SCF/c-KIT Signaling Increased Mucin2 Production by Maintaining Atoh1 Expression in Mucinous Colorectal Adenocarcinoma
Abstract
:1. Introduction
2. Results
2.1. Mucinous Colorectal Adenocarcinoma (MCA) Was Much More Efficiently Induced in WT Mice than in Wadsm/m Mice
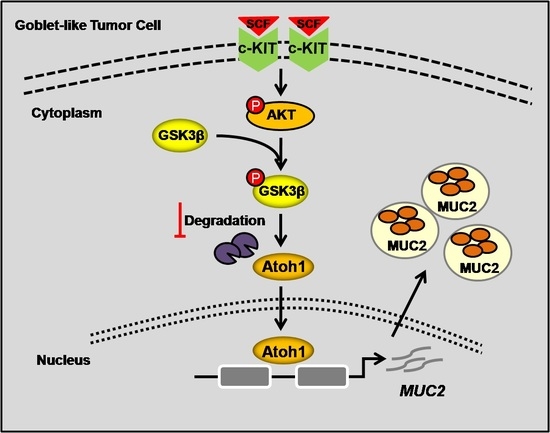
2.2. Highly-Activated c-KIT Signaling Promoted Mucin 2 (MUC2) Expression via Increasing Atonal Homologue 1 (Atoh1)
2.3. Stem Cell Factor (SCF)/c-KIT Signaling Increased Atoh1 via Protein Kinase B (AKT) and c-Jun N-Terminal Kinase (JNK) Pathway In Vitro
2.4. c-KIT and Atoh1 Were Increased in Human MCA Tissues
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. MCA Murine Model
4.3. Patients
4.4. Cell Culture
4.5. Cryosectioning and Immunofluorescent Staining
4.6. Paraffin-Sectioning and Immunohistochemical Staining
4.7. Alcian Blue/Periodic Acid-Schiff (PAS) Staining
4.8. Western Blot
4.9. Overexpression of c-KIT
4.10. RNA Exraction and Real-Time PCR
4.11. ONCOMINE Database Analysis
4.12. Statistics
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| MCA | Mucinous colorectal adenocarcinoma |
| CRC | Colorectal cancer |
| AOM | Azoxymethane |
| DSS | Dextran sodium sulfate |
| RTK | Receptor tyrosine kinase |
| MUC2 | Mucin 2 |
| rhSCF | Recombinant human stem cell factor |
| Atoh1 | Atonal homologue 1 |
| AKT | Protein Kinase B |
| GSK3β | Glycogen synthase kinase 3β |
| CDX2 | Caudal-related homeobox transcription factor |
| Spdef | SAM pointed domain containing ETS transcription factor |
References
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; IARC: Lyon, France, 2014; pp. 93–95. [Google Scholar]
- Hamilton, S.R.; Rubio, C.A.; Vogelstein, B.; Sobin, L.H.; Kudo, S.; Fogt, F.; Riboli, E.; Winawer, S.J.; Nakamura, S.; Goldgar, D.E.; et al. Carcinoma of the colon and rectum. In WHO Classification of Tumours of the Digestive System; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC: Lyon, France, 2010; pp. 134–146. [Google Scholar]
- Nitsche, U.; Zimmermann, A.; Späth, C.; Müller, T.; Maak, M.; Schuster, T.; Slotta-Huspenina, J.; Käser, S.A.; Michalski, C.W.; Janssen, K.P.; et al. Mucinous and Signet-Ring Cell Colorectal Cancers Differ from Classical Adenocarcinomas in Tumor Biology and Prognosis. Ann. Surg. 2013, 258, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Bananzadeh, A.M.; Salek, R.; Zare-Bandamiri, M.; Kermani, A.T.; Mohammadianpanah, M. Prognostic significance of mucinous histologic subtype on oncologic outcomes in patients with colorectal cancer. Ann. Coloproctol. 2017, 33, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Debunne, H.; Ceelen, W. Mucinous differentiation in colorectal cancer: Molecular, histological and clinical aspects. Acta Chir. Belg. 2013, 113, 385–390. [Google Scholar] [PubMed]
- Tanaka, H.; Deng, G.; Matsuzaki, K.; Kakar, S.; Kim, G.E.; Miura, S.; Sleisenger, M.H.; Kim, Y.S. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int. J. Cancer 2006, 118, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Yamauchi, M.; Nishihara, R.; Kim, S.A.; Mima, K.; Sukawa, Y.; Li, T.; Yasunari, M.; Zhang, X.; Wu, K.; et al. Prognostic significance and molecular features of signet-ring cell and mucinous components in colorectal carcinoma. Ann. Surg. Oncol. 2015, 22, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Kuo, W.H.; Chen, F.L.; Lee, M.Y.; Ruan, A.; Tyan, Y.S.; Hsu, J.D.; Chiang, H.; Han, C.P. Identification of the coexisting HER2 gene amplification and novel mutations in the HER2 protein-overexpressed mucinous epithelial ovarian cancer. Ann. Surg. Oncol. 2011, 18, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J. Mucinous epithelial ovarian carcinoma. Ann. Oncol. 2016, 27 (Suppl. 1), i53–i57. [Google Scholar] [CrossRef] [PubMed]
- Hurbin, A.; Wislez, M.; Busser, B.; Antoine, M.; Tenaud, C.; Rabbe, N.; Dufort, S.; de Fraipont, F.; Moro-Sibilot, D.; Cadranel, J.; et al. Insulin-like growth factor-1 receptor inhibition overcomes gefitinib resistance in mucinous lung adenocarcinoma. J. Pathol. 2011, 225, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Brustmann, H. Immunohistochemical detection of human telomerase reverse transcriptase (hTERT) and c-KIT in serous ovarian carcinoma: A clinicopathologic study. Gynecol. Oncol. 2005, 98, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Ohike, N.; Sato, M.; Hisayuki, T.; Imataka, H.; Sato, S.; Wada, Y.; Saito, K.; Takahashi, M.; Tajiri, T.; Kunimura, T.; et al. Immunohistochemical analysis of nestin and c-KIT and their significance in pancreatic tumors. Pathol. Int. 2007, 57, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Bellone, G.; Smirne, C.; Carbone, A.; Buffolino, A.; Scirelli, T.; Prati, A.; Solerio, D.; Pirisi, M.; Valente, G.; Nano, M.; et al. KIT/stem cell factor expression in premalignant and malignant lesions of the colon mucosa in relationship to disease progression and outcomes. Int. J. Oncol. 2006, 29, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.C.; Karl, T.A.; Kalisky, T.; Gupta, S.K.; O’Brien, C.A.; Longacre, T.A.; van de Rijn, M.; Quake, S.R.; Clarke, M.F.; Rothenberg, M.E. KIT signaling promotes growth of colon xenograft tumors in mice and is up-regulated in a subset of human colon cancers. Gastroenterology 2015, 149, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yang, S.; Shen, P.; Sun, H.; Xiao, J.; Wang, Y.; Wu, B.; Ji, F.; Yan, J.; Xue, H.; et al. c-KIT signaling promotes proliferation and invasion of colorectal mucinous adenocarcinoma in a murine model. Oncotarget 2015, 6, 27037–27048. [Google Scholar] [CrossRef] [PubMed]
- Lungulescu, C.V.; Răileanu, S.; Afrem, G.; Ungureanu, B.S.; Florescu, D.N.; Gheonea, I.A.; Șovăilă, S.; Crăițoiu, Ș. Histochemical and immunohistochemical study of mucinous rectal carcinoma. J. Med. Life 2017, 10, 139–143. [Google Scholar] [PubMed]
- Tozawa, E.; Ajioka, Y.; Watanabe, H.; Nishikura, K.; Mukai, G.; Suda, T.; Kanoh, T.; Hatakeyama, K. Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: Is mucinous carcinoma a distinct histological entity? Pathol. Res. Pract. 2007, 203, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Bresalier, R.S. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- VanDussen, K.L.; Samuelson, L.C. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev. Biol. 2010, 346, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Bermingham, N.A.; Finegold, M.J.; Zoghbi, H.Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2001, 294, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Park, E.T.; Oh, H.K.; Gum, J.R., Jr.; Crawley, S.C.; Kakar, S.; Engel, J.; Leow, C.C.; Gao, W.Q.; Kim, Y.S. HATH1 expression in mucinous cancers of the colorectum and related lesions. Clin. Cancer Res. 2006, 12, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Nakamura, T.; Okamoto, R.; Kanai, T.; Watanabe, M. Reciprocal targeting of Hath1 and β-catenin by Wnt glycogen synthase kinase 3β in human colon cancer. Gastroenterology 2007, 132, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Colorectal Cancers: An Update on Their Molecular Pathology. Cancers 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Limburg, P.J.; Baron, J.A.; Burnett-Hartman, A.N.; Weisenberger, D.J.; Laird, P.W.; Sinicrope, F.A.; Rosty, C.; Buchanan, D.D.; Potter, J.D.; et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 2015, 148, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilly, A.K.; Lee, Y.J.; Zeh, H.J.; Guo, Z.S.; Bartlett, D.L.; Choudry, H.A. Targeting hypoxia-mediated mucin 2 production as a therapeutic strategy for mucinous tumors. Transl. Res. 2016, 169, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Tsuchiya, K.; Kano, Y.; Horita, N.; Hibiya, S.; Hayashi, R.; Kitagaki, K.; Negi, M.; Itoh, E.; Akashi, T.; et al. Atonal homolog 1 protein stabilized by tumor necrosis factor α induces high malignant potential in colon cancer cell line. Cancer Sci. 2015, 106, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Tsuchiya, K.; Zheng, X.; Horita, N.; Fukushima, K.; Hibiya, S.; Yamauchi, Y.; Nishimura, T.; Hinohara, K.; Gotoh, N.; et al. The acquisition of malignant potential in colon cancer is regulated by the stabilization of Atonal homolog 1 protein. Biochem. Biophys. Res. Commun. 2013, 432, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.M.; Chia, L.A.; Kosinski, C.M.; Kuo, C.J. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol. Life Sci. 2011, 68, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, H.; Sakamoto, H.; Hayakawa, H.; Arao, Y.; Satoh, K.; Nokubi, M.; Sugano, K. The intestine-specific homeobox gene Cdx2 induces expression of the basic helix–loop–helix transcription factor Math1. Differentiation 2006, 74, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Gregorieff, A.; Stange, D.E.; Kujala, P.; Begthel, H.; van den Born, M.; Korving, J.; Peters, P.J.; Clevers, H. The ETS-domain transcription factor Spdef promotes maturation of goblet and paneth cell in the intestinal epithelium. Gastroenterology 2009, 137, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.H.; Chung, E.; Li, Z.; Wan, Y.W.; Mahe, M.M.; Chen, M.S.; Noah, T.K.; Bell, K.N.; Yalamanchili, H.K.; Klisch, T.J.; et al. Transcriptional Regulation by ATOH1 and its Target SPDEF in the Intestine. Cell Mol. Gastroenterol. Hepatol. 2016, 3, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Mandić, A.D.; Bennek, E.; Verdier, J.; Zhang, K.; Roubrocks, S.; Davis, R.J.; Denecke, B.; Gassler, N.; Streetz, K.; Kel, A.; et al. c-Jun N-terminal kinase 2 promotes enterocyte survival and goblet cell differentiation in the inflamed intestine. Mucosal Immunol. 2017, 10, 1211–1223. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, P.; Yang, S.; Sun, H.; Li, G.; Wu, B.; Ji, F.; Sun, T.; Zhou, D. SCF/c-KIT Signaling Increased Mucin2 Production by Maintaining Atoh1 Expression in Mucinous Colorectal Adenocarcinoma. Int. J. Mol. Sci. 2018, 19, 1541. https://doi.org/10.3390/ijms19051541
Shen P, Yang S, Sun H, Li G, Wu B, Ji F, Sun T, Zhou D. SCF/c-KIT Signaling Increased Mucin2 Production by Maintaining Atoh1 Expression in Mucinous Colorectal Adenocarcinoma. International Journal of Molecular Sciences. 2018; 19(5):1541. https://doi.org/10.3390/ijms19051541
Chicago/Turabian StyleShen, Ping, Shu Yang, Haimei Sun, Guilan Li, Bo Wu, Fengqing Ji, Tingyi Sun, and Deshan Zhou. 2018. "SCF/c-KIT Signaling Increased Mucin2 Production by Maintaining Atoh1 Expression in Mucinous Colorectal Adenocarcinoma" International Journal of Molecular Sciences 19, no. 5: 1541. https://doi.org/10.3390/ijms19051541