome. Cochrane Database Syst. Rev. 2015, 9, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Dhanotiya, C.; Malviya, N. Physicochemical characterization and determination of free radical scavenging activity of rutin-phospholipid complex. Int. J. Pharm. Sci. Res. 2012, 3, 909–913. [Google Scholar]
- Wang, X.; Zhao, X.; Feng, T.; Jin, G.; Li, Z. Rutin prevents high glucose-induced renal glomerular endothelial hyperpermeability by inhibiting the ROS/Rhoa/ROCK signaling pathway. Planta Med. 2016, 82, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2011, 41, 492–499. [Google Scholar] [CrossRef]
- Yoshikuni, Y. Inhitibion of intestinal α-glucosidase activity and postprandial hyperglycemia by moranoline and its N-alkyl derivatives. Agric. Biol. Chem. 1988, 52, 121–128. [Google Scholar]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Filter aided proteome preparation (FASP). Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Jia, T.Z.; Cao, Q.C.; Tian, F.; Ying, W.T. A Crude 1-DNJ Extract from Home Made Bombyx Batryticatus Inhibits Diabetic Cardiomyopathy-Associated Fibrosis in db/db Mice and Reduces Protein N-Glycosylation Levels. Int. J. Mol. Sci. 2018, 19, 1699. https://doi.org/10.3390/ijms19061699
Zhao Q, Jia TZ, Cao QC, Tian F, Ying WT. A Crude 1-DNJ Extract from Home Made Bombyx Batryticatus Inhibits Diabetic Cardiomyopathy-Associated Fibrosis in db/db Mice and Reduces Protein N-Glycosylation Levels. International Journal of Molecular Sciences. 2018; 19(6):1699. https://doi.org/10.3390/ijms19061699
Chicago/Turabian StyleZhao, Qing, Tian Zhu Jia, Qi Chen Cao, Fang Tian, and Wan Tao Ying. 2018. "A Crude 1-DNJ Extract from Home Made Bombyx Batryticatus Inhibits Diabetic Cardiomyopathy-Associated Fibrosis in db/db Mice and Reduces Protein N-Glycosylation Levels" International Journal of Molecular Sciences 19, no. 6: 1699. https://doi.org/10.3390/ijms19061699




