Protective Effects of 2-Amino-5,6-dihydro-4H-1,3-thiazine and Its Derivative against Radiation-Induced Hematopoietic and Intestinal Injury in Mice
Abstract
:1. Introduction
2. Results
2.1. 2-ADT and 2-AADT Improved the Survival of Mice Exposed to a Lethal Dose of Irradiation
2.2. 2-ADT and 2-AADT Promoted Recovery of Hematopoietic System
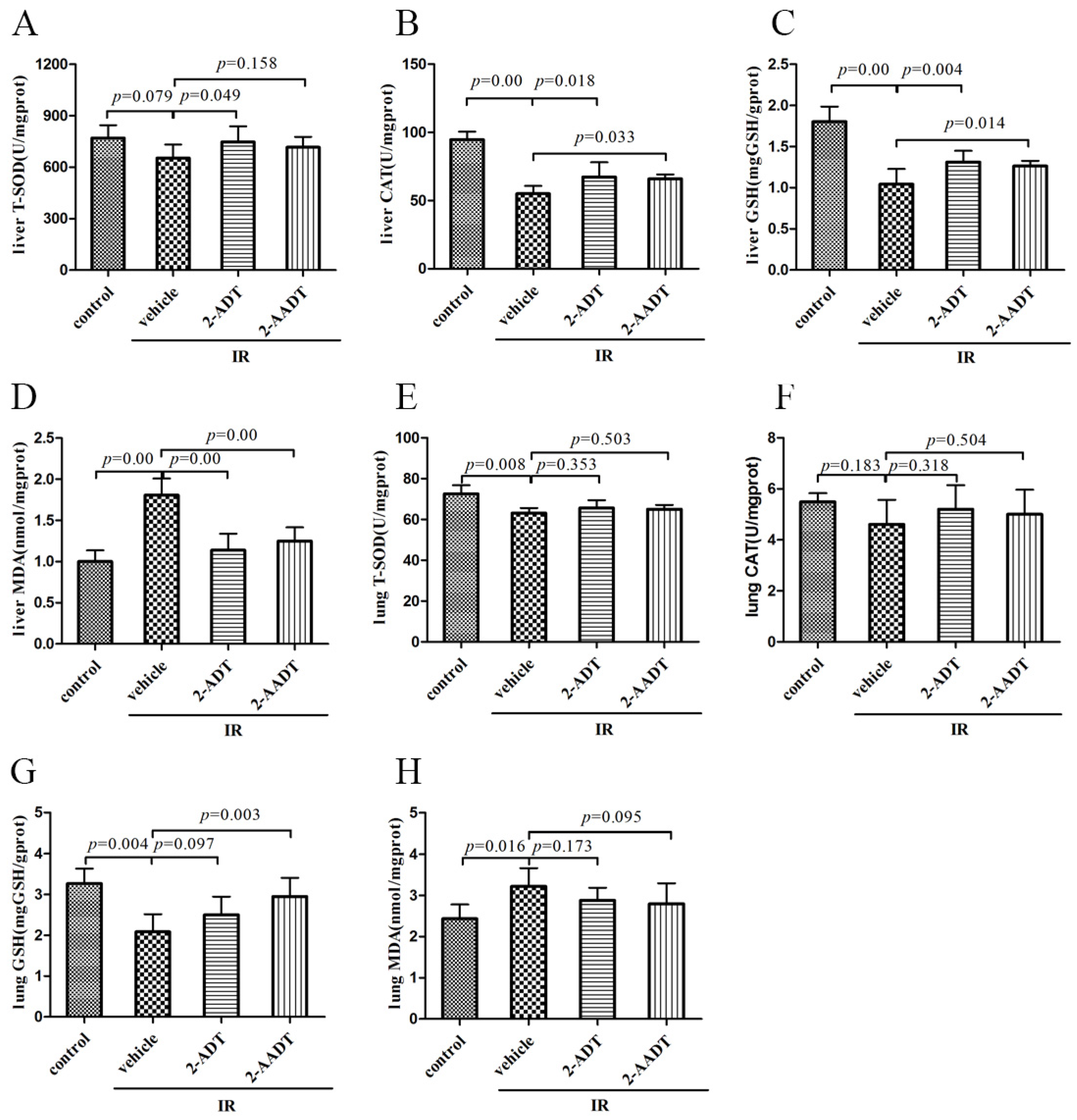
2.3. 2-ADT and 2-AADT Enhanced the Activity of Various Antioxidant Enzymes and Reduced Lipid Peroxidation
2.4. 2-ADT and 2-AADT Attenuated Radiation-Induced Elevation of NO in Plasma
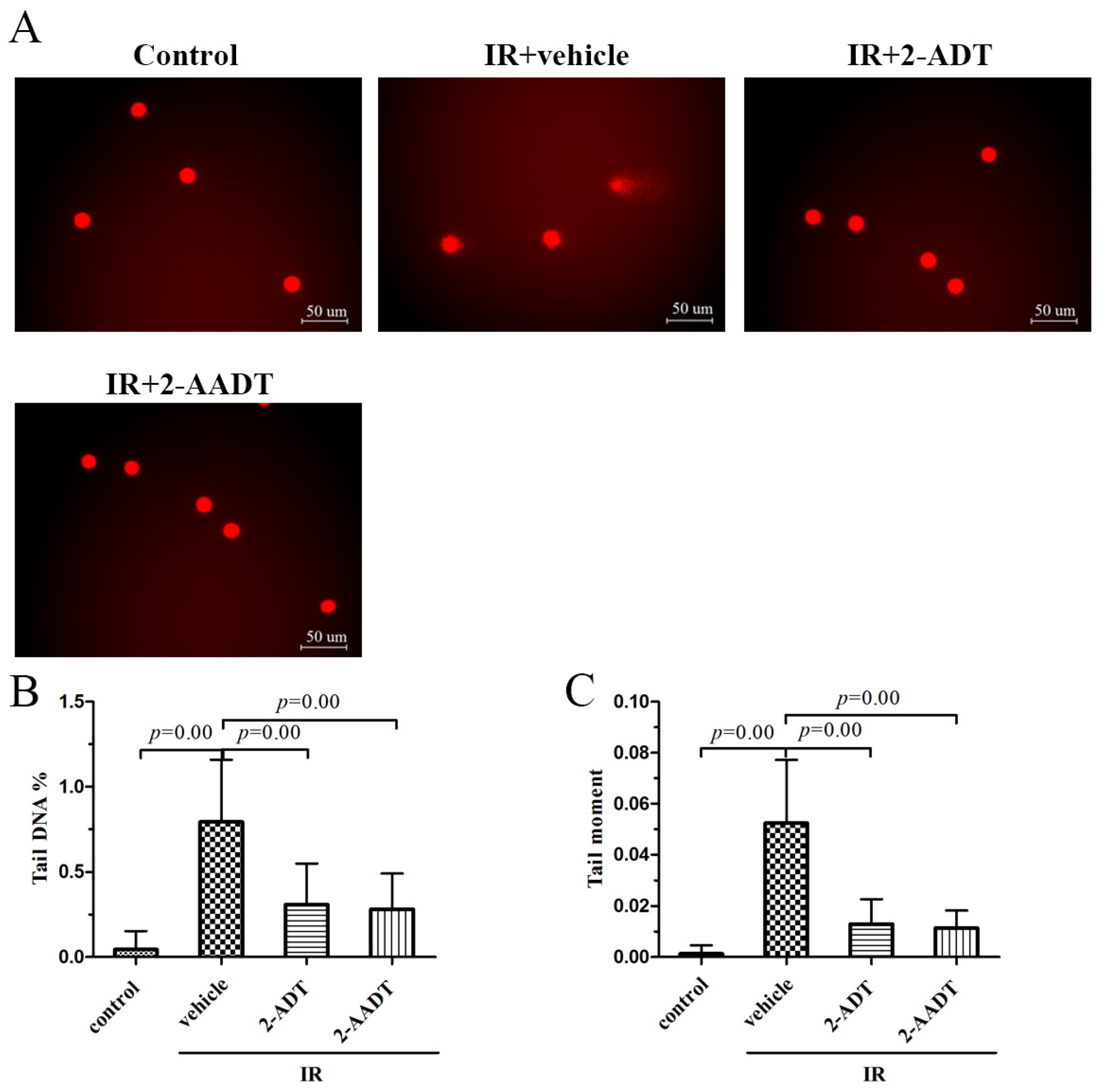
2.5. 2-ADT and 2-AADT Alleviated Radiation-Induced DNA Damage to Peripheral Blood Lymphocytes
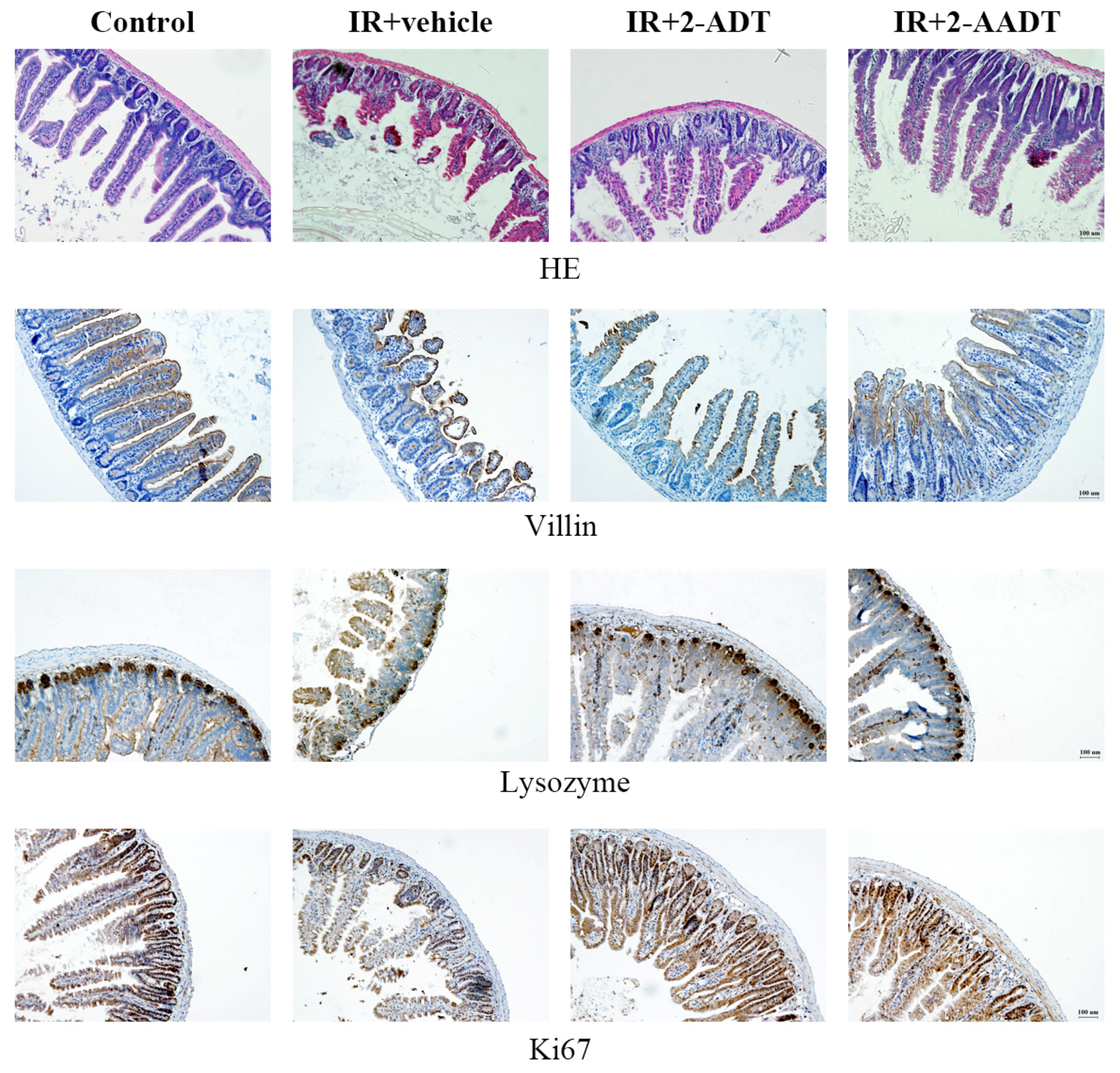
2.6. 2-ADT and 2-AADT Ameliorated Small Intestinal Injury in Mice after Abdominal Irradiation
2.7. 2-ADT and 2-AADT Promoted the Proliferation of Vill Enterocytes, Paneth Cells and Ki67 Transient Amplifying Cells in the Small Intestine of Mice after Abdominal Irradiation
2.8. 2-ADT and 2-AADT Attenuated Radiation-Induced Nitrotyrosine Expression in the Small Intestine after Abdominal Irradiation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Mice and Irradiation
4.3. Survival Study
4.4. Determination of the Effect of 2-ADT and 2-AADT on Radiation Induced Hematopoietic System Injury
4.4.1. Hematological Parameters
4.4.2. Colony Forming Assay
4.4.3. Bone Marrow Nucleated Cells and DNA Content
4.5. Biochemical Determination
4.6. Plasma NOx Detection
4.7. Comet Assay
4.8. Determination of the Effects of 2-ADT and 2-AADT on Intestinal Injury in Irradiated Mice
4.8.1. Histopathology
4.8.2. Immunohistochemistry
4.9. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pratheeshkumar, P.; Raphael, T.J.; Kuttan, G. Protective role of perillic acid against radiation-induced oxidative stress, cytokine profile, DNA damage, and intestinal toxicity in mice. J. Environ. Pathol. Toxicol. Oncol. 2010, 29, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Babicova, A.; Havlinova, Z.; Hroch, M.; Rezacova, M.; Pejchal, J.; Vavrova, J.; Chladek, J. In vivo study of radioprotective effect of NO-synthase inhibitors and acetyl-L-carnitine. Physiol. Res. 2013, 62, 701–710. [Google Scholar] [PubMed]
- Voevodskaya, N.V.; Vanin, A.F. Gamma-irradiation potentiates L-arginine-dependent nitric oxide formation in mice. Biochem. Biophys. Res. Commun. 1992, 186, 1423–1428. [Google Scholar] [CrossRef]
- Babicova, A.; Havlinova, Z.; Pejchal, J.; Tichy, A.; Rezacova, M.; Vavrova, J.; Chladek, J. Early changes in L-arginine-nitric oxide metabolic pathways in response to the whole-body gamma irradiation of rats. Int. J. Radiat. Biol. 2011, 87, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamekguzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Ricciardolo, F.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric Oxide in Health and Disease of the Respiratory System. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef] [PubMed]
- Giaid, A.; Lehnert, S.M.; Chehayeb, B.; Chehayeb, D.; Kaplan, I.; Shenouda, G. Inducible Nitric Oxide Synthase and Nitrotyrosine in Mice With Radiation-Induced Lung Damage. Am. J. Clin. Oncol. 2003, 26, e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogenand radiation-induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754. [Google Scholar] [CrossRef] [PubMed]
- Brach, M.A.; Hass, R.; Sherman, M.L.; Gunji, H.; Weichselbaum, R.; Kufe, D. Ionizing Radiation Induces Expression and Binding Activity of the Nuclear Factor KB. J. Clin. Investig. 1991, 88, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Brown, S.A.; Yu, T.; Chen, G.; Barve, S.; Kang, B.C.; Thompson, J.S. A high dose of ionizing radiation induces tissue-specific activation of nuclear factor-kappaB in vivo. Radiat. Res. 1999, 151, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [PubMed]
- Liebmann, J.; DeLuca, A.M.; Coffin, D.; Keefer, L.K.; Venzon, D.; Wink, D.A.; Mitchell, J.B. In vivo radiation protection by nitric oxide modulation. Cancer Res. 1994, 54, 3365–3368. [Google Scholar] [PubMed]
- Michurina, T.; Krasnov, P.; Balazs, A.; Nakaya, N.; Vasilieva, T.; Kuzin, B.; Khrushchov, N.; Mulligan, R.C.; Enikolopov, G. Nitric oxide is a regulator of hematopoietic stem cell activity. Mol. Ther. 2004, 10, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Filimonova, M.V.; Shevchenko, L.I.; Trofimova, T.P.; Makarchuk, V.M.; Shevchuk, A.S.; Lushnikova, G.A. On the mechanism of radioprotective effect of NO-synthase inhibitors. Radiat. Biol. Radioecol. 2014, 54, 500–506. [Google Scholar]
- Meer, C.V.D.; Bekkum, D.W.V. The Mechanism of Radiation Protection by Histamine and Other Biological Amines, International Journal of Radiation Biology, Informa Healthcare. Int. J. Radiat. Biol. 1959, 1, 5–23. [Google Scholar] [CrossRef]
- Prewitt, R.L.; Musacchia, X.J. Mechanisms of radio-protection by catecholamines in the hamster (Mesocricetus auratus). Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1975, 27, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Allalunis-Turner, M.J.; Walden, T.L.; Sawich, C. Induction of marrow hypoxia by radioprotective agents. Radiat. Res. 1989, 118, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Proskuryakov, S.Y.; Filimonova, M.V.; Verkhovskii, Y.G.; Konoplyannikov, A.G.; Mandrugin, A.A.; Fedoseev, V.M.; Skvortsov, V.G. Effect of NO synthase inhibitor 2-amino-5,6-dihydro-4H-1,3-thiazine on endotoxin-induced changes in hemodynamic parameters and respiration in rats. Bull. Exp. Biol. Med. 2004, 138, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Filimonova, M.V.; Trofimova, T.P.; Borisova, G.S.; Mandrugin, A.A. Antihypotensive activity of 2-acetylamino-5,6-dihydro-4H-1,3-thiazine for an endotoxic shock model in rats. Pharm. Chem. J. 2012, 46, 210–212. [Google Scholar] [CrossRef]
- Trofimova, T.P.; Zefirova, O.N.; Mandrugin, A.A.; Fedoseev, V.M.; Peregud, D.I.; Onufriev, M.V.; Gulyaeva, N.V.; Proskuryakov, S.Y. Synthesis and study of NOS-inhibiting activity of 2-N-acylamino-5,6-dihydro-4H-1,3-thiazine. Mosc. Univ. Chem. Bull. 2008, 10, 274–277. [Google Scholar] [CrossRef]
- Park, E.; Ahn, G.N.; Lee, N.H.; Kim, J.M.; Yun, J.S.; Hyun, J.W.; Jeon, Y.J.; Wie, M.B.; Lee, Y.J.; Park, J.W.; et al. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Lett. 2008, 582, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondrial-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, A.Y.; Mohammadi, M.T.; Sarami, F.M.; Raouf, S.J. Captopril and Valsartan May Improve Cognitive Function Through Potentiation of the Brain Antioxidant Defense System and Attenuation of Oxidative/Nitrosative Damage in STZ-Induced Dementia in Rat. Adv. Pharm. Bull. 2016, 6, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Desai, D.; Kumar, V. Effect of MgCl2 stress on germination, plant growth, chlorophyll content, proline content and lipid peroxidation in sorghum cultivars. J. Stress Phys. Biochem. 2012, 8, 169–178. [Google Scholar]
- Sowmithra, K.; Shetty, N.J.; Jha, S.K.; Chaubey, R.C. Evaluation of genotoxicity of the acute gamma radiation on earthworm Eisenia fetida using single cell gel electrophoresis technique (Comet Assay). Genet. Toxicol. Environ. 2015, 794, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Z.; Gong, W.; Zhang, H.W.; Wang, Y.; Du, L.Q.; Xu, C.; Wang, Q.; Zhao, H.; Liu, Q.; Fan, F.Y. Human mesenchymal stem cells promote survival and prevent intestinal damage in a mouse model of radiation injury. RSC Adv. 2016, 6, 65105–65111. [Google Scholar] [CrossRef]
- Whittle, B.J. Nitric oxide in physiology and pathology. Mol. Asp. Med. 1995, 27, 727–737. [Google Scholar] [CrossRef]
- Mandrugin, A.A.; Konstantinova, M.M.; Nekrasova, I.V.; Tarasenko, A.G. Study of the mechanism of the radioprotective activity of 2-amino-5,6-dihydro-4H-1,3-thiazine, 4-methyl- and 2-amino-2-thiazoline hydrobromides. Radiobiologiia 1977, 17, 590–592. [Google Scholar] [PubMed]
- Téoule, R. Radiation-induced DNA Damage and Its Repair. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1987, 51, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Billiar, T.R. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv. Pharmacol. 1995, 34, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Crow, J.P.; Beckman, J.S. Reactions between Nitric Oxide, Superoxide, and Peroxynitrite: Footprints of Peroxynitrite in Vivo. Adv. Pharmacol. 1995, 34, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, N.K.; Guner, Y.S.; Hunter, C.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. the role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Leitão, R.F.; Brito, G.A.; Oriá, R.B.; Braga-Neto, M.B.; Bellaguarda, E.A.; Silva, J.V.; Gomes, A.S.; Lima-Júnior, R.C.; Siqueira, F.J.; Freire, R.S.; et al. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol. 2011, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, F.; Zhang, Y.; Shen, X. Protective effects of rosmarinic acid against radiation-induced damage to the hematopoietic system in mice. J. Radiat. Res. 2016, 57, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Jing, Y.; Song, S.; Yang, J.; Wang, J.Y.; Xue, X.; Min, Y.; Park, G.; Shen, X.; Sun, Y.M.; et al. Catalytic topological insulator Bi2Se3 nanoparticles for in vivo protection against ionizing radiation. Nanomedicine 2017, 13, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Gutzki, F.M.; Stichtenoth, D.O. Circulating and excretory nitrite and nitrate as indicators of nitric oxide synthesis in humans: Methods of analysis. Eur. J. Clin. Pharmacol. 2006, 62, 51–59. [Google Scholar] [CrossRef]
- Yang, W.; Sun, Z.; Yang, B.; Wang, Q. Nrf2-Knockout Protects from Intestinal Injuries in C57BL/6J Mice Following Abdominal Irradiation with gamma Rays. Int. J. Mol. Sci. 2017, 18, 1656. [Google Scholar] [CrossRef] [PubMed]
- Acipayam, C.; Bayram, I.; Daglioglu, K.; Doran, F.; Yilmaz, S.; Sezgin, G.; Totan Ateş, B.; Ozkan, A.; Tanyeli, A. the protective effect of hesperidin on methotrexate-induced intestinal epithelial damage in rats: An experimental study. Med. Princ. Pract. 2014, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.; Wang, F.S.; Lin, I.H.; Yang, K.D. Aminoguanidine alleviates radiation-induced small-bowel damage through its antioxidant effect. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 237–244. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Kong, S.; Yang, F.; Xu, W. Protective Effects of 2-Amino-5,6-dihydro-4H-1,3-thiazine and Its Derivative against Radiation-Induced Hematopoietic and Intestinal Injury in Mice. Int. J. Mol. Sci. 2018, 19, 1530. https://doi.org/10.3390/ijms19051530
Li Y, Kong S, Yang F, Xu W. Protective Effects of 2-Amino-5,6-dihydro-4H-1,3-thiazine and Its Derivative against Radiation-Induced Hematopoietic and Intestinal Injury in Mice. International Journal of Molecular Sciences. 2018; 19(5):1530. https://doi.org/10.3390/ijms19051530
Chicago/Turabian StyleLi, Yuanyuan, Shaofan Kong, Fujun Yang, and Wenqing Xu. 2018. "Protective Effects of 2-Amino-5,6-dihydro-4H-1,3-thiazine and Its Derivative against Radiation-Induced Hematopoietic and Intestinal Injury in Mice" International Journal of Molecular Sciences 19, no. 5: 1530. https://doi.org/10.3390/ijms19051530



