Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells
Abstract
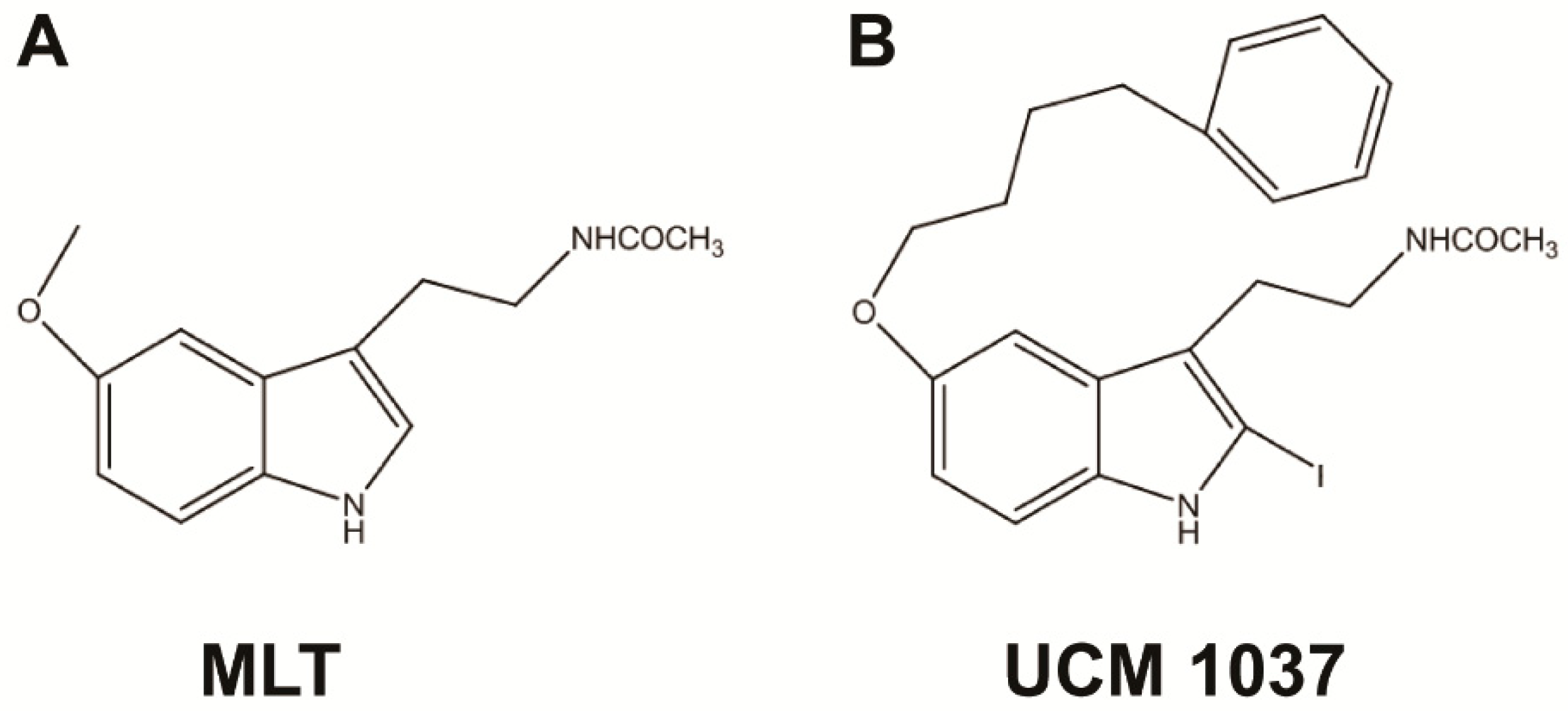
:1. Introduction
2. Results
2.1. Expression of Melatonin Receptors in Prostate Cancer Cells
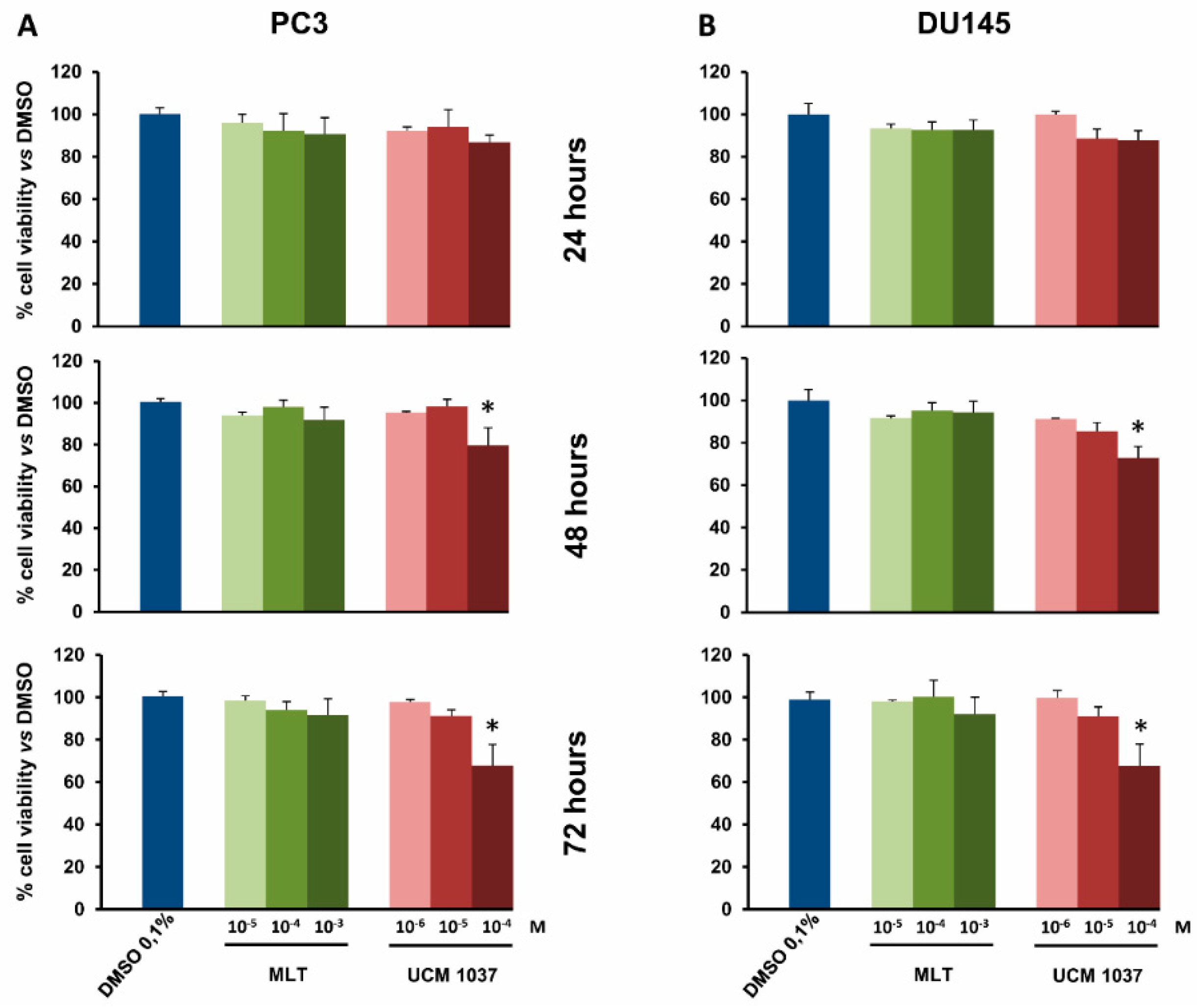
2.2. Melatonin Analogue Effects on Prostate Cancer Cell Growth
2.3. Effects of Melatonin Analogue on Cell Cycle Distribution
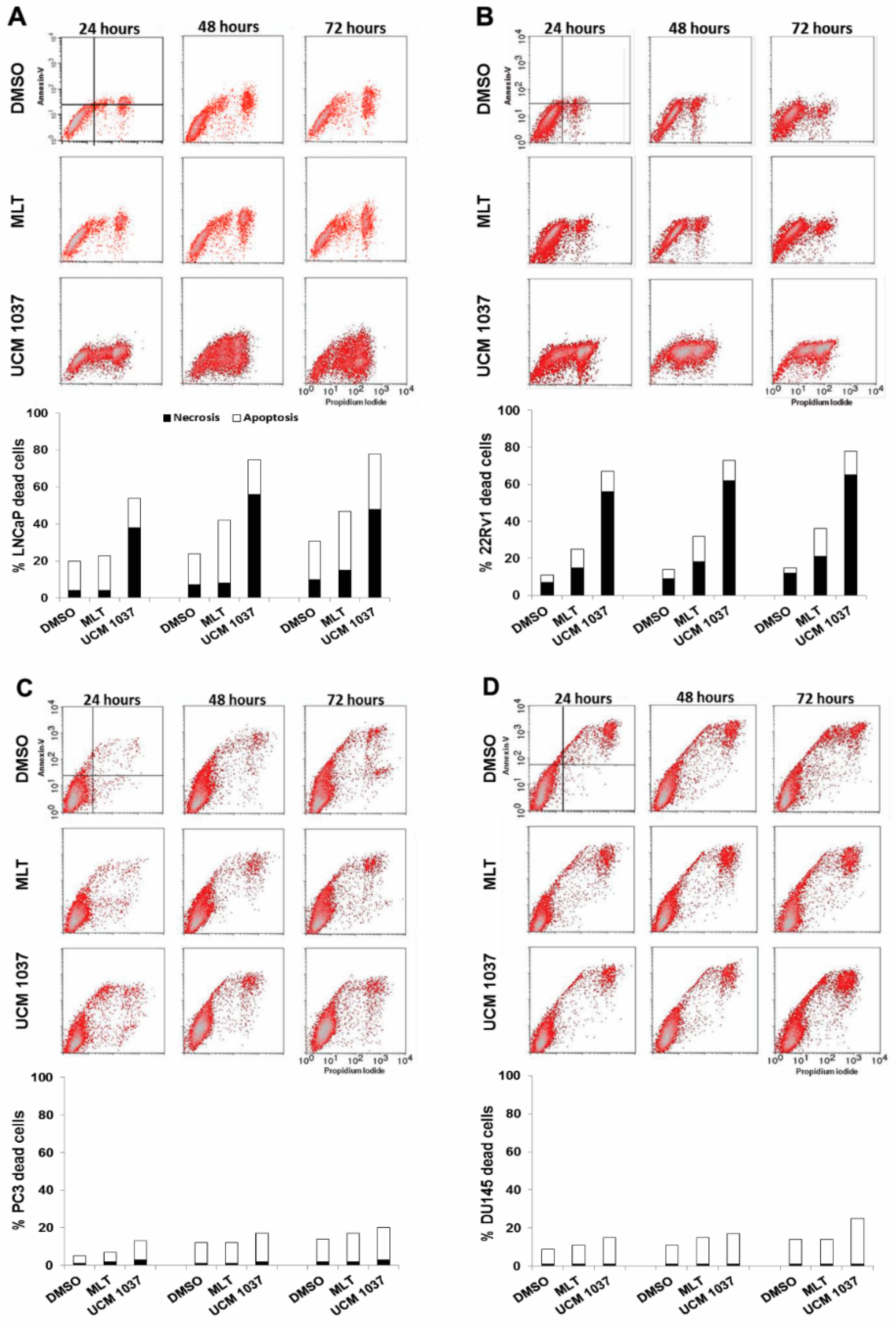
2.4. Cytotoxicity of Melatonin Analogue on Prostate Cancer Cells
2.5. Melatonin Analogue Effects on AR and Akt Levels
3. Discussion
4. Materials and Methods
4.1. Cells Culture and Reagents
4.2. Cellular Proliferation and Viability Assay
4.3. Cell Cycle Analysis by Flow Cytometry
4.4. Detection of Apoptosis and Necrosis by Annexin-V/Propidium Iodide Assay
4.5. Western Blot Analysis
4.6. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, L.G.; Markt, S.C.; Rider, J.R.; Haneuse, S.; Fall, K.; Schernhammer, E.S.; Tamimi, R.M.; Flynn-Evans, E.; Batista, J.L.; Launer, L.; et al. Urinary Melatonin Levels, Sleep Disruption, and Risk of Prostate Cancer in Elderly Men. Eur. Urol. 2015, 67, 191–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, S.Y.; Huang, S.P.; Bao, B.Y.; Wu, M.T. Urinary melatonin-sulfate/cortisol ratio and the presence of prostate cancer: A case-control study. Sci. Rep. 2016, 8, 29606. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Audinot, V.; Ferry, G.; Delagrange, P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol. Sci. 2005, 26, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J. 2010, 24, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.C.; Siu, S.W.; Fong, S.W.; Shiu, S.Y. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: Association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate 2001, 46, 52–61. [Google Scholar] [CrossRef]
- Tam, C.W.; Mo, C.W.; Yao, K.M.; Shiu, S.Y. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: Implications for prostate cancer chemoprevention. J. Pineal Res. 2007, 42, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Cheng, Q.; Guardiola-Lemaître, B.; Schuster-Klein, C.; Dong, C.; Lai, L.; Hill, S.M. In vitro and in vivo antitumor activity of melatonin receptor agonists. J. Pineal Res. 2010, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Zlotos, D.P.; Jockers, R.; Cecon, E.; Rivara, S.; Witt-Enderby, P.A. MT1 and MT2 Melatonin Receptors: Ligands, Models, Oligomers, and Therapeutic Potential. J. Med. Chem. 2014, 57, 3161–3185. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Lucini, V.; Dugnani, S.; Calastretti, A.; Spadoni, G.; Bedini, A.; Rivara, S.; Mor, M.; Canti, G.; Scaglione, F.; et al. Antiproliferative and pro-apoptotic activity of melatonin analogues on melanoma and breast cancer cells. Oncotarget 2017, 8, 68338–68353. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, M.G. Investigating the relationship between the cell cycle and apoptosis using flow cytometry. J. Immunol. Methods 2002, 265, 73–80. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Hevia, D.; Mayo, J.C.; Gonzalez-Menendez, P.; Coppo, L.; Lu, J.; Holmgren, A.; Sainz, R.M. Thioredoxin 1 modulates apoptosis induced by bioactive compounds in prostate cancer cells. Redox Biol. 2017, 12, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, J.; Liu, M.; Yeung, S.; Chang, A.; Chow, M.S.; Pon, D.; Huang, Y. Nutraceuticals for prostate cancer chemoprevention: From molecular mechanisms to clinical application. Expert Opin. Investig. Drugs 2013, 22, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.W.; Lau, K.W.; Tam, P.C.; Shiu, S.Y. Melatonin and prostate cancer cell proliferation: Interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate 2002, 52, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.C.; Tam, P.C.; Brown, G.M.; Pang, S.F.; Shiu, S.Y. Potential involvement of mt1 receptor and attenuated sex steroid-induced calcium influx in the direct anti-proliferative action of melatonin on androgen-responsive LNCaP human prostate cancer cells. J. Pineal Res. 2000, 29, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Gilad, E.; Laufer, M.; Matzkin, H.; Zisapel, N. Melatonin receptors in PC3 human prostate tumor cells. J. Pineal Res. 1999, 26, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Marelli, M.M.; Limonta, P.; Maggi, R.; Motta, M.; Moretti, R.M. Growth-inhibitory activity of melatonin on human androgen-independent DU145 prostate cancer cells. Prostate 2000, 45, 238–244. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Tan, D.X.; León, J.; Manchester, L.; Reiter, R.J. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate 2005, 63, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.; Negrín, G.; Estévez, F.; Loro, J.; Reiter, R.J.; Quintana, J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J. Pineal Res. 2010, 49, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Recio, J.; Sánchez-Barceló, E.J. Modulation of the length of the cell cycle time of MCF-7 human breast cancer cells by melatonin. Life Sci. 1996, 58, 811–816. [Google Scholar] [CrossRef]
- Rimler, A.; Culig, Z.; Lupowitz, Z.; Zisapel, N. Nuclear exclusion of the androgen receptor by melatonin. J. Steroid Biochem. Mol. Biol. 2002, 81, 77–84. [Google Scholar] [CrossRef]
- Sampson, S.R.; Lupowitz, Z.; Braiman, L.; Zisapel, N. Role of protein kinase Calpha in melatonin signal transduction. Mol. Cell. Endocrinol. 2006, 252, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.W.; Shiu, S. Functional interplay between melatonin receptor-mediated antiproliferative signaling and androgen receptor signaling in human prostate epithelial cells: Potential implications for therapeutic strategies against prostate cancer. J. Pineal Res. 2011, 51, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Hevia, D.; Quiros-Gonzalez, I.; Rodriguez-Garcia, A.; Gonzalez-Menendez, P.; Cepas, V.; Gonzalez-Pola, I.; Sainz, R.M. IGFBP3 and MAPK/ERK signaling mediates melatonin-induced antitumor activity in prostate cancer. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Zhao, L.; Xi, C.; Li, H.; Shen, G.; Liu, H.; Zhang, S.; Sun, L. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res. Cardiol. 2016, 111, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Z.; Liu, W.; Wang, J.; He, X.; Huang, H.; Zhang, J.; Yang, Z. Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget 2017, 8, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Sun, G.; Ma, T.; Zhong, F.; Wei, W. Melatonin overcomes apoptosis resistance in human hepatocellular carcinoma by targeting survivin and XIAP. J. Pineal Res. 2013, 55, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Chen, D.L.; Wang, D.S.; Chen, L.Z.; Mo, H.Y.; Sheng, H.; Bai, L.; Wu, Q.N.; Yu, H.E.; Xie, D.; et al. Melatonin enhances sensitivity to fluorouracil in oesophageal squamous cell carcinoma through inhibition of Erk and Akt pathway. Cell Death Dis. 2016, 7, e2432. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Lisi, E.; Bizzarri, M. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J. Pineal Res. 2011, 50, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, S.J.; Luo, J.H.; Zhang, H.; Wang, R.X.; Liu, H.; Li, L.; Zhang, Z.G.; Zhou, R.X. Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol. Rep. 2018, 39, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Ruoff, R.; Kahoud, N.; Franke, T.F.; Logan, S.K. Androgen receptor levels are upregulated by Akt in prostate cancer. Endocr. Relat. Cancer 2011, 18, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Calastretti, A.; Gatti, G.; Quaresmini, C.; Bevilacqua, A. Down-modulation of Bcl-2 sensitizes PTEN-mutated prostate cancer cells to starvation and taxanes. Prostate 2014, 74, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sramkoski, R.M.; Pretlow, T.G., 2nd; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calastretti, A.; Gatti, G.; Lucini, V.; Dugnani, S.; Canti, G.; Scaglione, F.; Bevilacqua, A. Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1505. https://doi.org/10.3390/ijms19051505
Calastretti A, Gatti G, Lucini V, Dugnani S, Canti G, Scaglione F, Bevilacqua A. Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells. International Journal of Molecular Sciences. 2018; 19(5):1505. https://doi.org/10.3390/ijms19051505
Chicago/Turabian StyleCalastretti, Angela, Giuliana Gatti, Valeria Lucini, Silvana Dugnani, Gianfranco Canti, Francesco Scaglione, and Annamaria Bevilacqua. 2018. "Melatonin Analogue Antiproliferative and Cytotoxic Effects on Human Prostate Cancer Cells" International Journal of Molecular Sciences 19, no. 5: 1505. https://doi.org/10.3390/ijms19051505




