Signal Transduction in Plant–Nematode Interactions
Abstract
:1. Introduction
2. Cellular Signal Transduction in Plants
3. Nematode-Associated Molecular Patterns (NAMPs) and Signaling for Nematode Resistance
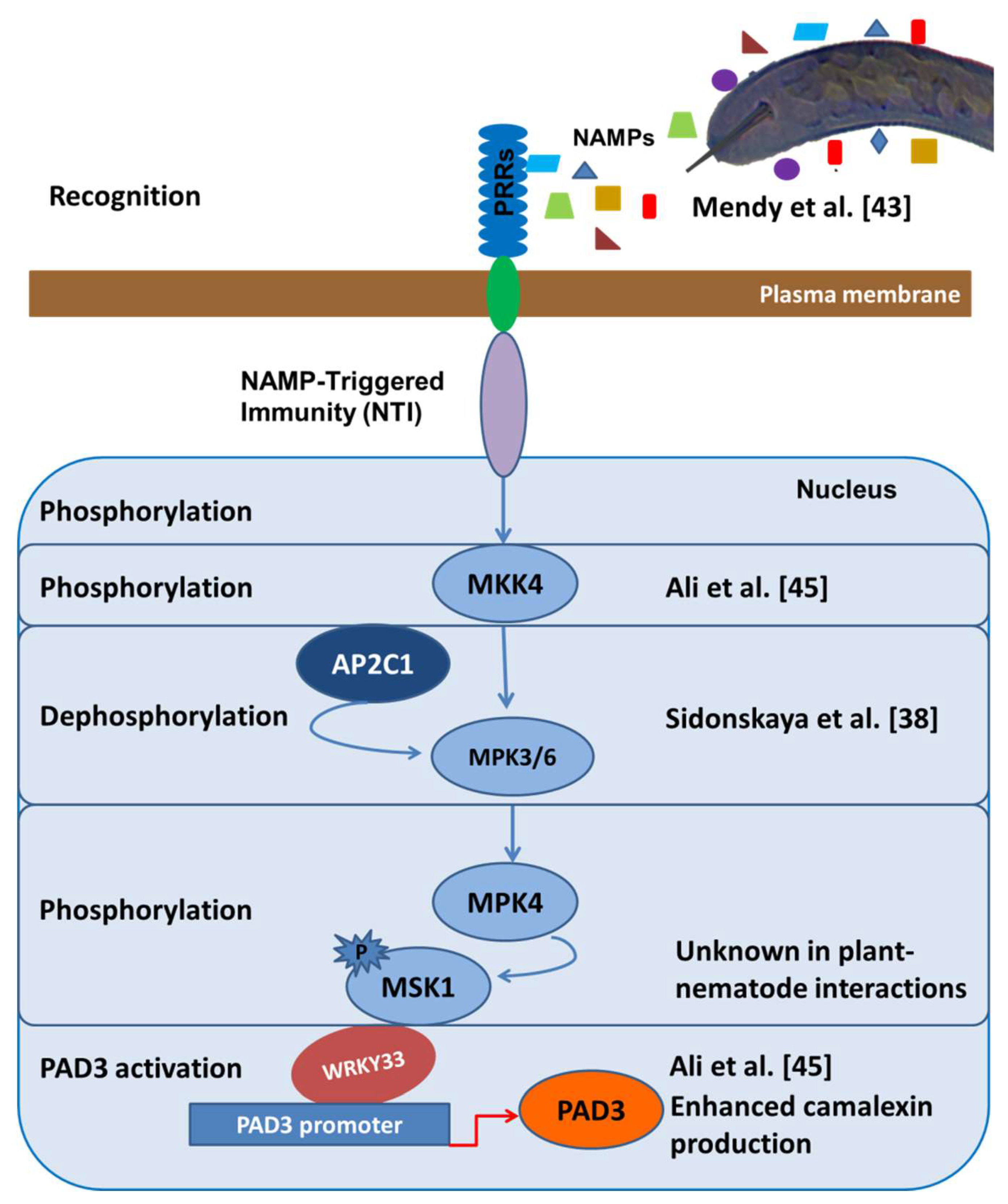
4. Phytoalexin Pathway and NAMP-Triggered Immunity (NTI)
5. Nematode Effector Proteins
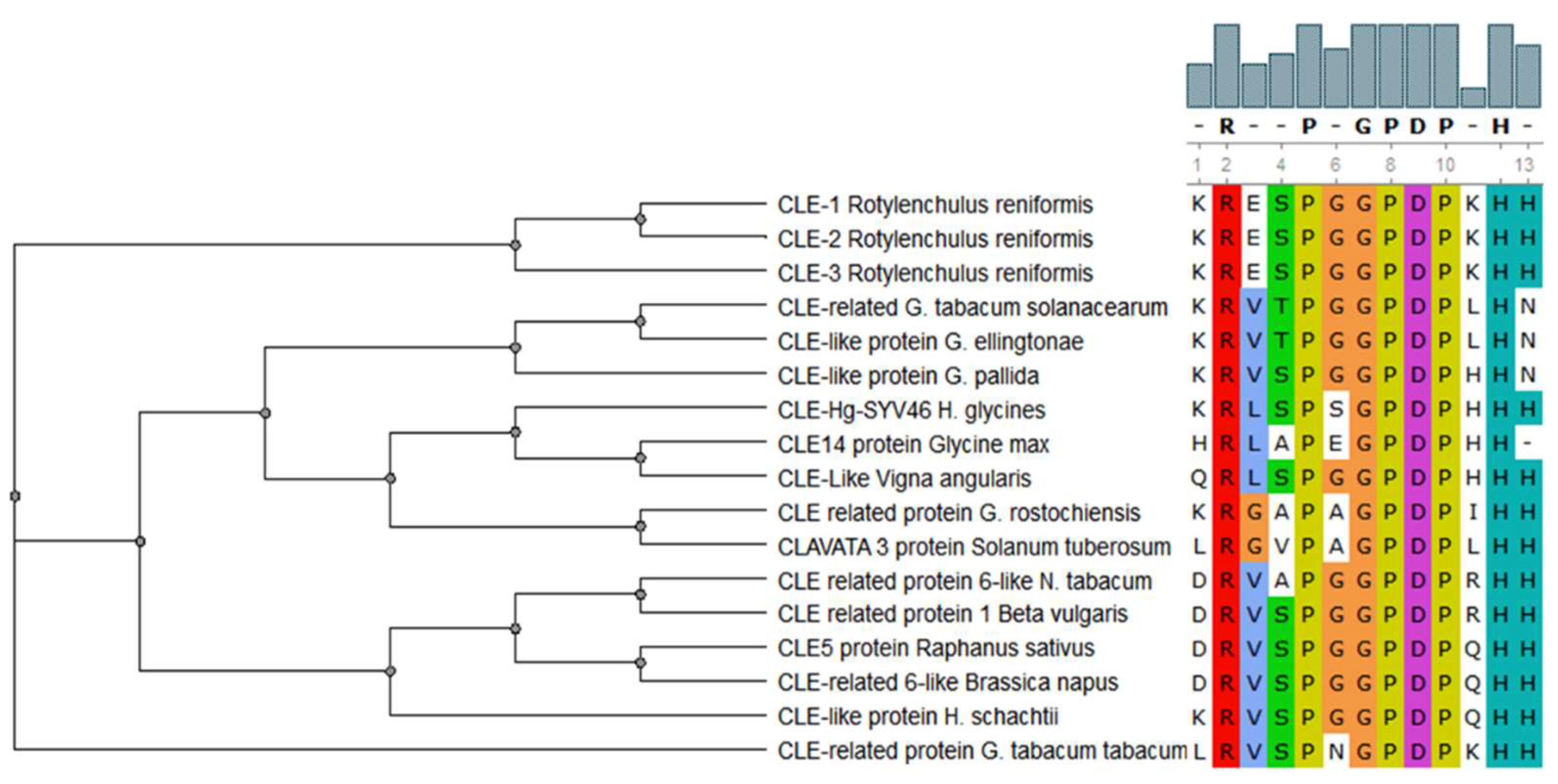
6. CLE Signaling and Parasitic Success of Nematodes
7. Nematode Effectors Are Targeted to Plant Cell Nuclei to Manipulate Host Functions
8. Nematode Effectors Mimic Defense Responses in Host Plants
9. Roles of SA/JA, Auxin, and Cytokinin Signaling in Plant–Nematode Interactions
10. Parasitic Nematodes Modulate ROS Signaling and PCD in Plants
11. Nematodes Modulate RNA Silencing Pathways to Promote Infection Process
12. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Abbas, A.; Azeem, F.; Javed, N.; Bohlmann, H. Plant–nematode Interactions: From Genomics to Metabolomics. Int. J. Agric. Biol. 2015, 17, 1071–1082. [Google Scholar]
- Ali, M.A.; Azeem, F.; Abbas, A.; Joyia, F.A.; Li, H.; Dababat, A. Transgenic strategies for enhancement of nematode resistance in plants. Front. Plant Sci. 2017, 8, 750. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Azeem, F.; Li, H.; Bohlmann, H. Smart Parasitic Nematodes Use Multifaceted Strategies to Parasitize Plants. Front. Plant Sci. 2017, 8, 1699. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The Good, the Bad, and the Ugly of Rhizosphere Microbiome. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 253–290. [Google Scholar]
- Wyss, U.; Grundler, F.M.W. Feeding-Behavior of Sedentary Plant Parasitic Nematodes. Neth. J. Plant. Pathol. 1992, 98, 165–173. [Google Scholar] [CrossRef]
- Golinowski, W.; Grundler, F.M.W.; Sobczak, M. Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 1996, 194, 103–116. [Google Scholar] [CrossRef]
- Endo, B.Y.; Wyss, U. Ultrastructure of Cuticular Exudations in Parasitic Juvenile Heterodera-Schachtii, as Related to Cuticle Structure. Protoplasma 1992, 166, 67–77. [Google Scholar] [CrossRef]
- Grundler, F.M.W.; Sobczak, M.; Lange, S. Defence responses of Arabidopsis thaliana during invasion and feeding site induction by the plant-parasitic nematode Heterodera glycines. Physiol. Mol. Plant Pathol. 1997, 50, 419–429. [Google Scholar] [CrossRef]
- Bellafiore, S.; Briggs, S.P. Nematode effectors and plant responses to infection. Curr. Opin. Plant Biol. 2010, 13, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, G.; Mitchum, M.G. How nematodes manipulate plant development pathways for infection. Curr. Opin. Plant Biol. 2011, 14, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, G.; Fenoll, C. Gene expression in nematode feeding sites. Ann. Rev. Phytopathol. 2002, 40, 191–219. [Google Scholar] [CrossRef] [PubMed]
- Holbein, J.; Grundler, F.M.W.; Siddique, S. Plant basal resistance to nematodes: An update. J. Exp. Bot. 2016, 67, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Klessig, D.F. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biol. 2016, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.C.; Dubreuil, G.; Quentin, M.; Perfus-Barbeoch, L.; Lecomte, P.; de Almeida Engler, J.; Abad, P.; Rosso, M.N.; Favery, B. Root-knot nematodes manipulate plant cell functions during a compatible interaction. J. Plant Physiol. 2008, 165, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T. Cellular Signaling Pathways and Posttranslational Modifications Mediated by Nematode Effector Proteins. Plant Physiol. 2015, 169, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Baum, T. Manipulation of Plant Cells by Cyst and Root-Knot Nematode Effectors. Mol. Plant Microbe Interact 2012, 26, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Howe, P.; Maier, T.R.; Baum, T.J. Arabidopsis Small RNAs and Their Targets During Cyst Nematode Parasitism. Mol. Plant Microbe Interact 2008, 21, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.K.; Xie, Q.G.; Mantelin, S.; Bishnoi, U.; Girke, T.; Navarre, D.A.; Kaloshian, I. Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol. Plant Microbe Interact 2008, 21, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Branch, C.; Hwang, C.F.; Navarre, D.A.; Williamson, V.M. Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol. Plant Microbe Interact 2004, 17, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Absmanner, B.; Stadler, R.; Hammes, U.Z. Phloem development in nematode-induced feeding sites: The implications of auxin and cytokinin. Front. Plant Sci. 2013, 4, 241. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Stael, S.; Kmiecik, P.; Willems, P.; Van Der Kelen, K.; Coll, N.S.; Teige, M.; Van Breusegem, F. Plant innate immunity—Sunny side up? Trends Plant Sci. 2015, 20, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone Crosstalk in Plant Disease and Defense: More Than Just jasmonate-salicylate Antagonism. Ann. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Ann. Rev. Cell Developmental Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.P.; Zipfel, C. Plant PRRs and the Activation of Innate Immune Signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From Pathogen Perception to Robust Defense. Ann. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, H.; Li, X.; Li, Y.; Cui, H.; Wen, C.-K.; Tang, X.; Su, Z.; Zhou, J.-M. Effector-Triggered and Pathogen-Associated Molecular Pattern–Triggered Immunity Differentially Contribute to Basal Resistance to Pseudomonas syringae. Mol. Plant Microbe Interact 2010, 23, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andolfo, G.; Ercolano, M.R. Plant Innate Immunity Multicomponent Model. Front. Plant Sci. 2015, 6, 987. [Google Scholar] [CrossRef] [PubMed]
- Hamamouch, N.; Li, C.Y.; Seo, P.J.; Park, C.M.; Davis, E.L. Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol. Plant Pathol. 2011, 12, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, P.K.; Ithal, N.; Recknor, J.; Maier, T.; Nettleton, D.; Baum, T.J.; Mitchum, M.G. The Soybean Rhg1 Locus for Resistance to the Soybean Cyst Nematode Heterodera glycines Regulates the Expression of a Large Number of Stress- and Defense-Related Genes in Degenerating Feeding Cells. Plant Physiol. 2011, 155, 1960–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddique, S.; Matera, C.; Radakovic, Z.S.; Shamim Hasan, M.; Gutbrod, P.; Rozanska, E.; Sobczak, M.; Angel Torres, M.; Grundler, F.M. Parasitic worms stimulate host NADPH oxidases to produce reactive oxygen species that limit plant cell death and promote infection. Sci. Signal 2014, 7, ra33. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, P.; Manohar, M.; von Reuss, S.H.; Chen, S.; Koch, A.; Kaplan, F.; Choe, A.; Micikas, R.J.; Wang, X.; Kogel, K.-H.; et al. Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. Commun. 2015, 6, 7795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidonskaya, E.; Schweighofer, A.; Shubchynskyy, V.; Kammerhofer, N.; Hofmann, J.; Wieczorek, K.; Meskiene, I. Plant resistance against the parasitic nematodeHeterodera schachtiiis mediated by MPK3 and MPK6 kinases, which are controlled by the MAPK phosphatase AP2C1 in Arabidopsis. J. Exp. Bot. 2016, 67, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; von Reuss, S.H.; Kogan, D.; Gasser, R.B.; Platzer, E.G.; Schroeder, F.C.; Sternberg, P.W. Ascaroside Signaling Is Widely Conserved among Nematodes. Curr. Biol. 2012, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Panda, O.; Akagi, A.E.; Artyukhin, A.B.; Judkins, J.C.; Le, H.H.; Mahanti, P.; Cohen, S.M.; Sternberg, P.W.; Schroeder, F.C. Biosynthesis of Modular Ascarosides in C. elegans. Angew. Chem. Int. Ed. 2017, 56, 4729–4733. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Srinivasan, J.; Mahanti, P.; Ajredini, R.; Durak, O.; Nimalendran, R.; Sternberg, P.W.; Teal, P.E.A.; Schroeder, F.C.; Edison, A.S.; et al. Ascaroside Expression in Caenorhabditis elegans Is Strongly Dependent on Diet and Developmental Stage. PLoS ONE 2011, 6, e17804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Wei, Y.; Zhou, J.; Zhang, W.; Qin, P.; Chinta, S.; Kong, X.; Liu, Y.; Yu, H.; et al. Ascarosides coordinate the dispersal of a plant-parasitic nematode with the metamorphosis of its vector beetle. Nat. Commun. 2016, 7, 12341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendy, B.; Wang’ombe, M.W.; Radakovic, Z.S.; Holbein, J.; Ilyas, M.; Chopra, D.; Holton, N.; Zipfel, C.; Grundler, F.M.W.; Siddique, S. Arabidopsis leucine-rich repeat receptor–like kinase NILR1 is required for induction of innate immunity to parasitic nematodes. PLoS Pathog. 2017, 13, e1006284. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Ali, J.G.; Helder, J.; van der Putten, W.H. Ecology and Evolution of Soil Nematode Chemotaxis. J. Chem. Ecol. 2012, 38, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Wieczorek, K.; Kreil, D.P.; Bohlmann, H. The beet cyst nematode Heterodera schachtii modulates the expression of WRKY transcription factors in syncytia to favour its development in Arabidopsis roots. PLoS ONE 2014, 9, e102360. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Abbas, A.; Kreil, D.P.; Bohlmann, H. Overexpression of the transcription factor RAP2.6 leads to enhanced callose deposition in syncytia and enhanced resistance against the beet cyst nematode Heterodera schachtii in Arabidopsis roots. BMC Plant Biol. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Fiil, B.K.; Mundy, J.; Petersen, M. Downstream targets of WRKY33. Plant Signal Behav. 2008, 3, 1033–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Szakasits, D.; Heinen, P.; Wieczorek, K.; Hofmann, J.; Wagner, F.; Kreil, D.P.; Sykacek, P.; Grundler, F.M.; Bohlmann, H. The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J. 2009, 57, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Hussey, R.S.; Baum, T.J.; Bakker, J.; Schots, A. Nematode parasitism genes. Ann. Rev. Phytopathol. 2000, 38, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Hussey, R.S. Disease-Inducing Secretions of Plant-Parasitic Nematodes. Ann. Rev. Phytopathol. 1989, 27, 123–141. [Google Scholar] [CrossRef]
- Hussey, R.S.; Davis, E.L.; Baum, T.J. Secrets in secretions: Genes that control nematode parasitism of plants. Braz. J. Plant Physiol. 2002, 14, 183–194. [Google Scholar] [CrossRef]
- Burgess, T.L.; Kelly, R.B. Constitutive and Regulated Secretion of Proteins. Ann. Rev. Cell Biol. 1987, 3, 243–293. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, B.; De Meutter, J.; Tytgat, T.; Van Montagu, M.; Coomans, A.; Gheysen, G. Secretions of plant-parasitic nematodes: A molecular update. Gene 2004, 332, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.L.; Allen, R.; Maier, T.; Davis, E.L.; Baum, T.J.; Hussey, R.S. The parasitome of the phytonematode Heterodera glycines. Mol. Plant Microbe Interact 2003, 16, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Bellafiore, S.; Shen, Z.X.; Rosso, M.N.; Abad, P.; Shih, P.; Briggs, S.P. Direct Identification of the Meloidogyne incognita Secretome Reveals Proteins with Host Cell Reprogramming Potential. PLoS Pathog. 2008, 4, e1000192. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Plattner, S.; Radakovic, Z.; Wieczorek, K.; Elashry, A.; Grundler, F.M.; Ammelburg, M.; Siddique, S.; Bohlmann, H. An Arabidopsis ATPase gene involved in nematode-induced syncytium development and abiotic stress responses. Plant J. 2013, 74, 852–866. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Hussey, R.S.; Baum, T.J. Getting to the roots of parasitism by nematodes. Trends Parasitol. 2004, 20, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Mitchum, M.G. Nematodes. Sophisticated parasites of legumes. Plant Physiol. 2005, 137, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Mitchum, M.G.; Wang, X.H.; Davis, E.L. Diverse and conserved roles of CLE peptides. Curr. Opin. Plant Biol. 2008, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.N.; Skriver, K. Ligand mimicry? Plant-parasitic nematode polypeptide with similarity to CLAVATA3. Trends Plant Sci. 2003, 8, 55–57. [Google Scholar] [CrossRef]
- Fisher, K.; Turner, S. PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr. Biol. 2007, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fiers, M. CLE peptide signaling during plant development. Protoplasma 2009, 240, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Replogle, A.; Wang, J.Y.; Bleckmann, A.; Hussey, R.S.; Baum, T.J.; Sawa, S.; Davis, E.L.; Wang, X.H.; Simon, R.; Mitchum, M.G. Nematode CLE signaling in Arabidopsis requires CLAVATA2 and CORYNE. Plant J. 2011, 65, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Replogle, A.; Wang, J.; Paolillo, V.; Smeda, J.; Kinoshita, A.; Durbak, A.; Tax, F.E.; Wang, X.; Sawa, S.; Mitchum, M.G. Synergistic interaction of CLAVATA1, CLAVATA2, and RECEPTOR-LIKE PROTEIN KINASE 2 in cyst nematode parasitism of Arabidopsis. Mol. Plant Microbe Interact 2013, 26, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lang, P.; Chronis, D.; Zhang, S.; De Jong, W.S.; Mitchum, M.G.; Wang, X. In planta processing and glycosylation of a nematode CLAVATA3/ENDOSPERM SURROUNDING REGION-like effector and its interaction with a host CLAVATA2-like receptor to promote parasitism. Plant Physiol. 2015, 167, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Ni, J.; Denver, R.; Wang, X.H.; Clark, S.E. Mechanisms of Molecular Mimicry of Plant CLE Peptide Ligands by the Parasitic Nematode Globodera rostochiensis. Plant Physiol. 2011, 157, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyohara, S.; Sawa, S. CLE signaling systems during plant development and nematode infection. Plant Cell Physiol. 2012, 53, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J.; Gardner, M.; Fukuda, H.; Kondo, Y.; Etchells, J.P.; Wang, X.; Mitchum, M.G. Identification of cyst nematode B-type CLE peptides and modulation of the vascular stem cell pathway for feeding cell formation. PLoS Pathog. 2017, 13, e1006142. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Howe, P.J.; Maier, T.R.; Hussey, R.S.; Mitchum, M.G.; Davis, E.L.; Baum, T.J. Arabidopsis Spermidine Synthase Is Targeted by an Effector Protein of the Cyst Nematode Heterodera schachtii. Plant Physiol. 2010, 152, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Jaouannet, M.; Perfus-Barbeoch, L.; Deleury, E.; Magliano, M.; Engler, G.; Vieira, P.; Danchin, E.G.; Da Rocha, M.; Coquillard, P.; Abad, P.; et al. A root-knot nematode-secreted protein is injected into giant cells and targeted to the nuclei. New Phytol. 2012, 194, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhuo, K.; Wu, P.; Cui, R.; Zhang, L.H.; Liao, J. A novel effector protein, MJ-NULG1a, targeted to giant cell nuclei plays a role in Meloidogyne javanica parasitism. Mol. Plant Microbe Interact 2013, 26, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, S.; Ledger, T.N.; Laffaire, J.B.; Piotte, C.; Abad, P.; Rosso, M.N. Direct identification of stylet secreted proteins from root-knot nematodes by a proteomic approach. Mol. Biochem. Parasitol. 2002, 121, 205–211. [Google Scholar] [CrossRef]
- Jaubert, S.; Milac, A.L.; Petrescu, A.J.; de Almeida-Engler, J.; Abad, P.; Rosso, M.N. In planta secretion of a calreticulin by migratory and sedentary stages of root-knot nematode. Mol. Plant Microbe Interact 2005, 18, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Jaouannet, M.; Magliano, M.; Arguel, M.J.; Gourgues, M.; Evangelisti, E.; Abad, P.; Rosso, M.N. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol. Plant Microbe Interact 2013, 26, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Zhuo, K.; Luo, M.; Sun, L.H.; Liao, J.L. Molecular cloning and characterization of a calreticulin cDNA from the pinewood nematode Bursaphelenchus xylophilus. Exp. Parasitol. 2011, 128, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Juvale, P.S.; Piya, S.; Maier, T.R.; Rambani, A.; Rice, J.H.; Mitchum, M.G.; Davis, E.L.; Hussey, R.S.; Baum, T.J. The Cyst Nematode Effector Protein 10A07 Targets and Recruits Host Posttranslational Machinery to Mediate Its Nuclear Trafficking and to Promote Parasitism in Arabidopsis. Plant Cell 2015, 27, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hamamouch, N.; Li, C.Y.; Hewezi, T.; Hussey, R.S.; Baum, T.J.; Mitchum, M.G.; Davis, E.L. A nematode effector protein similar to annexins in host plants. J. Exp. Bot. 2010, 61, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, S.; Liu, Q.; Niu, J.; Liu, P.; Zhao, J.; Jian, H. An ANNEXIN-Like Protein from the Cereal Cyst Nematode Heterodera avenae Suppresses Plant Defense. PLoS ONE 2015, 10, e0122256. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liu, P.; Liu, Q.; Chen, C.; Guo, Q.; Yin, J.; Yang, G.; Jian, H. Msp40 effector of root-knot nematode manipulates plant immunity to facilitate parasitism. Sci. Rep. 2016, 6, 19443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, P.; Matthews, B.F. Regulatory interplay between soybean root and soybean cyst nematode during a resistant and susceptible reaction. BMC Plant BIol. 2014, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Postma, W.; Tytgat, T.; Prins, P.; Qin, L.; Overmars, H.; Vossen, J.; Spiridon, L.N.; Petrescu, A.J.; Goverse, A.; et al. A Secreted SPRY Domain-Containing Protein (SPRYSEC) from the Plant-Parasitic Nematode Globodera rostochiensis Interacts with a CC-NB-LRR Protein from a Susceptible Tomato. Mol. Plant Microbe Interact 2009, 22, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Postma, W.J.; Slootweg, E.J.; Rehman, S.; Finkers-Tomczak, A.; Tytgat, T.O.; van Gelderen, K.; Lozano-Torres, J.L.; Roosien, J.; Pomp, R.; van Schaik, C.; et al. The effector SPRYSEC-19 of Globodera rostochiensis suppresses CC-NB-LRR-mediated disease resistance in plants. Plant Physiol. 2012, 160, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Castagnone-Sereno, P.; Semblat, J.P.; Castagnone, C. Modular architecture and evolution of the map-1 gene family in the root-knot nematode Meloidogyne incognita. Mol. Genet Genom. 2009, 282, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.N.; Bekal, S.; Domier, L.L.; Niblack, T.L.; Noel, G.R.; Smyth, C.A. Selection of Heterodera glycines chorismate mutase-1 alleles on nematode-resistant soybean. Mol. Plant Microbe Interact 2005, 18, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Bekal, S.; Niblack, T.L.; Lambert, K.N. A chorismate mutase from the soybean cyst nematode Heterodera glycines shows polymorphisms that correlate with virulence. Mol. Plant Microbe Interact 2003, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Gleason, C.A.; Liu, Q.L.; Williamson, V.M. Silencing a candidate nematode effector gene corresponding to the tomato resistance gene Mi-1 leads to acquisition of virulence. Mol. Plant Microbe Interact 2008, 21, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Torres, J.L.; Wilbers, R.H.P.; Gawronski, P.; Boshoven, J.C.; Finkers-Tomczak, A.; Cordewener, J.H.G.; America, A.H.P.; Overmars, H.A.; Van’t Klooster, J.W.; Baranowski, L.; et al. Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc. Natl. Acad. Sci. USA 2012, 109, 10119–10124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, M.A.; Koropacka, K.; Grenier, E.; Jaubert, M.J.; Blanchard, A.; Goverse, A.; Smant, G.; Moffett, P. The Cyst Nematode SPRYSEC Protein RBP-1 Elicits Gpa2-and RanGAP2-Dependent Plant Cell Death. PLoS Pathog. 2009, 5, e1000564. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Esquibet, M.; Fouville, D.; Grenier, E. Ranbpm homologue genes characterised in the cyst nematodes Globodera pallida and Globodera ‘mexicana’. Physiol. Mol. Plant Pathol. 2005, 67, 15–22. [Google Scholar] [CrossRef]
- Semblat, J.P.; Rosso, M.N.; Hussey, R.S.; Abad, P.; Castagnone-Sereno, P. Molecular cloning of a cDNA encoding an amphid-secreted putative avirulence protein from the root-knot nematode Meloidogyne incognita. Mol. Plant Microbe Interact 2001, 14, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, J.; Grenier, E.; Esquibet, M.; Hamel, L.P.; Moffett, P.; Manzanares-Dauleux, M.J.; Kerlan, M.C. Evolution and variability of Solanum RanGAP2, a cofactor in the incompatible interaction between the resistance protein GPA2 and the Globodera pallida effector Gp-RBP-1. BMC Evol. Biol. 2013, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Shields, J.; Allen, R.; Hussey, R.S. Molecular cloning and characterisation of a venom allergen AG5-like cDNA from Meloidogyne incognita. Int. J. Parasitol. 2000, 30, 77–81. [Google Scholar] [CrossRef]
- Wang, X.H.; Replogle, A.; Davis, E.L.; Mitchum, M.G. The tobacco Cel7 gene promoter is auxin-responsive and locally induced in nematode feeding sites of heterologous plants. Mol. Plant Pathol. 2007, 8, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Koh, Y.H.; Moon, Y.S.; Lee, S.H. Molecular properties of a venom allergen-like protein suggest a parasitic function in the pinewood nematode Bursaphelenchus xylophilus. Int. J. Parasitol. 2012, 42, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Torres, J.L.; Wilbers, R.H.P.; Warmerdam, S.; Finkers-Tomczak, A.; Diaz-Granados, A.; van Schaik, C.C.; Helder, J.; Bakker, J.; Goverse, A.; Schots, A.; et al. Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors. PLoS Pathog. 2014, 10, e1004569. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Magne, M.; Chen, S.; Cote, O.; Stare, B.G.; Obradovic, N.; Jamshaid, L.; Wang, X.; Belair, G.; Moffett, P. Analysis of putative apoplastic effectors from the nematode, Globodera rostochiensis, and identification of an expansin-like protein that can induce and suppress host defenses. PLoS ONE 2015, 10, e0115042. [Google Scholar] [CrossRef] [PubMed]
- Iberkleid, I.; Vieira, P.; de Almeida Engler, J.; Firester, K.; Spiegel, Y.; Horowitz, S.B. Fatty acid-and retinol-binding protein, Mj-FAR-1 induces tomato host susceptibility to root-knot nematodes. PLoS ONE 2013, 8, e64586. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Thorpe, P.; Jones, J.T. Suppression of Plant Defences by Plant-Parasitic Nematodes. 2015, 73, 325–337. [Google Scholar]
- Liu, S.; Kandoth, P.K.; Warren, S.D.; Yeckel, G.; Heinz, R.; Alden, J.; Yang, C.; Jamai, A.; El-Mellouki, T.; Juvale, P.S.; et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 2012, 492, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.F.; Beard, H.; MacDonald, M.H.; Kabir, S.; Youssef, R.M.; Hosseini, P.; Brewer, E. Engineered resistance and hypersusceptibility through functional metabolic studies of 100 genes in soybean to its major pathogen, the soybean cyst nematode. Planta 2013, 237, 1337–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wen, Z.; Li, W.; Zhang, Y.; Zhang, L.; Dai, H.; Wang, D.; Xu, R. Genome-wide association study for soybean cyst nematode resistance in Chinese elite soybean cultivars. Mol. Breed. 2017, 37, 60. [Google Scholar] [CrossRef]
- Grunewald, W.; Cannoot, B.; Friml, J.; Gheysen, G. Parasitic Nematodes Modulate PIN-Mediated Auxin Transport to Facilitate Infection. PLoS Pathog. 2009, 5, e1000266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald, W.; van Noorden, G.; van Isterdael, G.; Beeckman, T.; Gheysen, G.; Mathesius, U. Manipulation of Auxin Transport in Plant Roots during Rhizobium Symbiosis and Nematode Parasitism. Plant Cell 2009, 21, 2553–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehara, T.; Sugiyama, S.; Matsuura, H.; Arie, T.; Masuta, C. Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol. 2010, 51, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, E.; Alba, E.; Montes, M.J.; Delibes, A.; Lopez-Brana, I. Analysis of ascorbate peroxidase genes expressed in resistant and susceptible wheat lines infected by the cereal cyst nematode, Heterodera avenae. Plant Cell Rep. 2010, 29, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Chronis, D.; Kenning, C.; Peret, B.; Hewezi, T.; Davis, E.L.; Baum, T.J.; Hussey, R.; Bennett, M.; Mitchum, M.G. The Novel Cyst Nematode Effector Protein 19C07 Interacts with the Arabidopsis Auxin Influx Transporter LAX3 to Control Feeding Site Development. Plant Physiol. 2011, 155, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Doyle, E.A.; Lambert, K.N. Meloidogyne javanicaChorismate Mutase 1 Alters Plant Cell Development. Mol. Plant Microbe Interact 2003, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Piya, S.; Richard, G.; Rice, H.J. Spatial and temporal expression patterns of auxin response transcription factors in the syncytium induced by the beet cyst nematode Heterodera schachtii in Arabidopsis. Mol. Plant Pathol. 2014, 15, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Beneventi, M.A.; da Silva, O.B., Jr.; de Sa, M.E.; Firmino, A.A.; de Amorim, R.M.; Albuquerque, E.V.; da Silva, M.C.; da Silva, J.P.; Campos Mde, A.; Lopes, M.J.; et al. Transcription profile of soybean-root-knot nematode interaction reveals a key role of phythormones in the resistance reaction. BMC Genom. 2013, 14, 322. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Radakovic, Z.S.; De La Torre, C.M.; Chronis, D.; Novák, O.; Ramireddy, E.; Holbein, J.; Matera, C.; Hütten, M.; Gutbrod, P.; et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc. Natl. Acad. Sci. USA 2015, 112, 12669–12674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, C.; Dutta, T.K.; Banakar, P.; Rao, U. Comparing the defence-related gene expression changes upon root-knot nematode attack in susceptible versus resistant cultivars of rice. Sci. Rep. 2016, 6, 22846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Kjemtrup-Lovelace, S.; Li, C.; Luo, Y.; Chen, L.P.; Song, B.-H. Comparative RNA-Seq Analysis Uncovers a Complex Regulatory Network for Soybean Cyst Nematode Resistance in Wild Soybean (Glycine soja). Sci. Rep. 2017, 7, 9699. [Google Scholar] [CrossRef] [PubMed]
- Gillet, F.-X.; Bournaud, C.; de Souza Júnior, J.D.A.; Fatima Grossi-de-Sa, M. Plant-parasitic nematodes: Towards understanding molecular players in stress responses. Ann. Bot. 2017, 119, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic Acquired Resistance. Ann. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Shah, J.; Klessig, D.F. Salicylic acid and disease resistance in plants. Trends Plant Sci. 1997, 2, 266–274. [Google Scholar] [CrossRef]
- Draper, J. Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 1997, 2, 162–165. [Google Scholar] [CrossRef]
- Overmyer, K.; Brosche, M.; Kangasjarvi, J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Pathogen-induced, NADPH oxidase–derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Hanse, B.; Mitreva, M.; Vanholme, B.; Bakker, J.; Smant, G. Mining the secretome of the root-knot nematode Meloidogyne chitwoodi for candidate parasitism genes. Mol. Plant Pathol. 2008, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.G.; Jesuino, R.S.; Dantas Ada, S.; Brigido Mde, M.; Felipe, M.S. Oxidative stress response in Paracoccidioides brasiliensis. Genet. Mol. Res. 2005, 4, 409–429. [Google Scholar] [PubMed]
- Robertson, L.; Robertson, W.M.; Sobczak, M.; Helder, J.; Tetaud, E.; Ariyanayagam, M.R.; Ferguson, M.A.J.; Fairlamb, A.; Jones, J.T. Cloning, expression and functional characterisation of a peroxiredoxin from the potato cyst nematode Globedera rostochiensis. Mol. Biochem. Parasit. 2000, 111, 41–49. [Google Scholar] [CrossRef]
- Sunkar, R. MicroRNAs in Plant Development and Stress Responses; Springer: Heidelberg/Berlin, Germany, 2012; Volume 15. [Google Scholar]
- Tian, B.; Wang, S.; Todd, T.C.; Johnson, C.D.; Tang, G.; Trick, H.N. Genome-wide identification of soybean microRNA responsive to soybean cyst nematodes infection by deep sequencing. BMC Genom. 2017, 18, 572. [Google Scholar] [CrossRef] [PubMed]
- Hewezi, T.; Maier, T.R.; Nettleton, D.; Baum, T.J. The Arabidopsis MicroRNA396-GRF1/GRF3 Regulatory Module Acts as a Developmental Regulator in the Reprogramming of Root Cells during Cyst Nematode Infection. Plant Physiol. 2012, 159, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, S.; Liu, D.; Duan, Y.; Dong, W. Identification of soybean microRNAs involved in soybean cyst nematode infection by deep sequencing. PLoS ONE 2012, 7, e39650. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Shukla, N.; Joshi, G.; VijayaKumar, C.; Jagannath, A.; Agarwal, M.; Goel, S.; Kumar, A. Genome-wide identification and characterization of miRNAome from tomato (Solanum lycopersicum) roots and root-knot nematode (Meloidogyne incognita) during susceptible interaction. PLoS ONE 2017, 12, e0175178. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, G.; Vanholme, B. RNAi from plants to nematodes. Trends Biotechnol. 2007, 25, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Paiva, G.; Proença, D.N.; Francisco, R.; Verissimo, P.; Santos, S.S.; Fonseca, L.; Abrantes, I.M.O.; Morais, P.V. Nematicidal Bacteria Associated to Pinewood Nematode Produce Extracellular Proteases. PLoS ONE 2013, 8, e79705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klink, V.P.; Matthews, B.F. Emerging approaches to broaden resistance of soybean to soybean cyst nematode as supported by gene expression studies. Plant Physiol. 2009, 151, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.R.; Hewezi, T.; Peng, J.; Baum, T.J. Isolation of whole esophageal gland cells from plant-parasitic nematodes for transcriptome analyses and effector identification. Mol. Plant Microbe Interact 2013, 26, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Andolfo, G.; Iovieno, P.; Frusciante, L.; Ercolano, M.R. Genome-Editing Technologies for Enhancing Plant Disease Resistance. Front. Plant. Sci. 2016, 7, 1813. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.A.; Anjam, M.S.; Nawaz, M.A.; Lam, H.-M.; Chung, G. Signal Transduction in Plant–Nematode Interactions. Int. J. Mol. Sci. 2018, 19, 1648. https://doi.org/10.3390/ijms19061648
Ali MA, Anjam MS, Nawaz MA, Lam H-M, Chung G. Signal Transduction in Plant–Nematode Interactions. International Journal of Molecular Sciences. 2018; 19(6):1648. https://doi.org/10.3390/ijms19061648
Chicago/Turabian StyleAli, Muhammad Amjad, Muhammad Shahzad Anjam, Muhammad Amjad Nawaz, Hon-Ming Lam, and Gyuhwa Chung. 2018. "Signal Transduction in Plant–Nematode Interactions" International Journal of Molecular Sciences 19, no. 6: 1648. https://doi.org/10.3390/ijms19061648






