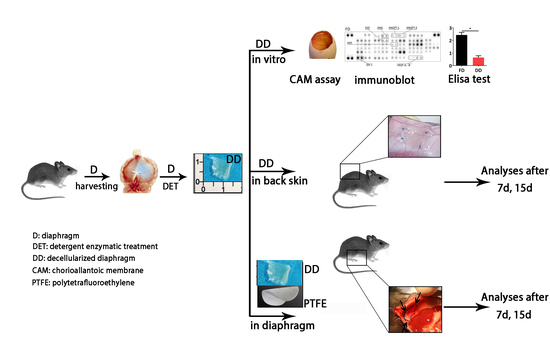
Decellularized Diaphragmatic Muscle Drives a Constructive Angiogenic Response In Vivo
Abstract
:1. Introduction
2. Results
2.1. Diaphragm-Derived Decellularized Matrix Retains Angiogenic Potential
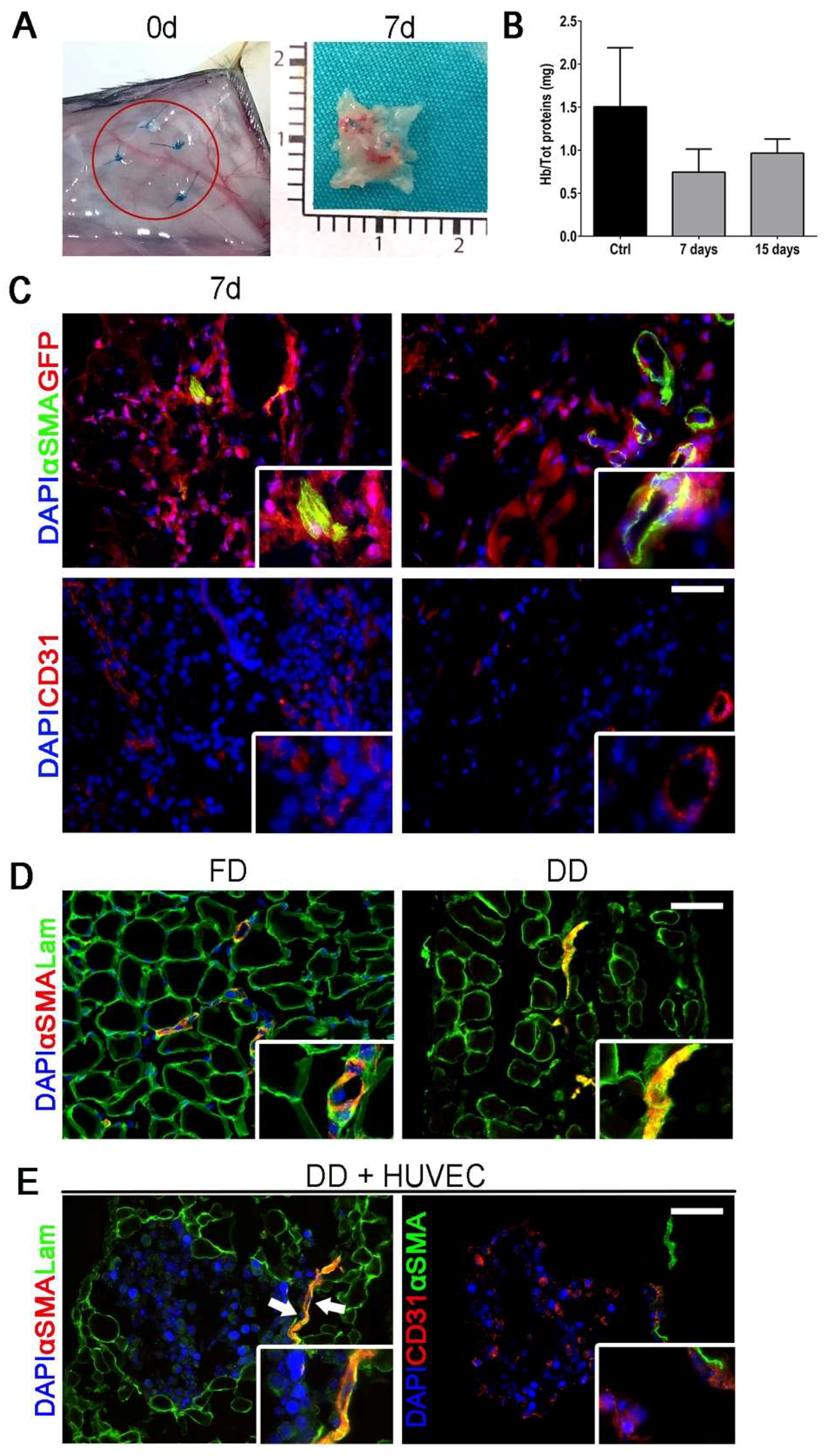
2.2. Cell-Scaffold Interaction
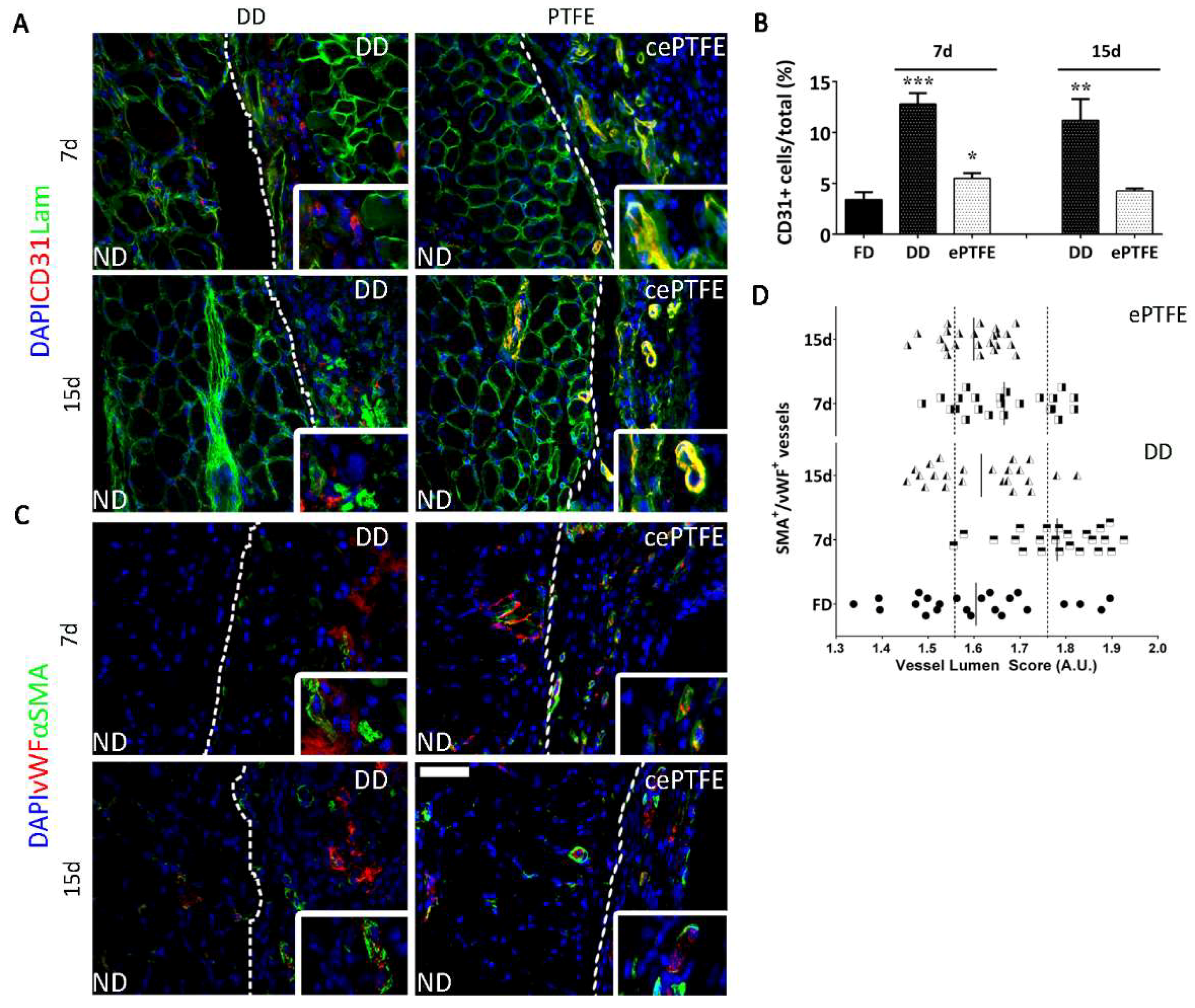
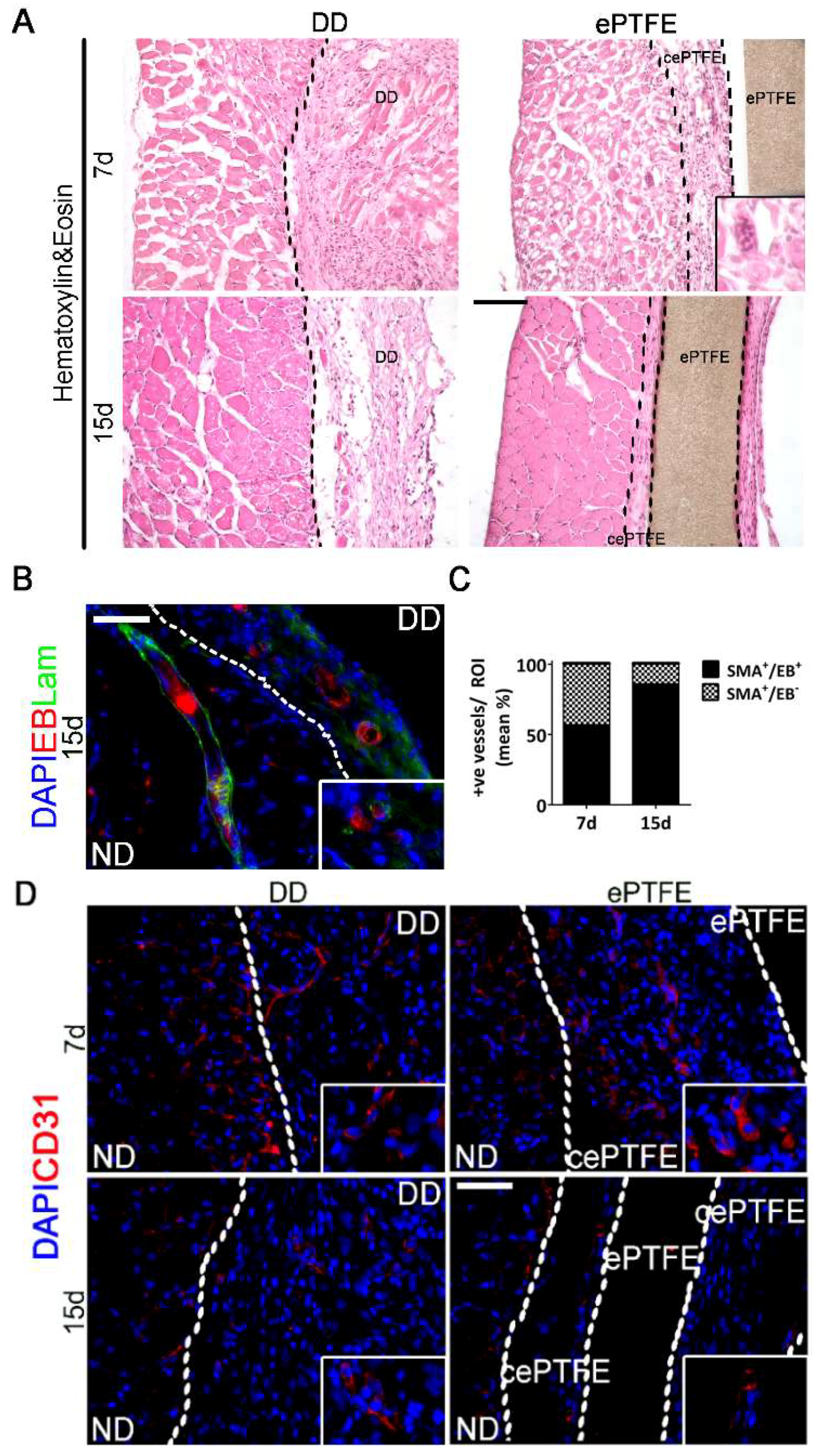
2.3. Angiogenic Response to Orthotopic Transplantation of DD vs. ePTFE
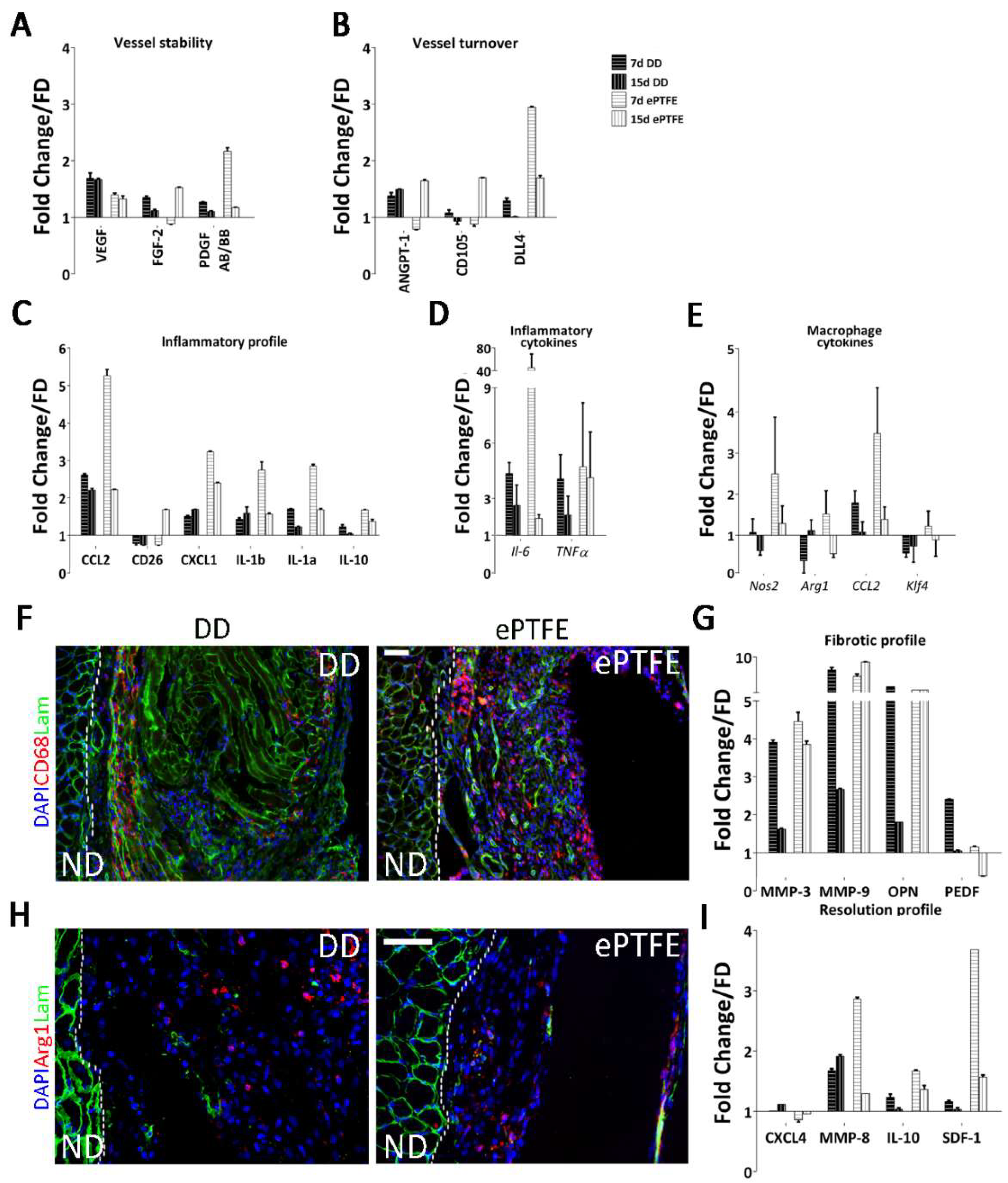
2.4. Molecular Profiling of Host-Scaffold Interaction
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Diaphragm Decellularization
4.3. Chicken Chorioallantoic Membrane Assay
4.4. Proteome Profiler Angiogenesis Array
4.5. ELISA Test
4.6. Subcutaneous Implantation
4.7. Hemoglobin Quantification
4.8. Evans Blue Injection
4.9. Human Umbilical Vein Endothelial Cells Culture and Seeding
4.10. Orthotopic Implantation
4.11. Histological Stain and Immunofluorescence
4.12. Vessel Size Analysis
4.13. Real Time PCR
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| DD | Decellularized diaphragm |
| ePTFE | Expanded polytetrafluoroethylene |
| cePTFE | Capsule Expanded polytetrafluoroethylene |
| ECM | Extracellular matrix |
| CAM | Chorion allantoic membrane |
| ND | Native diaphragm |
References
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Bellini, S.; Teoli, D.; de Coppi, P.; Ribatti, D.; Nico, B.; Simonato, E.; Gamba, P.G.; Nussdorfer, G.G.; Morpurgo, M.; et al. In vitro and in vivo evaluation of acellular diaphragmatic matrices seeded with muscle precursors cells and coated with VEGF silica gels to repair muscle defect of the diaphragm. J. Biomed. Mater. Res. Part A 2009, 89A, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; García, J.R.; Paez, J.I.; Singh, A.; Phelps, E.A.; Weis, S.; Shafiq, Z.; Shekaran, A.; del Campo, A.; García, A.J. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 2014, 14, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Gholobova, D.; Decroix, L.; van Muylder, V.; Desender, L.; Gerard, M.; Carpentier, G.; Vandenburgh, H.; Thorrez, L. Endothelial Network Formation Within Human Tissue-Engineered Skeletal Muscle. Tissue Eng. Part A 2015, 21, 150901071945000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.L.; Hudon, V.; Berthod, F.; Germain, L.; Auger, F.A. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am. J. Transplant. 2005, 5, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering complex tissues. Sci. Transl. Med. 2012, 4, 160rv12. [Google Scholar] [CrossRef] [PubMed]
- Baranski, J.D.; Chaturvedi, R.R.; Stevens, K.R.; Eyckmans, J.; Carvalho, B.; Solorzano, R.D.; Yang, M.T.; Miller, J.S.; Bhatia, S.N.; Chen, C.S. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. USA 2013, 110, 7586–7591. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Hochberg, M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973, 138, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Prevascularization in tissue engineering: Current concepts and future directions. Biotechnol. Adv. 2015. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.S.; Kristiansen, M.; Kristensen, L.P.; Larsen, K.H.; Nielsen, M.O.; Christiansen, H.; Nehlin, J.; Andersen, J.S.; Kassem, M. Decellularized Matrix from Tumorigenic Human Mesenchymal Stem Cells Promotes Neovascularization with Galectin-1 Dependent Endothelial Interaction. PLoS ONE 2011, 6, e21888. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.C.; Pandolfi, V.; McFetridge, P.S. Novel human-derived extracellular matrix induces in vitro and in vivo vascularization and inhibits fibrosis. Biomaterials 2015, 49, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Totonelli, G.; Maghsoudlou, P.; Georgiades, F.; Garriboli, M.; Koshy, K.; Turmaine, M.; Ashworth, M.; Sebire, N.J.; Pierro, A.; Eaton, S.; et al. Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration. Pediatr. Surg. Int. 2013, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Hudlicka, O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: Involvement of VEGF and metalloproteinases. Angiogenesis 2003, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Christov, C.; Chretien, F.; Abou-Khalil, R.; Bassez, G.; Vallet, G.; Authier, F.-J.; Bassaglia, Y.; Shinin, V.; Tajbakhsh, S.; Chazaud, B.; et al. Muscle Satellite Cells and Endothelial Cells: Close Neighbors and Privileged Partners. Mol. Biol. Cell 2007, 18, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, C.; Rizzi, R.; Biondo, A.; Longa, E.; Mascaro, A.; Shapira-, K.; Kossovar, O.; Benedetti, S.; Salvatori, M.L.; Santoleri, S.; et al. In vivo generation of a mature and functional artificial skeletal muscle. EMBO Mol. Med. 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jank, B.J.; Xiong, L.; Moser, P.T.; Guyette, J.P.; Ren, X.; Cetrulo, C.L.; Leonard, D.A.; Fernandez, L.; Fagan, S.P.; Ott, H.C. Engineered composite tissue as a bioartificial limb graft. Biomaterials 2015, 61, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; Engelmayr, G.C.; Fontanella, A.N.; Palmer, G.M.; Bursac, N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 5508–5513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Marini, R.; van Blitterswijk, C.A.; Mulligan, R.C.; D’Amore, P.A.; et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Shimizu-Motohashi, Y.; Asakura, A. Angiogenesis as a novel therapeutic strategy for Duchenne muscular dystrophy through decreased ischemia and increased satellite cells. Front. Physiol. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Uchida, C.; Nwadozi, E.; Hasanee, A.; Olenich, S.; Olfert, I.M.; Haas, T.L. Muscle-derived vascular endothelial growth factor regulates microvascular remodelling in response to increased shear stress in mice. Acta Physiol. 2015, 214, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, M.J.; Molema, G.; Plantinga, J.; Moorlag, H.; van Luyn, M.J. Neovascularization and vascular markers in a foreign body reaction to subcutaneously implanted degradable biomaterial in mice. Angiogenesis 2002, 5, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kwee, B.J.; Mooney, D.J. Manipulating the Intersection of Angiogenesis and Inflammation. Ann. Biomed. Eng. 2015, 43, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; Urbani, L.; Alvarez-Fallas, M.E.; Franzin, C.; Dedja, A.; Bertin, E.; Zuccolotto, G.; Rosato, A.; Pavan, P.; Elvassore, N.; et al. Improvement of diaphragmatic performance through orthotopic application of decellularized extracellular matrix patch. Biomaterials 2016, 74, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Romao, R.L.P.; Nasr, A.; Chiu, P.P.L.; Langer, J.C. What is the best prosthetic material for patch repair of congenital diaphragmatic hernia? Comparison and meta-analysis of porcine small intestinal submucosa and polytetrafluoroethylene. J. Pediatr. Surg. 2012, 47, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Vailhé, B.; Vittet, D.; Feige, J.J. In vitro models of vasculogenesis and angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Bland, E.; Dréau, D.; Burg, K.J.L. Overcoming hypoxia to improve tissue-engineering approaches to regenerative medicine. J. Tissue Eng. Regen. Med. 2013, 7, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Bornstein, P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 2003, 89, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Salza, R. Matricryptins and matrikines: Biologically active fragments of the extracellular matrix. Exp. Dermatol. 2014, 23, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.C.; Calle, E.A.; Dzieciatkowska, M.; Niklason, L.E.; Hansen, K.C. Quantification of Extracellular Matrix Proteins from a Rat Lung Scaffold to Provide a Molecular Readout for Tissue Engineering. Mol. Cell. Proteom. 2015, 1, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Uygun, B.E.; Geerts, S.; Ozer, S.; Scalf, M.; Gilpin, S.E.; Ott, H.C.; Yarmush, M.L.; Smith, L.M.; Welham, N.V.; et al. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials 2016, 75, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Caralt, M.; Uzarski, J.S.; Iacob, S.; Obergfell, K.P.; Berg, N.; Bijonowski, B.M.; Kiefer, K.M.; Ward, H.H.; Wandinger-Ness, A.; Miller, W.M.; et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am. J. Transplant. 2015, 15, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Hoganson, D.M.; O’Doherty, E.M.; Owens, G.E.; Harilal, D.O.; Goldman, S.M.; Bowley, C.M.; Neville, C.M.; Kronengold, R.T.; Vacanti, J.P. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials 2010, 31, 6730–6737. [Google Scholar] [CrossRef] [PubMed]
- Reing, J.E.; Zhang, L.; Myers-Irvin, J.; Cordero, K.E.; Freytes, D.O.; Heber-Katz, E.; Bedelbaeva, K.; McIntosh, D.; Dewilde, A.; Braunhut, S.J.; et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng. Part A 2009, 15, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Moens, S.; Goveia, J.; Stapor, P.C.; Cantelmo, A.R.; Carmeliet, P. The multifaceted activity of VEGF in angiogenesis—Implications for therapy responses. Cytokine Growth Factor Rev. 2014, 25, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Latroche, C.; Gitiaux, C.; Chrétien, F.; Desguerre, I.; Mounier, R.; Chazaud, B. Skeletal Muscle Microvasculature: A Highly Dynamic Lifeline. Physiology 2015, 30, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, E.; Kowalewska, M.; Markowska-Zagrajek, A.; Kowalski, K.; Archacka, K.; Zimowska, M.; Grabowska, I.; Czerwińska, A.M.; Czarnecka-Góra, M.; Stremińska, W.; et al. Sdf-1 (CXCL12) improves skeletal muscle regeneration via the mobilisation of Cxcr4 and CD34 expressing cells. Biol. Cell 2012, 104, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Kwee, B.J.; Lewin, S.A.; Raimondo, T.; Mehta, M.; Mooney, D.J. Local Delivery of VEGF and SDF Enhances Endothelial Progenitor Cell Recruitment and Resultant Recovery from Ischemia. Tissue Eng. Part A 2015, 21, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Alitalo, K.; Annex, B.H.; Augustin, H.G.; Beam, C.; Berk, B.C.; Byzova, T.; Carmeliet, P.; Chilian, W.; Cooke, J.P.; et al. State-of-the-Art Methods for Evaluation of Angiogenesis and Tissue Vascularization: A Scientific Statement From the American Heart Association. Circ. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Htay, A.; Santos, W.D.; Gillies, G.T.; Fillmore, H.L.; Sholley, M.M.; Broaddus, W.C. In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. J. Neurooncol. 2009, 92, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; de Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.-L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C.; et al. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Vigodarzere, G.C.; Mantero, S. Skeletal muscle tissue engineering: Strategies for volumetric constructs. Front. Physiol. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Garg, K.; Ward, C.L.; Corona, B.T. Asynchronous inflammation and myogenic cell migration limit muscle tissue regeneration mediated by acellular scaffolds. Inflamm. Cell Signal. 2014, 6–8. [Google Scholar] [CrossRef]
- De Coppi, P.; Deprest, J. Regenerative medicine for congenital diaphragmatic hernia: Regeneration for repair. Eur. J. Pediatr. Surg. 2012, 22, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Grethel, E.J.; Cortes, R.A.; Wagner, A.J.; Clifton, M.S.; Lee, H.; Farmer, D.L.; Harrison, M.R.; Keller, R.L.; Nobuhara, K.K. Prosthetic patches for congenital diaphragmatic hernia repair: Surgisis vs Gore-Tex. J. Pediatr. Surg. 2006, 41, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wälchli, T.; Mateos, J.M.; Weinman, O.; Babic, D.; Regli, L.; Hoerstrup, S.P.; Gerhardt, H.; Schwab, M.E.; Vogel, J. Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain. Nat. Protoc. 2014, 10, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.B.; Sawyer, A.J.; Charokopos, A.; Skokos, E.a.; Kyriakides, T.R. Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFκB activation in the foreign body response. Acta Biomater. 2015, 11, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Therapeutic angiogenesis for ischemic disorders: What is missing for clinical benefits? Discov. Med. 2010, 9, 179–184. [Google Scholar] [PubMed]
- Korn, C.; Augustin, H.G. Born to Die: Blood vessel regression research coming of age. Circulation 2012, 125, 3063–3065. [Google Scholar] [CrossRef] [PubMed]
- Boersema, G.S.A.; Grotenhuis, N.; Bayon, Y.; Lange, J.F.; Bastiaansen-Jenniskens, Y.M. The Effect of Biomaterials Used for Tissue Regeneration Purposes on Polarization of Macrophages. BioRes. Open Access 2016, 5, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.M.; Lowdell, M.W.; Urbani, L.; Ansari, T.; Burns, A.J.; Turmaine, M.; North, J.; Sibbons, P.; Seifalian, A.M.; Wood, K.J.; et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA 2013, 110, 14360–14365. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.V.; Yang, H.L.; Giachelli, C.M.; Scatena, M. Engineering macrophages to control the inflammatory response and angiogenesis. Exp. Cell Res. 2015, 339, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Kreuger, J.; Phillipson, M. Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat. Rev. Drug Discov. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Anders, H.J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014, 2014, 756078. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G. Tissue inhibitors of metalloproteinases in cell signaling: Metalloproteinase-independent biological activities. Sci. Signal. 2008, 1, re6. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, M.; Parks, W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014, 7, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chiang, Y.-P.; Chuang, C.-K.; Chen, S.-L.; Hsieh, J.-W.; Lan, Y.-W.; Tsao, Y.-P. PEDF-derived peptide promotes skeletal muscle regeneration through its mitogenic effect on muscle progenitor cells. Am. J. Physiol. Cell Physiol. 2015, 309, C159–C168. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Wasmuth, H.E. Chemokines in tissue fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, S.M.; Ploeger, D.T.A.; Popa, E.R.; Bank, R.A. Macrophage phenotypes in the collagen-induced foreign body reaction in rats. Acta Biomater. 2013, 9, 6502–6510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, P.; Cui, R.; Zhang, M.; Li, H.; Qian, C.; Sheng, C.; Qu, S.; Bu, L. Eosinophils Reduce Chronic Inflammation in Adipose Tissue by Secreting Th2 Cytokines and Promoting M2 Macrophages Polarization. Int. J. Endocrinol. 2015, 2015, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Ponce, M.L.; Kleinmann, H.K. The chick chorioallantoic membrane as an in vivo angiogenesis model. Curr. Protoc. Cell Biol. 2003, 19. [Google Scholar] [CrossRef]
- Drabkin, D.L. The standardization of hemoglobin measurement. Am. J. Med. Sci. 1948, 215, 110. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.; Monaghan, M.G.; Brauchle, E.; Berrio, D.C.; Chantepie, S.; Papy-Garcia, D.; Schenke-Layland, K.; Pandit, A. Modulation of inflammation and angiogenesis and changes in ECM GAG-activity via dual delivery of nucleic acids. Biomaterials 2015, 69, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Brudno, Y.; Ennett-Shepard, A.B.; Chen, R.R.; Aizenberg, M.; Mooney, D.J. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 2013, 34, 9201–9209. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarèz Fallas, M.E.; Piccoli, M.; Franzin, C.; Sgrò, A.; Dedja, A.; Urbani, L.; Bertin, E.; Trevisan, C.; Gamba, P.; Burns, A.J.; et al. Decellularized Diaphragmatic Muscle Drives a Constructive Angiogenic Response In Vivo. Int. J. Mol. Sci. 2018, 19, 1319. https://doi.org/10.3390/ijms19051319
Alvarèz Fallas ME, Piccoli M, Franzin C, Sgrò A, Dedja A, Urbani L, Bertin E, Trevisan C, Gamba P, Burns AJ, et al. Decellularized Diaphragmatic Muscle Drives a Constructive Angiogenic Response In Vivo. International Journal of Molecular Sciences. 2018; 19(5):1319. https://doi.org/10.3390/ijms19051319
Chicago/Turabian StyleAlvarèz Fallas, Mario Enrique, Martina Piccoli, Chiara Franzin, Alberto Sgrò, Arben Dedja, Luca Urbani, Enrica Bertin, Caterina Trevisan, Piergiorgio Gamba, Alan J. Burns, and et al. 2018. "Decellularized Diaphragmatic Muscle Drives a Constructive Angiogenic Response In Vivo" International Journal of Molecular Sciences 19, no. 5: 1319. https://doi.org/10.3390/ijms19051319