NAD(P)H Oxidase Activity in the Small Intestine Is Predominantly Found in Enterocytes, Not Professional Phagocytes
Abstract
:1. Introduction
2. Results
2.1. Dynamic and Functional Intravital Imaging in Villi of the Small Intestine
2.2. NAD(P)H Metabolism in Myeloid Cells of the Small Intestine under Steady State Conditions
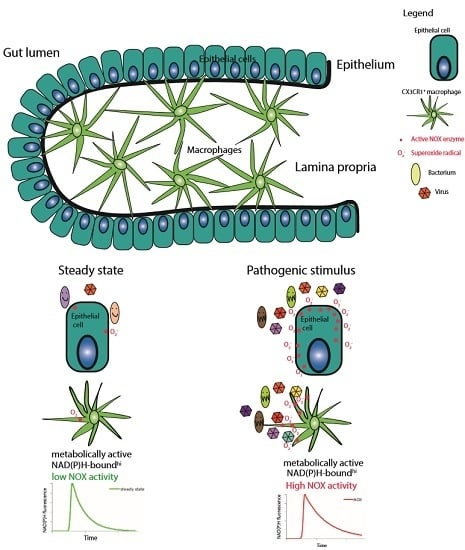
2.3. NOX Activity is Present in Epithelial Cells of the Small Intestine in Steady State
2.4. NOX Isotype Expression in Various Cellular Compartments of the Small Intestine
2.5. NAD(P)H-Metabolism in the Small Intestine in Response to Inflammatory Stimuli
3. Discussion
4. Materials and Methods
4.1. Multi-Photon Microscope Setup for Fluorescence Lifetime Imaging (FLIM)
4.2. Mice
4.3. Intravital Imaging of the Small Intestine
4.4. In Vitro Activation
4.5. Cell Sorting
4.6. RT-PCR
4.7. Immunofluorescence
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EGFP | Enhanced green fluorescent protein |
| FLIM | Fluorescence lifetime imaging |
| GI | Gastro-intestinal |
| IEL | intraepithelial lymphocytes |
| LP | lamina propria |
| MHC | Major histocompatibility complex |
| NAD(P)H | Nicotinamide Adenine Dinucleotide (Phosphate) Dehydrogenase |
| NOX | NADPH oxidases |
| PMA | Phorbol myristate acetate |
| ROS | Reactive oxygen species (ROS) |
| TCSPC | Time-correlated single photon counter |
| Ti:Sa | Titanium-sapphire |
| TLR | Toll-like receptor |
References
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.; Kraft, M.; Roth, K.; Riedel, R.; Lammerding, D.; Hauser, A.E. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal Immunol. 2016, 9, 83–97. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xu, W.; Santini, P.A.; Polydorides, A.D.; Chiu, A.; Estrella, J.; Shan, M.; Chadburn, A.; Villanacci, V.; Plebani, A.; et al. Intestinal bacteria trigger t cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine April. Immunity 2007, 26, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; He, B.; Chiu, A.; Chadburn, A.; Shan, M.; Buldys, M.; Ding, A.; Knowles, D.M.; Santini, P.A.; Cerutti, A. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor slpi. Nat. Immunol. 2007, 8, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Mileti, E.; Matteoli, G.; Chieppa, M.; Rescigno, M. Intestinal epithelial cells promote colitis-protective regulatory t-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009, 2, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, M.; Chieppa, M.; Salucci, V.; Avogadri, F.; Sonzogni, A.; Sampietro, G.M.; Nespoli, A.; Viale, G.; Allavena, P.; Rescigno, M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005, 6, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.C.; Zaph, C.; Troy, A.E.; Du, Y.; Guild, K.J.; Comeau, M.R.; Artis, D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009, 206, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ivanov, I.I. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol. Cell Biol. 2013, 91, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Laky, K.; Lefrancois, L.; Lingenheld, E.G.; Ishikawa, H.; Lewis, J.M.; Olson, S.; Suzuki, K.; Tigelaar, R.E.; Puddington, L. Enterocyte expression of interleukin 7 induces development of gammadelta T cells and peyer’s patches. J. Exp. Med. 2000, 191, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.; Willis, C.R.; et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000, 191, 771–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cepek, K.L.; Shaw, S.K.; Parker, C.M.; Russell, G.J.; Morrow, J.S.; Rimm, D.L.; Brenner, M.B. Adhesion between epithelial cells and T lymphocytes mediated by e-cadherin and the alpha e beta 7 integrin. Nature 1994, 372, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Leishman, A.J.; Naidenko, O.V.; Attinger, A.; Koning, F.; Lena, C.J.; Xiong, Y.; Chang, H.C.; Reinherz, E.; Kronenberg, M.; Cheroutre, H. T cell responses modulated through interaction between cd8alphaalpha and the nonclassical mhc class I molecule, TL. Science 2001, 294, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Perry, V.H.; Gordon, S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: Macrophages associated with epithelia. Anat. Rec. 1984, 210, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Pull, S.L.; Doherty, J.M.; Mills, J.C.; Gordon, J.I.; Stappenbeck, T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 2005, 102, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, E.; Jung, S. Intestinal macrophages: Well educated exceptions from the rule. Trends Immunol. 2013, 34, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. Intestinal macrophages—Specialised adaptation to a unique environment. Eur. J. Immunol. 2011, 41, 2494–2498. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Aliberti, J.; Graemmel, P.; Sunshine, M.J.; Kreutzberg, G.W.; Sher, A.; Littman, D.R. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000, 20, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, C.C.; Mowat, A.M. The monocyte-macrophage axis in the intestine. Cell. Immunol. 2014, 291, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, E.; Varol, C.; Farache, J.; Elmaliah, E.; Satpathy, A.T.; Friedlander, G.; Mack, M.; Shpigel, N.; Boneca, I.G.; Murphy, K.M.; et al. Ly6c hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 2012, 37, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Saurer, L.; Schenk, M.; Dickgreber, N.; Mueller, C. CX3CR1 defines functionally distinct intestinal mononuclear phagocyte subsets which maintain their respective functions during homeostatic and inflammatory conditions. Eur. J. Immunol. 2011, 41, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Niesner, R.; Narang, P.; Spiecker, H.; Andresen, V.; Gericke, K.H.; Gunzer, M. Selective detection of NADPH oxidase in polymorphonuclear cells by means of nad(p)h-based fluorescence lifetime imaging. J. Biophys. 2008, 2008, 602639. [Google Scholar] [CrossRef] [PubMed]
- Mossakowski, A.A.; Pohlan, J.; Bremer, D.; Lindquist, R.; Millward, J.M.; Bock, M.; Pollok, K.; Mothes, R.; Viohl, L.; Radbruch, M.; et al. Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol. 2015, 130, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, H.; Bremer, D.; Guenther, R.; Cseresnyes, Z.; Lindquist, R.; Hauser, A.E.; Niesner, R. Ongoing oxidative stress causes subclinical neuronal dysfunction in the recovery phase of EAE. Front. Immunol. 2016, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, H.; Mothes, R.; Bremer, D.; Seifert, S.; Kohler, R.; Pohlan, J.; Ostendorf, L.; Gunther, R.; Leben, R.; Stenzel, W.; et al. Analyzing nicotinamide adenine dinucleotide phosphate oxidase activation in aging and vascular amyloid pathology. Front. Immunol. 2017, 8, 844. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, F.L.; Kohler, R.; Bayat-Sarmadi, J.; Bayerl, S.; Hauser, A.E.; Niesner, R.; Luch, A.; Cseresnyes, Z. Cell shape characterization and classification with discrete Fourier transforms and self-organizing maps. Cytometry A 2018, 93, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Caton, M.L.; Smith-Raska, M.R.; Reizis, B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J. Exp. Med. 2007, 204, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Luche, H.; Weber, O.; Nageswara Rao, T.; Blum, C.; Fehling, H.J. Faithful activation of an extra-bright red fluorescent protein in “knock-in” cre-reporter mice ideally suited for lineage tracing studies. Eur. J. Immunol. 2007, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. NADPH oxidase: An update. Blood 1999, 93, 1464–1476. [Google Scholar] [PubMed]
- Bremer, D.; Leben, R.; Mothes, R.; Radbruch, H.; Niesner, R. Method to detect the cellular source of over-activated NADPH oxidases using NADPH fluorescence lifetime imaging. Curr. Protoc. Cytom. 2017, 80, 9.52.1–9.52.14. [Google Scholar] [PubMed]
- Dolowschiak, T.; Chassin, C.; Ben Mkaddem, S.; Fuchs, T.M.; Weiss, S.; Vandewalle, A.; Hornef, M.W. Potentiation of epithelial innate host responses by intercellular communication. PLoS Pathog. 2010, 6, e1001194. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D.; Neish, A.S. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu. Rev. Pathol. 2014, 9, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. Phagocytes and oxidative stress. Am. J. Med. 2000, 109, 33–44. [Google Scholar] [CrossRef]
- Yokota, H.; Tsuzuki, A.; Shimada, Y.; Imai, A.; Utsumi, D.; Tsukahara, T.; Matsumoto, M.; Amagase, K.; Iwata, K.; Nakamura, A.; et al. NOX1/NADPH oxidase expressed in colonic macrophages contributes to the pathogenesis of colonic inflammation in trinitrobenzene sulfonic acid-induced murine colitis. J. Pharmacol. Exp. Ther. 2017, 360, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Welak, S.R.; Rentea, R.M.; Teng, R.J.; Heinzerling, N.; Biesterveld, B.; Liedel, J.L.; Pritchard, K.A., Jr.; Fredrich, K.M.; Gourlay, D.M. Intestinal NADPH oxidase 2 activity increases in a neonatal rat model of necrotizing enterocolitis. PLoS ONE 2014, 9, e115317. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Smythies, L.E.; Mosteller-Barnum, M.; Sibley, D.A.; Russell, M.W.; Merger, M.; Sellers, M.T.; Orenstein, J.M.; Shimada, T.; Graham, M.F.; et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IGA-mediated activities. J. Immunol. 2001, 167, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Smythies, L.E.; Sellers, M.; Clements, R.H.; Mosteller-Barnum, M.; Meng, G.; Benjamin, W.H.; Orenstein, J.M.; Smith, P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Investig. 2005, 115, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Smythies, L.E.; Shen, R.; Greenwell-Wild, T.; Gliozzi, M.; Wahl, S.M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011, 4, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Rugtveit, J.; Haraldsen, G.; Hogasen, A.K.; Bakka, A.; Brandtzaeg, P.; Scott, H. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14+L1+ monocyte derived cells. Gut 1995, 37, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.J.; Riley, G.P.; Morgan, K.; Miller, R.; Hunter, J.O.; Middleton, S.J. The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J. Clin. Pathol. 2001, 54, 293–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denning, T.L.; Wang, Y.C.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing t cell responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Muller, W.; Sparwasser, T.; Forster, R.; Pabst, O. Intestinal tolerance requires gut homing and expansion of foxp3+ regulatory t cells in the lamina propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.-W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Müller, W.; et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H. Starting at the beginning: New perspectives on the biology of mucosal T cells. Annu. Rev. Immunol. 2004, 22, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Lim, H.W.; Andrisani, O.M.; Broxmeyer, H.E.; Kim, C.H. Vitamin a metabolites induce gut-homing foxp3+ regulatory T cells. J. Immunol. 2007, 179, 3724–3733. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Smulian, A.G.; Greaves, D.R.; Hogan, S.P.; Herbert, D.R. TGF-beta limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur. J. Immunol. 2011, 41, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Hershberg, R.M.; Framson, P.E.; Cho, D.H.; Lee, L.Y.; Kovats, S.; Beitz, J.; Blum, J.S.; Nepom, G.T. Intestinal epithelial cells use two distinct pathways for hla class ii antigen processing. J. Clin. Investig. 1997, 100, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Bland, P. MHC class II expression by the gut epithelium. Immunol. Today 1988, 9, 174–178. [Google Scholar] [CrossRef]
- Hershberg, R.M.; Mayer, L.F. Antigen processing and presentation by intestinal epithelial cells—Polarity and complexity. Immunol. Today 2000, 21, 123–128. [Google Scholar] [CrossRef]
- Pascual-Reguant, A.; Bayat Sarmadi, J.; Baumann, C.; Noster, R.; Cirera-Salinas, D.; Curato, C.; Pelczar, P.; Huber, S.; Zielinski, C.E.; Lohning, M.; et al. TH17 cells express ST2 and are controlled by the alarmin IL-33 in the small intestine. Mucosal Immunol. 2017, 10, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, S.H.; Niesner, R.; Cseresnyes, Z.; Radbruch, H.; Pohlan, J.; Brandenburg, S.; Czabanka, M.A.; Vajkoczy, P. Time lapse in vivo microscopy reveals distinct dynamics of microglia-tumor environment interactions-a new role for the tumor perivascular space as highway for trafficking microglia. Glia 2016, 64, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Rinnenthal, J.L.; Börnchen, C.; Radbruch, H.; Andresen, V.; Mossakowski, A.; Siffrin, V.; Seelemann, T.; Spiecker, H.; Moll, I.; Herz, J.; et al. Parallelized TCSPC for dynamic intravital fluorescence lifetime imaging: Quantifying neuronal dysfunction in neuroinflammation. PLoS ONE 2013, 8, e60100. [Google Scholar] [CrossRef] [PubMed]
- Farache, J.; Koren, I.; Milo, I.; Gurevich, I.; Kim, K.-W.; Zigmond, E.; Furtado, G.C.; Lira, S.A.; Shakhar, G. Luminal bacteria recruit cd103(+) dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 2013, 38, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Akdis, M.; Andra, I.; Annunziato, F.; Bacher, P.; Barnaba, V.; Battistini, L.; Bauer, W.M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2017, 47, 1584–1797. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindquist, R.L.; Bayat-Sarmadi, J.; Leben, R.; Niesner, R.; Hauser, A.E. NAD(P)H Oxidase Activity in the Small Intestine Is Predominantly Found in Enterocytes, Not Professional Phagocytes. Int. J. Mol. Sci. 2018, 19, 1365. https://doi.org/10.3390/ijms19051365
Lindquist RL, Bayat-Sarmadi J, Leben R, Niesner R, Hauser AE. NAD(P)H Oxidase Activity in the Small Intestine Is Predominantly Found in Enterocytes, Not Professional Phagocytes. International Journal of Molecular Sciences. 2018; 19(5):1365. https://doi.org/10.3390/ijms19051365
Chicago/Turabian StyleLindquist, Randall L., Jannike Bayat-Sarmadi, Ruth Leben, Raluca Niesner, and Anja E. Hauser. 2018. "NAD(P)H Oxidase Activity in the Small Intestine Is Predominantly Found in Enterocytes, Not Professional Phagocytes" International Journal of Molecular Sciences 19, no. 5: 1365. https://doi.org/10.3390/ijms19051365