The Initiation of Th2 Immunity Towards Food Allergens
Abstract
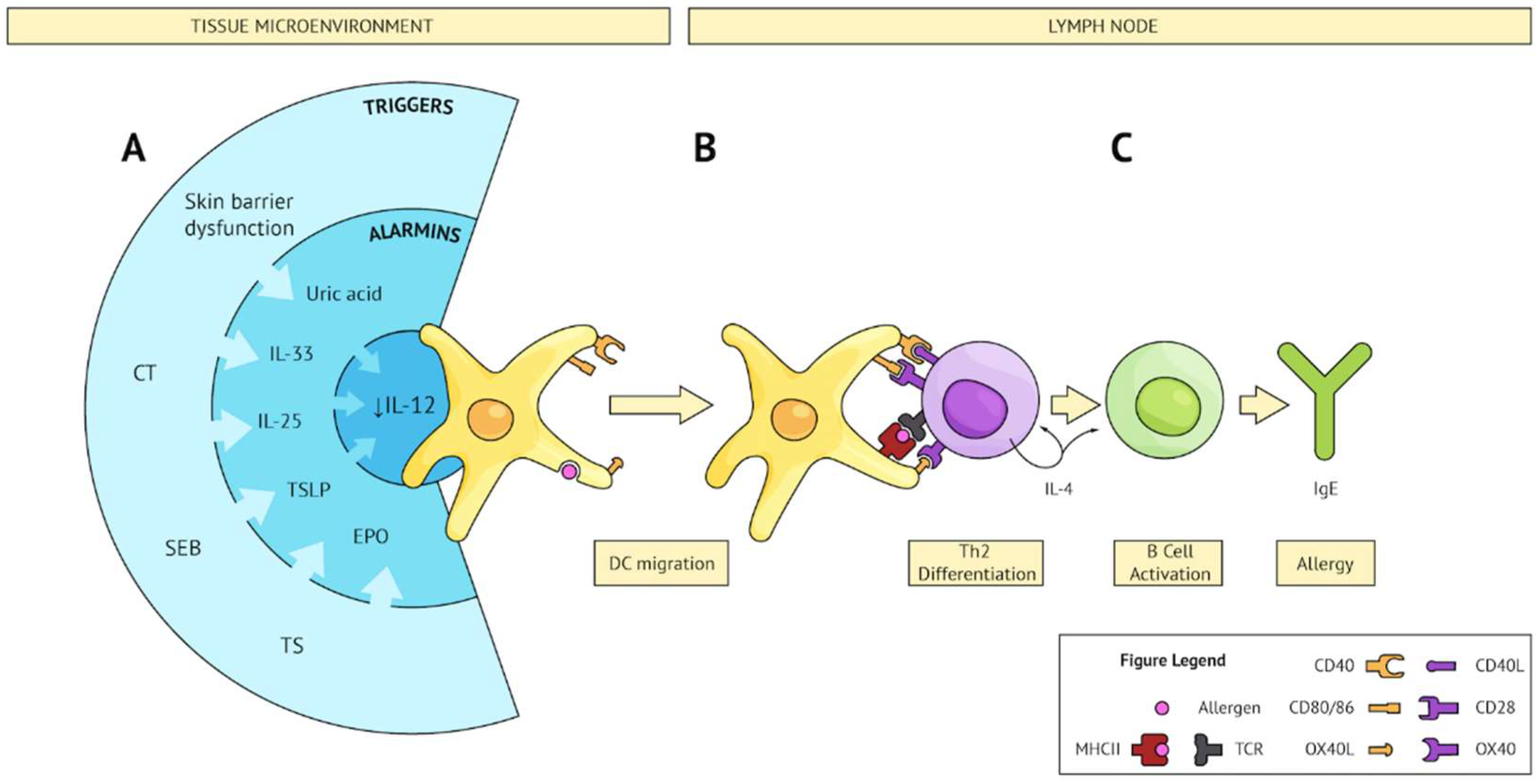
:1. Introduction
2. On the Acquisition of a Th2 Identity
3. On the Innocuousness of Food Allergens
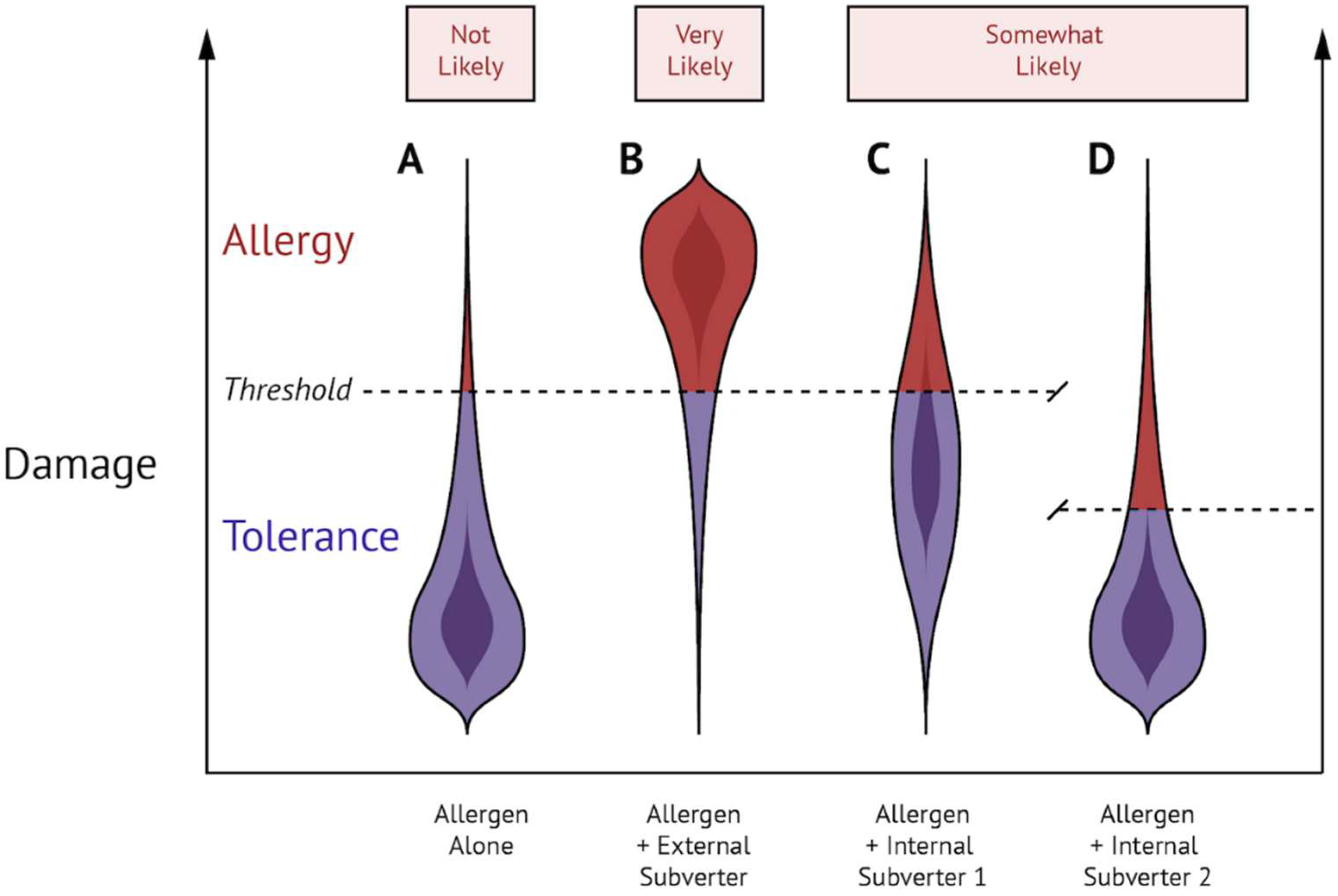
4. External Subverters of the Steady State
5. Converging Pathways Leading to Th2 Sensitization
6. Internal Subverters of the Steady State
7. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Paul, W.E.; Zhu, J. How Are Th2-Type Immune Responses Initiated and Amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Karp, C.L. Guilt by Intimate Association: What Makes an Allergen an Allergen? J. Allergy Clin. Immunol. 2010, 125, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; ONeill, L.A.J.; Xavier, R.J. Trained Immunity: A Program of Innate Immune Memory in Health and Disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Cher, D.; Coffman, R.L. Two Types of Mouse Helper T Cell Clone: Differences in B Cell Help and Lymphokine Synthesis. In Molecular Basis of Lymphokine Action; Humana Press: New York, NY, USA, 1987; pp. 149–159. [Google Scholar]
- Romagnani, S. Human Th1 and Th2 Subsets: Doubt No More. Immunol. Today 1991, 12, 256–257. [Google Scholar] [CrossRef]
- Romagnani, S. Immunologic Influences on Allergy and the Th1/Th2 Balance. J. Allergy Clin. Immunol. 2004, 113, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Le Gros, G. Generation of Interleukin 4 (IL-4)-Producing Cells in Vivo and in Vitro: IL-2 and IL-4 Are Required for in Vitro Generation of IL-4-Producing Cells. J. Exp. Med. 1990, 172, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; Weinberg, A.D.; English, M.; Huston, G. IL-4 Directs the Development of Th2-like Helper Effectors. J. Immunol. 1990, 145, 3796–3806. [Google Scholar] [PubMed]
- Liao, W.; Lin, J.-X.; Leonard, W.J. IL-2 Family Cytokines: New Insights into the Complex Roles of IL-2 as a Broad Regulator of T Helper Cell Differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. History of Interleukin-4. Cytokine 2015, 75, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Zhu, J.; Paul, W.E. Independent Roles for IL-2 and GATA-3 in Stimulating Naive CD4+ T Cells to Generate a Th2-Inducing Cytokine Environment. J. Exp. Med. 2005, 202, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Van Panhuys, N.; Tang, S.-C.; Prout, M.; Camberis, M.; Scarlett, D.; Roberts, J.; Hu-Li, J.; Paul, W.E.; Le Gros, G. In Vivo Studies Fail to Reveal a Role for IL-4 or STAT6 Signaling in Th2 Lymphocyte Differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 12423–12428. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.-Y.; Truitt, M.L.; Ho, I.-C. GATA-3 Deficiency Abrogates the Development and Maintenance of T Helper Type 2 Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Min, B.; Hu-Li, J.; Watson, C.J.; Grinberg, A.; Wang, Q.; Killeen, N.; Urban, J.F.; Guo, L.; Paul, W.E. Conditional Deletion of Gata3 Shows Its Essential Function in Th1-Th2 Responses. Nat. Immunol. 2004, 5, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Rajewsky, K.; Muller, W. Generation and Analysis of Interleukin-4 Deficient Mice. Science 1991, 254, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Noben-Trauth, N.; Shultz, L.D.; Brombacher, F.; Urban, J.F.; Gu, H.; Paul, W.E. An Interleukin 4 (IL-4)-Independent Pathway for CD4+ T Cell IL-4 Production Is Revealed in IL-4 Receptor-Deficient Mice. Proc. Natl. Acad. Sci. USA 1997, 94, 10838–10843. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tanaka, T.; Shi, W.; Matsumoto, M.; Minami, M.; Kashiwamura, S.; Nakanishi, K.; Yoshida, N.; Kishimoto, T.; Akira, S. Essential Role of Stat6 in IL-4 Signalling. Nature 1996, 380, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H.; Schindler, U.; Smiley, S.T.; Grusby, M.J. Stat6 Is Required for Mediating Responses to IL-4 and for the Development of Th2 Cells. Immunity 1996, 4, 313–319. [Google Scholar] [CrossRef]
- Shimoda, K.; van Deursent, J.; Sangster, M.Y.; Sarawar, S.R.; Carson, R.T.; Tripp, R.A.; Chu, C.; Quelle, F.W.; Nosaka, T.; Vignali, D.A.A.; et al. Lack of IL-4-Induced Th2 Response and IgE Class Switching in Mice with Disrupted State6 Gene. Nature 1996, 380, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; Chu, D.K.; Waserman, S.J.M. Initiation, Persistence and Exacerbation of Food Allergy. In Initiation, Persistence and Exacerbation of Food Allergy; CB, S.-W., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 121–144. [Google Scholar]
- Sun, J.; Arias, K.; Alvarez, D.; Fattouh, R.; Walker, T.; Goncharova, S.; Kim, B.; Waserman, S.; Reed, J.; Coyle, A.J.; et al. Impact of CD40 Ligand, B Cells, and Mast Cells in Peanut-Induced Anaphylactic Responses. J. Immunol. 2007, 179, 6696–6703. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Mohammed-Ali, Z.; Jiménez-Saiz, R.; Walker, T.D.; Goncharova, S.; Llop-Guevara, A.; Kong, J.; Gordon, M.E.; Barra, N.G.; Gillgrass, A.E.; et al. T Helper Cell IL-4 Drives Intestinal Th2 Priming to Oral Peanut Antigen, under the Control of OX40L and Independent of Innate-like Lymphocytes. Mucosal Immunol. 2014, 7, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. What Determines Th2 Differentiation, in Vitro and in Vivo? Immunol. Cell Biol. 2010, 88, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C. Structural Biology of Allergens. J. Allergy Clin. Immunol. 2000, 106, 228–238. [Google Scholar] [CrossRef] [PubMed]
- McClain, S.; Bowman, C.; Fernández-Rivas, M.; Ladics, G.S.; van Ree, R. Allergic Sensitization: Food- and Protein-Related Factors. Clin. Transl. Allergy 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, S.; Cantó, B.; Gómez, F.; Blanca, N.; Cuesta-Herranz, J.; Canto, G.; Blanca, M.; Rodríguez, R.; Villalba, M.; Palomares, O. Detailed Characterization of Act d 12 and Act d 13 from Kiwi Seeds: Implication in IgE Cross-Reactivity with Peanut and Tree Nuts. Allergy 2014, 69, 1481–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aas, K. What Makes an Allergen an Allergen. Allergy 1978, 33, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C.; Crameri, R. IgE-Binding Epitopes: A Reappraisal. Allergy 2011, 66, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens Are Distributed into Few Protein Families and Possess a Restricted Number of Biochemical Functions. J. Allergy Clin. Immunol. 2008, 121, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.A.; Griffiths-Jones, S.; Shewry, P.R.; Breiteneder, H.; Mills, E.N.C. Structural Relatedness of Plant Food Allergens with Specific Reference to Cross-Reactive Allergens: An in Silico Analysis. J. Allergy Clin. Immunol. 2005, 115, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.A.; Breiteneder, H.; Mills, E.N.C. Evolutionary Distance from Human Homologs Reflects Allergenicity of Animal Food Proteins. J. Allergy Clin. Immunol. 2007, 120, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of Processing Technologies on the Allergenicity of Food Products. Crit. Rev. Food Sci. Nutr. 2014, 55, 1902–1917. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J. Food Processing: Effects on Allergenicity. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Effects of Daily Food Processing on Allergenicity. Crit. Rev. Food Sci. Nutr. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food Processing and Allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Astwood, J.D.; Leach, J.N.; Fuchs, R.L. Stability of Food Allergens to Digestion in Vitro. Nat. Biotechnol. 1996, 14, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Satitsuksanoa, P.; Głobińska, A.; Jansen, K.; van de Veen, W.; Akdis, M. Modified Allergens for Immunotherapy. Curr. Allergy Asthma Rep. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Urisu, A.; Ando, H.; Morita, Y.; Wada, E.; Yasaki, T.; Yamada, K.; Komada, K.; Torii, S.; Goto, M.; Wakamatsu, T. Allergenic Activity of Heated and Ovomucoid-Depleted Egg White. J. Allergy Clin. Immunol. 1997, 100, 171–176. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Rupa, P.; Mine, Y. Immunomodulatory Effects of Heated Ovomucoid-Depleted Egg White in a BALB/c Mouse Model of Egg Allergy. J. Agric. Food Chem. 2011, 59, 13195–13202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husby, S.; Jensenius, J.C.; Svehag, S.-E. Passage of Undegraded Dietary Antigen into the Blood of Healthy Adults Further Characterization of the Kinetics of Uptake and the Size Distribution of the Antigen. Scand. J. Immunol. 1986, 24, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Jensenius, J.C.; Svehag, S.-E. Passage of Undegraded Dietary Antigen into the Blood of Healthy Adults. Scand. J. Immunol. 1985, 22, 83–92. [Google Scholar] [CrossRef] [PubMed]
- JanssenDuijghuijsen, L.M.; Wichers, H.J.; van Norren, K.; Keijer, J.; Baumert, J.L.; de Jong, G.A.H.; Witkamp, R.F.; Koppelman, S.J. Detection of Peanut Allergen in Human Blood after Consumption of Peanuts Is Skewed by Endogenous Immunoglobulins. J. Immunol. Methods 2017, 440, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Dirks, C.G.; Pedersen, M.H.; Platzer, M.H.; Bindslev-Jensen, C.; Skov, P.S.; Poulsen, L.K. Does Absorption across the Buccal Mucosa Explain Early Onset of Food-Induced Allergic Systemic Reactions? J. Allergy Clin. Immunol. 2005, 115, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J. Factors Associated with the Development of Peanut Allergy in Childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Ashley, S.E.; Tan, H.-T.T.; Vuillermin, P.; Dharmage, S.C.; Tang, M.L.K.; Koplin, J.; Gurrin, L.C.; Lowe, A.; Lodge, C.; Ponsonby, A.-L.; et al. The Skin Barrier Function Gene SPINK5 Is Associated with Challenge-Proven IgE-Mediated Food Allergy in Infants. Allergy 2017, 72, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Asai, Y.; Cordell, H.J.; Campbell, L.E.; Zhao, Y.; Liao, H.; Northstone, K.; Henderson, J.; Alizadehfar, R.; Ben-Shoshan, M.; et al. Loss-of-Function Variants in the Filaggrin Gene Are a Significant Risk Factor for Peanut Allergy. J. Allergy Clin. Immunol. 2011, 127, 661–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brough, H.A.; Liu, A.H.; Sicherer, S.; Makinson, K.; Douiri, A.; Brown, S.J.; Stephens, A.C.; Irwin McLean, W.H.; Turcanu, V.; Wood, R.A.; et al. Atopic Dermatitis Increases the Effect of Exposure to Peanut Antigen in Dust on Peanut Sensitization and Likely Peanut Allergy. J. Allergy Clin. Immunol. 2015, 135, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C.J. Innate Immune Recognition: Mechanisms and Pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Deifl, S.; Bohle, B. Factors Influencing the Allergenicity and Adjuvanticity of Allergens. Immunotherapy 2011, 3, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, B.; Shreffler, W.G. Innate Immunostimulatory Properties of Allergens and Their Relevance to Food Allergy. Semin. Immunopathol. 2012, 34, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, S.; Toda, M.; Vieths, S. What Makes an Allergen? Clin. Exp. Allergy 2015, 45, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Herre, J.; Gronlund, H.; Brooks, H.; Hopkins, L.; Waggoner, L.; Murton, B.; Gangloff, M.; Opaleye, O.; Chilvers, E.R.; Fitzgerald, K.; et al. Allergens as Immunomodulatory Proteins: The Cat Dander Protein Fel d 1 Enhances TLR Activation by Lipid Ligands. J. Immunol. 2013, 191, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat Amylase Trypsin Inhibitors Drive Intestinal Inflammation via Activation of Toll-like Receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo, G.; Quirce, S.; Diaz-Perales, A. Wheat Allergens Associated with Baker’s Asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 81–92. [Google Scholar] [PubMed]
- Jyonouchi, S.; Abraham, V.; Orange, J.S.; Spergel, J.M.; Gober, L.; Dudek, E.; Saltzman, R.; Nichols, K.E.; Cianferoni, A. Invariant Natural Killer T Cells from Children with versus without Food Allergy Exhibit Differential Responsiveness to Milk-Derived Sphingomyelin. J. Allergy Clin. Immunol. 2011, 128, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.E.H.; Louie, S.; Shi, H.N.; Nagler-Anderson, C. Toll-Like Receptor 4 Signaling by Intestinal Microbes Influences Susceptibility to Food Allergy. J. Immunol. 2004, 172, 6978–6987. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C.; Zheng, Y.; Domaradzki, M.; Li, X.-M.; Sampson, H.A. Role of TLR4 in Allergic Sensitization to Food Proteins in Mice. Allergy 2006, 61, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Chalcraft, K.; Mandur, T.S.; Jimenez-Saiz, R.; Walker, T.D.; Goncharova, S.; Gordon, M.E.; Naji, L.; Flader, K.; Larché, M.; et al. Comprehensive Metabolomics Identifies the Alarmin Uric Acid as a Critical Signal for the Induction of Peanut Allergy. Allergy 2015, 70, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; van der Goot, F.G. Did Cholera Toxin Finally Get Caught? Cell Host Microbe 2013, 13, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.; Hua, Y.-J.; Dahlgren, M.W.; Livingston, M.; Johansson-Lindbom, B.; Yrlid, U. Direct Interaction between Cholera Toxin and Dendritic Cells Is Required for Oral Adjuvant Activity. Eur. J. Immunol. 2013, 43, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Royer, P.-J.; Emara, M.; Yang, C.; Al-Ghouleh, A.; Tighe, P.; Jones, N.; Sewell, H.F.; Shakib, F.; Martinez-Pomares, L.; Ghaemmaghami, A.M. The Mannose Receptor Mediates the Uptake of Diverse Native Allergens by Dendritic Cells and Determines Allergen-Induced T Cell Polarization through Modulation of IDO Activity. J. Immunol. 2010, 185, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Shreffler, W.G.; Castro, R.R.; Kucuk, Z.Y.; Charlop-Powers, Z.; Grishina, G.; Yoo, S.; Burks, A.W.; Sampson, H.A. The Major Glycoprotein Allergen from Arachis hypogaea, Ara h 1, Is a Ligand of Dendritic Cell-Specific ICAM-Grabbing Nonintegrin and Acts as a Th2 Adjuvant In Vitro. J. Immunol. 2006, 177, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Tsai, T.-H.; Kawasaki, H.; Chen, C.-H.; Plunkett, B.; Lee, R.T.; Lee, Y.C.; Huang, S.-K. Antigen Coupled with Lewis-X Trisaccharides Elicits Potent Immune Responses in Mice. J. Allergy Clin. Immunol. 2007, 119, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger Receptors in Homeostasis and Immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, S.; Kautz, A.; Bohnert, V.; Knolle, P.A.; Kurts, C. Distinct Pathways of Antigen Uptake and Intracellular Routing in CD4 and CD8 T Cell Activation. Science 2007, 316, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Ilchmann, A.; Burgdorf, S.; Scheurer, S.; Waibler, Z.; Nagai, R.; Wellner, A.; Yamamoto, Y.; Yamamoto, H.; Henle, T.; Kurts, C.; et al. Glycation of a Food Allergen by the Maillard Reaction Enhances Its T-Cell Immunogenicity: Role of Macrophage Scavenger Receptor Class A Type I and II. J. Allergy Clin. Immunol. 2010, 125, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, M.; Wellner, A.; Gadermaier, G.; Ilchmann, A.; Briza, P.; Krause, M.; Nagai, R.; Burgdorf, S.; Scheurer, S.; Vieths, S.; et al. Ovalbumin Modified with Pyrraline, a Maillard Reaction Product, Shows Enhanced T-Cell Immunogenicity. J. Biol. Chem. 2014, 289, 7919–7928. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. Additional Effects of Dietary Advanced Glycation End Products. J. Allergy Clin. Immunol. 2017, 140, 319. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Masilamani, M.; Li, X.-M.; Sampson, H.A. The False Alarm Hypothesis: Food Allergy Is Associated with High Dietary Advanced Glycation End-Products and Proglycating Dietary Sugars That Mimic Alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Iijima, K.; Elias, M.K.; Seno, S.; Tojima, I.; Kobayashi, T.; Kephart, G.M.; Kurabayashi, M.; Kita, H. Airway Uric Acid Is a Sensor of Inhaled Protease Allergens and Initiates Type 2 Immune Responses in Respiratory Mucosa. J. Immunol. 2014, 192, 4032–4042. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Suzuki, M.; Hara, M.; Shimura, S.; Ochi, H.; Maruyama, N.; Matsuda, A.; Saito, H.; Nakae, S.; Suto, H.; et al. Subcutaneous Allergic Sensitization to Protease Allergen Is Dependent on Mast Cells but Not IL-33: Distinct Mechanisms between Subcutaneous and Intranasal Routes. J. Immunol. 2016, 196, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Stremnitzer, C.; Manzano-Szalai, K.; Willensdorfer, A.; Starkl, P.; Pieper, M.; König, P.; Mildner, M.; Tschachler, E.; Reichart, U.; Jensen-Jarolim, E. Papain Degrades Tight Junction Proteins of Human Keratinocytes In Vitro and Sensitizes C57BL/6 Mice via the Skin Independent of Its Enzymatic Activity or TLR4 Activation. J. Investig. Dermatol. 2015, 135, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The Immunology of Asthma. Nat. Immunol. 2014, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.I. Food Allergies in Developing and Emerging Economies: Need for Comprehensive Data on Prevalence Rates. Clin. Transl. Allergy 2012, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Soller, L.; Ben-Shoshan, M.; Harrington, D.W.; Knoll, M.; Fragapane, J.; Joseph, L.; St Pierre, Y.; La Vieille, S.; Wilson, K.; Elliott, S.J.; et al. Adjusting for Nonresponse Bias Corrects Overestimates of Food Allergy Prevalence. J. Allergy Clin. Immunol. Pract. 2015, 3, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Soller, L.; Ben-Shoshan, M.; Harrington, D.W.; Fragapane, J.; Joseph, L.; St. Pierre, Y.; Godefroy, S.B.; La Vieille, S.; Elliott, S.J.; Clarke, A.E. Overall Prevalence of Self-Reported Food Allergy in Canada. J. Allergy Clin. Immunol. 2012, 130, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; et al. The Prevalence of Food Allergy: A Meta-Analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: Epidemiology, Pathogenesis, Diagnosis, and Treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.K.; Mullins, R.J. Food Allergy: Is Prevalence Increasing? Intern. Med. J. 2017, 47, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Gajewska, B.U.; Alvarez, D.; Ritz, S.A.; Cundall, M.J.; Cates, E.C.; Coyle, A.J.; Gutierrez-Ramos, J.-C.; Inman, M.D.; Jordana, M.; et al. Inhalation of a Harmless Antigen (Ovalbumin) Elicits Immune Activation but Divergent Immunoglobulin and Cytokine Activities in Mice. Clin. Exp. Allergy 2002, 32, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Batty, J.E.; Turner, K.J. Inhibition of Specific IgE Responses in Mice by Pre-Exposure to Inhaled Antigen. Immunology 1981, 42, 409–417. [Google Scholar] [PubMed]
- Agrawal, R.; Woodfolk, J.A. Skin Barrier Defects in Atopic Dermatitis. Curr. Allergy Asthma Rep. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Hong, S.-W.; Han, D.; Yi, J.; Jung, J.; Yang, B.-G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary Antigens Limit Mucosal Immunity by Inducing Regulatory T Cells in the Small Intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Mowat, A.M. Oral Tolerance to Food Protein. Mucosal Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Worbs, T.; Bode, U.; Yan, S.; Hoffmann, M.W.; Hintzen, G.; Bernhardt, G.; Förster, R.; Pabst, O. Oral Tolerance Originates in the Intestinal Immune System and Relies on Antigen Carriage by Dendritic Cells. J. Exp. Med. 2006, 203, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Dunkin, D.; Berin, M.C.; Mayer, L. Allergic Sensitization Can Be Induced via Multiple Physiologic Routes in an Adjuvant-Dependent Manner. J. Allergy Clin. Immunol. 2011, 128, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Management of the Patient with Multiple Food Allergies. Curr. Allergy Asthma Rep. 2010, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Muratore, M.C.; Peltran, A.; Bonfante, G.; Silvestro, L.; Oggero, R.; Mussa, G.C. High Incidence of Adverse Reactions to Egg Challenge on First Known Exposure in Young Atopic Dermatitis Children: Predictive Value of Skin Prick Test and Radioallergosorbent Test to Egg Proteins. Clin. Exp. Allergy 2002, 32, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Wavrin, S.; Bernard, H.; Wal, J.-M.; Adel-Patient, K. Cutaneous or Respiratory Exposures to Peanut Allergens in Mice and Their Impacts on Subsequent Oral Exposure. Int. Arch. Allergy Immunol. 2014, 164, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Saiz, R.; Chu, D.K.; Mandur, T.S.; Walker, T.D.; Gordon, M.E.; Chaudhary, R.; Koenig, J.; Saliba, S.; Galipeau, H.J.; Utley, A.; et al. Lifelong Memory Responses Perpetuate Humoral Th2 Immunity and Anaphylaxis in Food Allergy. J. Allergy Clin. Immunol. 2017, 140, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Serebrisky, D.; Lee, S.Y.; Huang, C.K.; Bardina, L.; Schofield, B.H.; Stanley, J.S.; Burks, A.W.; Bannon, G.A.; Sampson, H.A. A Murine Model of Peanut Anaphylaxis: T- and B-Cell Responses to a Major Peanut Allergen Mimic Human Responses. J. Allergy Clin. Immunol. 2000, 106, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Snider, D.P.; Marshall, J.S.; Perdue, M.H.; Liang, H. Production of IgE Antibody and Allergic Sensitization of Intestinal and Peripheral Tissues after Oral Immunization with Protein Ag and Cholera Toxin. J. Immunol. 1994, 153, 647–657. [Google Scholar] [PubMed]
- Pablos-Tanarro, A.; Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Assessment of the Allergenic Potential of the Main Egg White Proteins in BALB/c Mice. J. Agric. Food Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Schofield, B.H.; Huang, C.K.; Kleiner, G.I.; Sampson, H.A. A Murine Model of IgE-Mediated Cow’s Milk Hypersensitivity. J. Allergy Clin. Immunol. 1999, 103 Pt 1, 206–214. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; Oettgen, H.C.; Chatila, T.A.; Geha, R.S.; Bryce, P.J. Food Allergy: Insights into Etiology, Prevention, and Treatment Provided by Murine Models. J. Allergy Clin. Immunol. 2014, 133, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Anjuère, F.; Luci, C.; Lebens, M.; Rousseau, D.; Hervouet, C.; Milon, G.; Holmgren, J.; Ardavin, C.; Czerkinsky, C. In Vivo Adjuvant-Induced Mobilization and Maturation of Gut Dendritic Cells after Oral Administration of Cholera Toxin. J. Immunol. 2004, 173, 5103–5111. [Google Scholar] [CrossRef] [PubMed]
- Shreedhar, V.K.; Kelsall, B.L.; Neutra, M.R. Cholera Toxin Induces Migration of Dendritic Cells from the Subepithelial Dome Region to T- and B-Cell Areas of Peyer’s Patches. Infect. Immun. 2003, 71, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Jimenez-Saiz, R.; Verschoor, C.P.; Walker, T.D.; Goncharova, S.; Llop-Guevara, A.; Shen, P.; Gordon, M.E.; Barra, N.G.; Bassett, J.D.; et al. Indigenous Enteric Eosinophils Control DCs to Initiate a Primary Th2 Immune Response in Vivo. J. Exp. Med. 2014, 211, 1657–1672. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Llop-Guevara, A.; Walker, T.D.; Flader, K.; Goncharova, S.; Boudreau, J.E.; Moore, C.L.; In, T.S.; Waserman, S.; Coyle, A.J.; et al. IL-33, but Not Thymic Stromal Lymphopoietin or IL-25, Is Central to Mite and Peanut Allergic Sensitization. J. Allergy Clin. Immunol. 2013, 131, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; He, S.-H.; Zheng, P.-Y.; Zhang, T.-Y.; Wang, B.-Q.; Yang, P.-C. Staphylococcal Enterotoxin B Increases TIM4 Expression in Human Dendritic Cells That Drives Naïve CD4 T Cells to Differentiate into Th2 Cells. Mol. Immunol. 2007, 44, 3580–3587. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus Aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Xing, Z.; Berin, C.M.; Soderholm, J.D.; Feng, B.; Wu, L.; Yeh, C. TIM-4 Expressed by Mucosal Dendritic Cells Plays a Critical Role in Food Antigen–Specific Th2 Differentiation and Intestinal Allergy. Gastroenterology 2007, 133, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ziegler, S.F. Intradermal Administration of IL-33 Induces Allergic Airway Inflammation. Sci. Rep. 2017, 7, 1706. [Google Scholar] [CrossRef] [PubMed]
- Kissner, T.L.; Moisan, L.; Mann, E.; Alam, S.; Ruthel, G.; Ulrich, R.G.; Rebek, M.; Rebek, J.; Saikh, K.U. A Small Molecule That Mimics the BB-Loop in the Toll Interleukin-1 (IL-1) Receptor Domain of MyD88 Attenuates Staphylococcal Enterotoxin B-Induced pro-Inflammatory Cytokine Production and Toxicity in Mice. J. Biol. Chem. 2011, 286, 31385–31396. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine That Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Larson, R.P.; Ziegler, S.F.; Geha, R.S. Mechanical Injury Polarizes Skin Dendritic Cells to Elicit a Th2 Response by Inducing Cutaneous Thymic Stromal Lymphopoietin Expression. J. Allergy Clin. Immunol. 2010, 126, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, L.M.; Gurish, M.F.; Burton, O.T.; Leisten, S.; Janssen, E.; Oettgen, H.C.; Beaupré, J.; Lewis, C.N.; Austen, K.F.; Schulte, S.; et al. Epicutaneous Sensitization Results in IgE-Dependent Intestinal Mast Cell Expansion and Food-Induced Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Goswami, R.; Benedé, S.; Grishina, G.; Dunkin, D.; Järvinen, K.M.; Maleki, S.J.; Sampson, H.A.; Berin, M.C. Skin Exposure Promotes a Th2-Dependent Sensitization to Peanut Allergens. J. Clin. Investig. 2014, 124, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Bruhs, A.; Proksch, E.; Schwarz, T.; Schwarz, A. Disruption of the Epidermal Barrier Induces Regulatory T Cells via IL-33 in Mice. J. Investig. Dermatol. 2018, 138, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Masuda, K.; Tamagawa-Mineoka, R.; Matsunaka, H.; Murakami, Y.; Yamashita, R.; Morita, E.; Katoh, N. Thymic Stromal Lymphopoietin Expression Is Increased in the Horny Layer of Patients with Atopic Dermatitis. Clin. Exp. Immunol. 2013, 171, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in Atopic Dermatitis: Expression Profiles and Modulation by Triggering Factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Callard, R.E.; Harper, J.I. The skin barrier, atopic dermititis and allergy: A role for Langerhans cells? Trends Immunol. 2007, 28, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Strid, J.; Hourihane, J.; Kimber, I.; Callard, R.; Strobel, S. Distruption of the stratum corenum allows potent epicutaneous immunization with protein antigens resulting in a dominant Th2 response. Eur. J. Immunol. 2004, 34, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Thelen, T.D.; Comeau, M.R.; Ziegler, S.F. Thymic Stromal Lymphopoietin–mediated Epicutaneous Inflammation Promotes Acute Diarrhea and Anaphylaxis. J. Clin. Investig. 2014, 124, 5442–5452. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Fukuoka, A.; Kabashima, K.; Ziegler, S.F.; Nakanishi, K.; Matsushita, K.; Yoshimoto, T. The Role of Basophils and Proallergic Cytokines, TSLP and IL-33, in Cutaneously Sensitized Food Allergy. Int. Immunol. 2014, 26, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, A.E.; Sattentau, Q.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to Food Allergens through Inflamed Skin Promotes Intestinal Food Allergy through the Thymic Stromal Lymphopoietin-Basophil Axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Mondoulet, L.; Blazquez, A.B.; Benhamou, P.-H.; Sampson, H.A.; Berin, M.C. Epicutaneous Immunotherapy Induces Gastrointestinal LAP+ regulatory T Cells and Prevents Food-Induced Anaphylaxis. J. Allergy Clin. Immunol. 2017, 139, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Dolence, J.J.; Kobayashi, T.; Iijima, K.; Krempski, J.; Drake, L.Y.; Dent, A.L.; Kita, H. Airway Exposure Initiates Peanut Allergy by Involving the IL-1 Pathway and T Follicular Helper Cells in Mice. J. Allergy Clin. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B. Regulation of IgE-Mediated Food Allergy by IL-9 Producing Mucosal Mast Cells and Type 2 Innate Lymphoid Cells. Immune Netw. 2016, 16, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ria, F.; Penna, G.; Adorini, L. Th1 Cells Induce and Th2 Inhibit Antigen-Dependent IL-12 Secretion by Dendritic Cells. Eur. J. Immunol. 1998, 28, 2003–2016. [Google Scholar] [CrossRef]
- Ito, T.; Wang, Y.-H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.-F.; Yao, Z.; Cao, W.; Liu, Y.-J. TSLP-Activated Dendritic Cells Induce an Inflammatory T Helper Type 2 Cell Response through OX40 Ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, A.B.; Berin, M.C. Gastrointestinal Dendritic Cells Promote Th2 Skewing via OX40L. J. Immunol. 2008, 180, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.S.; Straw, A.D.; Dalton, N.M.; Pearce, E.J. Cutting Edge: Th2 Response Induction by Dendritic Cells: A role for CD40. J. Immunol. 2002, 168, 537–540. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.S.; Patton, E.A.; La Flamme, A.C.; Araujo, M.I.; Huxtable, C.R.; Bauman, B.; Pearce, E.J. Impaired Th2 Development and Increased Mortality during Schistosoma mansoni Infection in the Absence of CD40/CD154 Interaction. J. Immunol. 2002, 168, 4643–4649. [Google Scholar] [CrossRef] [PubMed]
- Hussaarts, L.; Yazdanbakhsh, M.; Guigas, B. Priming Dendritic Cells for th2 Polarization: Lessons Learned from Helminths and Implications for Metabolic Disorders. Front. Immunol. 2014, 5, 499. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-G.; Du, Y.-M.; Yan, Z.-D.; Yan, J.; Zhuansun, Y.-X.; Chen, R.; Zhang, W.; Feng, S.-L.; Ran, P.-X. CD80 and CD86 Knockdown in Dendritic Cells Regulates Th1/Th2 Cytokine Production in Asthmatic Mice. Exp. Ther. Med. 2016, 11, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Carter, C.; Favila, M.; Polando, R.E.; Cotton, R.N.; Bogard Horner, K.; Condon, D.; Ballhorn, W.; Whitcomb, J.P.; Yadav, M.; Geister, R.L.; et al. Leishmania Major Inhibits IL-12 in Macrophages by Signalling through CR3 (CD11b/CD18) and down-Regulation of ETS-Mediated Transcription. Parasite Immunol. 2013, 35, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Soumelis, V.; Watanabe, N.; Ito, T.; Wang, Y.-H.; de Waal Malefyt, R.; Omori, M.; Zhou, B.; Ziegler, S.F. TSLP: An Epithelial Cell Cytokine That Regulates T Cell Differentiation by Conditioning Dendritic Cell Maturation. Annu. Rev. Immunol. 2007, 25, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Beddoe, T.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C. Structure, Biological Functions and Applications of the AB5 Toxins. Trends Biochem. Sci. 2010, 35, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Neilsen, C.V.; Hadsaitong, A.; Schleimer, R.P.; Luo, X.; Bryce, P.J. Impairing Oral Tolerance Promotes Allergy and Anaphylaxis: A New Murine Food Allergy Model. J. Allergy Clin. Immunol. 2009, 123, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Asahina, A.; Nakamura, K.; Tomura, M.; Fujiwara, H.; Tamaki, K. Granulocyte/macrophage Colony-Stimulating Factor Inhibits IL-12 Production of Mouse Langerhans Cells. J. Immunol. 2000, 164, 5113–5119. [Google Scholar] [CrossRef] [PubMed]
- Traidl-Hoffmann, C.; Mariani, V.; Hochrein, H.; Karg, K.; Wagner, H.; Ring, J.; Mueller, M.J.; Jakob, T.; Behrendt, H. Pollen-Associated Phytoprostanes Inhibit Dendritic Cell Interleukin-12 Production and Augment T Helper Type 2 Cell Polarization. J. Exp. Med. 2005, 201, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.M.; Kull, I.; Wickman, M.; Bergström, A. Antibiotic Use in Early Life and Development of Allergic Diseases: Respiratory Infection as the Explanation. Clin Exp Allergy 2010, 40. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, K.; Stecenko, A.; Sullivan, K.; Fitzpatrick, A. Relationship between Treatment with Antacid Medication and the Prevalence of Food Allergy in Children. Allergy Asthma Proc. 2013, 34, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Jensen-Jarolim, E. The Role of Protein Digestibility and Antacids on Food Allergy Outcomes. J. Allergy Clin. Immunol. 2008, 121, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Papathoma, E.; Triga, M.; Fouzas, S.; Dimitriou, G. Cesarean Section Delivery and Development of Food Allergy and Atopic Dermatitis in Early Childhood. Pediatr. Allergy Immunol. 2016, 27, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.M.; Hrusch, C.L.; Gozdz, J.; Igartua, C.; Pivniouk, V.; Murray, S.E.; Ledford, J.G.; Marques dos Santos, M.; Anderson, R.L.; Metwali, N.; et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N. Engl. J. Med. 2016, 375, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.; Yao, T.-C. The Genetics of Asthma and Allergic Disease: A 21st Century Perspective. Immunol. Rev. 2011, 242, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does Atopic Dermatitis Cause Food Allergy? A Systematic Review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Breuer, K. Role of Food Allergy in Atopic Dermatitis. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Srinivas, S.M. Food Allergy in Atopic Dermatitis. Indian J. Dermatol. 2016, 61, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the Frontline: Role in Skin Barrier Function and Disease. J. Cell Sci. 2009, 122 Pt 9, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Van den Oord, R.A.H.M.; Sheikh, A. Filaggrin Gene Defects and Risk of Developing Allergic Sensitisation and Allergic Disorders: Systematic Review and Meta-Analysis. BMJ 2009, 339, b2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, M.; Green, J.; Ferrie, R.; Queener, A.; Kaplan, M.H.; Cook-Mills, J.M. Mechanism for Initiation of Food Allergy: Dependence on Skin Barrier Mutations and Environmental Allergen Co-Stimulation. J. Allergy Clin. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yuki, T.; Tobiishi, M.; Kusaka-Kikushima, A.; Ota, Y.; Tokura, Y. Impaired Tight Junctions in Atopic Dermatitis Skin and in a Skin-Equivalent Model Treated with Interleukin-17. PLoS ONE 2016, 11, e0161759. [Google Scholar] [CrossRef] [PubMed]
- Negoro, T.; Orihara, K.; Irahara, T.; Nishiyama, H.; Hagiwara, K.; Nishida, R.; Takagi, H.; Satoh, K.; Yamamoto, Y.; Shimizu, S.; et al. Influence of SNPs in Cytokine-Related Genes on the Severity of Food Allergy and Atopic Eczema in Children. Pediatr. Allergy Immunol. 2006, 17, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Van der Pouw Kraan, T.; van Veen, A.; Boeije, L.; van Tuyl, S.; de Groot, E.; Stapel, S.; Bakker, A.; Verweij, C.; Aarden, L.; van der Zee, J. An IL-13 Promoter Polymorphism Associated with Increased Risk of Allergic Asthma. Genes Immun. 1999, 1, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Beaty, T.H.; Deindl, P.; Huang, S.-K.; Lau, S.; Sommerfeld, C.; Fallin, M.D.; Kao, W.H.L.; Wahn, U.; Nickel, R. Associations between Specific Serum IgE Response and 6 Variants within the Genes IL4, IL13, and IL4RA in German Children. J. Allergy Clin. Immunol. 2004, 113, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, C.D.; Pettersson, M.E.; Dubois, A.E.J.; Koppelman, G.H. Association of stat6 Gene Variants with Food Allergy Diagnosed by Double-Blind Placebo-Controlled Food Challenges. Allergy 2018. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, T.R.; Linane, A.; Moes, N.; Anover, S.; Mateo, V.; Rieux-Laucat, F.; Hermine, O.; Vijay, S.; Gambineri, E.; Cerf-Bensussan, N.; et al. Severe Food Allergy as a Variant of IPEX Syndrome Caused by a Deletion in a Noncoding Region of the FOXP3 Gene. Gastroenterology 2007, 132, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Burton, O.T.; Oettgen, H.C.; Chatila, T. IL-4 Production by Group 2 Innate Lymphoid Cells Promotes Food Allergy by Blocking Regulatory T-Cell Function. J. Allergy Clin. Immunol. 2016, 138, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A Microbiota Signature Associated with Experimental Food Allergy Promotes Allergic Sensitization and Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Szczawińska-Popłonyk, A.; Bręborowicz, A.; Ossowska, L. Food Allergy in Children with Hypogammaglobulinemia. Pediatr. Pol. 2012, 87, 444–448. [Google Scholar] [CrossRef]
- Tuano, K.S.; Orange, J.S.; Sullivan, K.; Cunningham-Rundles, C.; Bonilla, F.A.; Davis, C.M. Food Allergy in Patients with Primary Immunodeficiency Diseases: Prevalence within the US Immunodeficiency Network (USIDNET). J. Allergy Clin. Immunol. 2015, 135, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Neeland, M.R.; Koplin, J.J.; Dang, T.D.; Dharmage, S.C.; Tang, M.L.; Prescott, S.L.; Saffery, R.; Martino, D.J.; Allen, K.J. Early Life Innate Immune Signatures of Persistent Food Allergy. J. Allergy Clin. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kosoy, R.; Agashe, C.; Grishin, A.; Leung, D.Y.; Wood, R.A.; Sicherer, S.H.; Jones, S.M.; Burks, A.W.; Davidson, W.F.; Lindblad, R.W.; et al. Transcriptional Profiling of Egg Allergy and Relationship to Disease Phenotype. PLoS ONE 2016, 11, e0163831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Collier, F.; Naselli, G.; Saffery, R.; Tang, M.L.; Allen, K.J.; Ponsonby, A.-L.; Harrison, L.C.; Vuillermin, P.; BIS Investigator Group. Cord Blood Monocyte–derived Inflammatory Cytokines Suppress IL-2 and Induce Nonclassic “Th2-Type” Immunity Associated with Development of Food Allergy. Sci. Transl. Med. 2016, 8, 321ra8. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Tsai, H.-J.; Wang, X. Genetics of Food Allergy. Curr. Opin. Pediatr. 2009, 21, 770–776. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellenbogen, Y.; Jiménez-Saiz, R.; Spill, P.; Chu, D.K.; Waserman, S.; Jordana, M. The Initiation of Th2 Immunity Towards Food Allergens. Int. J. Mol. Sci. 2018, 19, 1447. https://doi.org/10.3390/ijms19051447
Ellenbogen Y, Jiménez-Saiz R, Spill P, Chu DK, Waserman S, Jordana M. The Initiation of Th2 Immunity Towards Food Allergens. International Journal of Molecular Sciences. 2018; 19(5):1447. https://doi.org/10.3390/ijms19051447
Chicago/Turabian StyleEllenbogen, Yosef, Rodrigo Jiménez-Saiz, Paul Spill, Derek K. Chu, Susan Waserman, and Manel Jordana. 2018. "The Initiation of Th2 Immunity Towards Food Allergens" International Journal of Molecular Sciences 19, no. 5: 1447. https://doi.org/10.3390/ijms19051447




