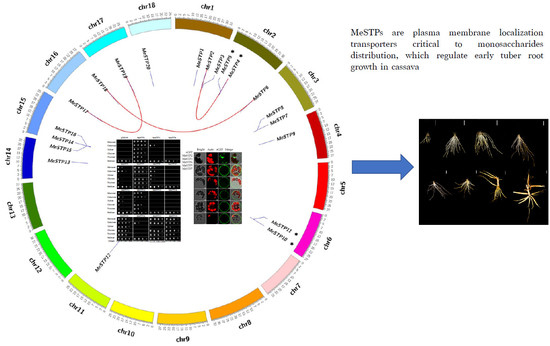
Genome-Wide Identification, Expression, and Functional Analysis of the Sugar Transporter Gene Family in Cassava (Manihot esculenta)
Abstract
:1. Introduction
2. Results
2.1. Identification of the STP Gene Family in Cassava
2.2. Sequence Structure Features of MeSTPs
2.3. Phylogenetic Analysis of MeSTP Proteins
2.4. Gene Duplication Analysis
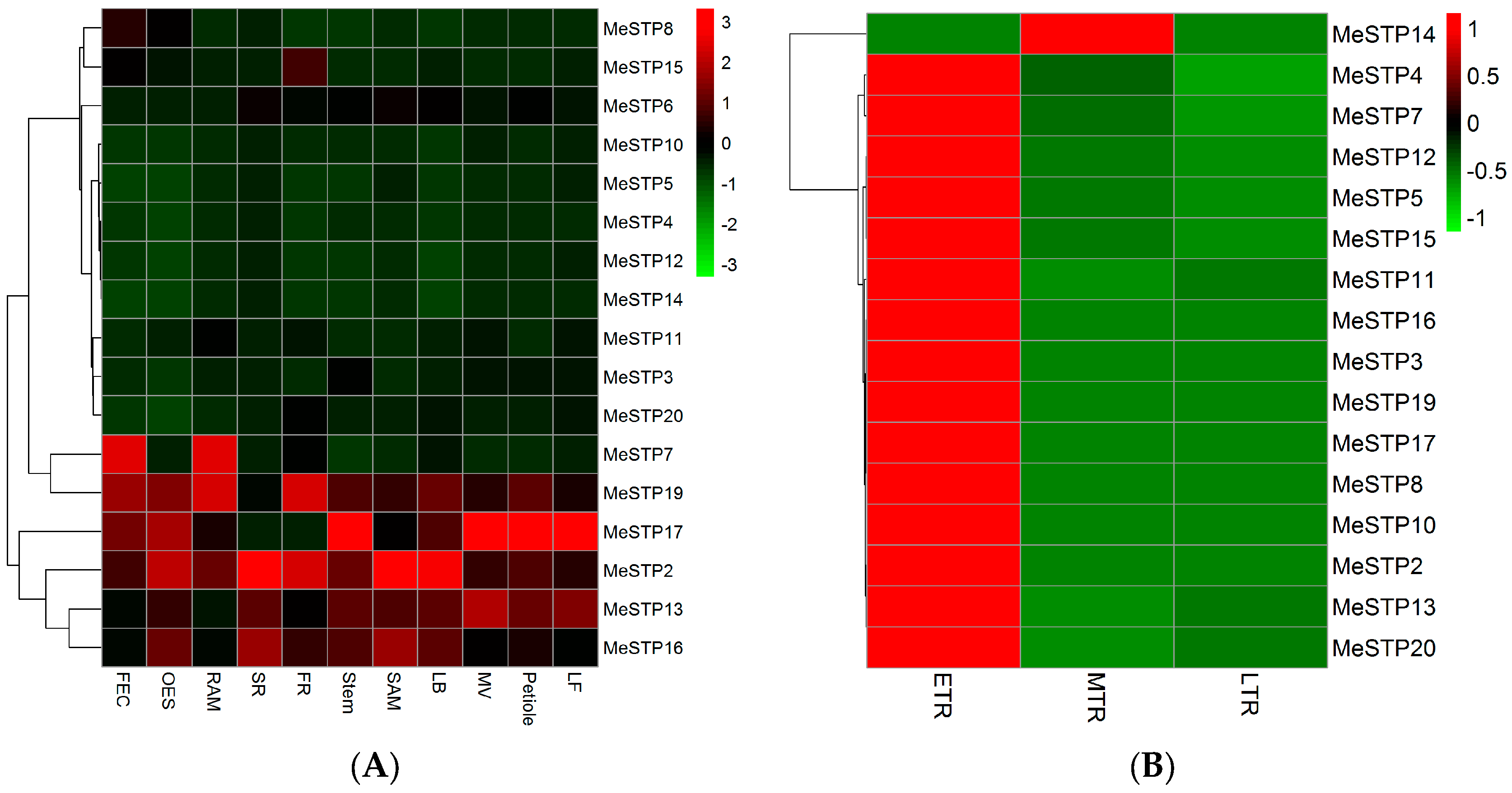
2.5. Expression Profiles of MeSTP Genes
2.6. Complementation Analysis of MeSTPs in a Yeast Strain Defective in Monosaccharide Uptake
2.7. Subcellular Localization of MeSTPs
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of the STP Genes in Cassava
4.3. Gene Structure, Conserved Motifs, and Phylogenetic Analysis
4.4. Gene Duplication Analysis
4.5. Expression Profiles of MeSTP Genes in RNA-Seq Data
4.6. Quantitative RT-PCR (qRT-PCR) Analysis of MeSTP Genes
4.7. Complementation Analysis of MeSTPs in Yeast (Saccharomyces cerevisiae)
4.8. Subcellular Localization of MeSTP
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smeekens, S.; Hellmann, H.A. Sugar sensing and signaling in plants. Front. Plant Sci. 2014, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, F.; Flügge, U.-I. Role of metabolite transporters in source-sink carbon allocation. Front. Plant Sci. 2013, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Yan, N. Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem. Sci. 2013, 38, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sauer, N.; Tanner, W. The hexose carrier from Chlorella: CDNA cloning of a eucaryotic H+-cotransporter. FEBS Lett. 1989, 259, 43–46. [Google Scholar] [CrossRef]
- Büttner, M. The Arabidopsis sugar transporter (AtSTP) family: An update. Plant Biol. 2010, 12, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, K.; Kasahara, M.; Yamaguchi, J. Characterization and expression of monosaccharide transporters (OsMSTs) in rice. Plant Cell Physiol. 2000, 41, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Thomas, M.A. The monosaccharide transporter gene family in Arabidopsis and rice: A history of duplications, adaptive evolution, and functional divergence. Mol. Biol. Evol. 2007, 24, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Afoufa-Bastien, D.; Medici, A.; Jeauffre, J.; Coutos-Thevenot, P.; Lemoine, R.; Atanassova, R.; Laloi, M. The Vitis vinifera sugar transporter gene family: Phylogenetic overview and macroarray expression profiling. BMC Plant Biol. 2010, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Reuscher, S.; Akiyama, M.; Yasuda, T.; Makino, H.; Aoki, K.; Shibata, D.; Shiratake, K. The sugar transporter inventory of tomato: Genome-wide identification and expression analysis. Plant Cell Physiol. 2014, 55, 1123–1141. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-M.; Zheng, D.-M.; Li, L.-T.; Qiao, X.; Wei, S.-W.; Bai, B.; Zhang, S.-L.; Wu, J. Genome-wide function, evolutionary characterization and expression analysis of sugar transporter family genes in pear (Pyrus bretschneideri Rehd). Plant Cell Physiol. 2015, 56, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Sherson, S.M.; Hemmann, G.; Wallace, G.; Forbes, S.; Germain, V.; Stadler, R.; Bechtold, N.; Sauer, N.; Smith, S.M. Monosaccharide/proton symporter AtSTP1 plays a major role in uptake and response of Arabidopsis seeds and seedlings to sugars. Plant J. 2000, 24, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Truernit, E.; Schmid, J.; Epple, P.; Illig, J.; Sauer, N. The sink-specific and stress-regulated Arabidopsis STP4 gene: Enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 1996, 8, 2169–2182. [Google Scholar] [CrossRef] [PubMed]
- Nørholm, M.H.; Nour-Eldin, H.H.; Brodersen, P.; Mundy, J.; Halkier, B.A. Expression of the Arabidopsis high-affinity hexose transporter STP13 correlates with programmed cell death. FEBS Lett. 2006, 580, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Büttner, M.; Truernit, E.; Baier, K.; Scholz-Starke, J.; Sontheim, M.; Lauterbach, C.; Huss, V.; Sauer, N. AtSTP3, a green leaf-specific, low affinity monosaccharide-H+ symporter of Arabidopsis thaliana. Plant Cell Environ. 2000, 23, 175–184. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M. Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 2002, 7, 352–356. [Google Scholar] [CrossRef]
- Yamada, K.; Saijo, Y.; Nakagami, H.; Takano, Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 2016, 354, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Consortium, I.C.G.M. High-resolution linkage map and chromosome-scale genome assembly for cassava (Manihot esculenta Crantz) from 10 populations. G3 Genes Genomes Genet. 2015, 5, 133–144. [Google Scholar]
- Ziska, L.H.; Runion, G.B.; Tomecek, M.; Prior, S.A.; Torbet, H.A.; Sicher, R. An evaluation of cassava, sweet potato and field corn as potential carbohydrate sources for bioethanol production in Alabama and Maryland. Biomass Bioenergy 2009, 33, 1503–1508. [Google Scholar] [CrossRef]
- Jansson, C.; Westerbergh, A.; Zhang, J.; Hu, X.; Sun, C. Cassava, a potential biofuel crop in (the) People’s Republic of China. Appl. Energy 2009, 86, S95–S99. [Google Scholar] [CrossRef]
- Wang, W.; Feng, B.; Xiao, J.; Xia, Z.; Zhou, X.; Li, P.; Zhang, W.; Wang, Y.; Møller, B.; Zhang, P. Cassava genome from a wild ancestor to cultivated varieties. Nat. Commun. 2013, 5, 5110. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.; Bernal, D.; Cortés, D.F.; Gómez-Vásquez, R.; Tohme, J.; Beeching, J.R. Towards identifying the full set of genes expressed during cassava post-harvest physiological deterioration. Plant Mol. Biol. 2007, 64, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; An, D.; Zhang, P. Expression profiling of cassava storage roots reveals an active process of glycolysis/gluconeogenesis. J. Integr. Plant Biol. 2011, 53, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Soto, M.; Restrepo, S.; Piégu, B.; Cooke, R.; Delseny, M.; Tohme, J.; Verdier, V. Gene expression profile in response to Xanthomonas axonopodis pv. manihotis infection in cassava using a cDNA microarray. Plant Mol. Biol. 2005, 57, 393–410. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Yang, J.; Zhang, P. Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genom. 2012, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Geng, M.T.; Wu, X.H.; Sun, C.; Wang, Y.-L.; Chen, X.; Shang, L.; Lu, X.-H.; Li, Z.; Li, R.M. Identification, Expression, and Functional Analysis of the Fructokinase Gene Family in Cassava. Int. J. Mol. Sci. 2017, 18, 2398. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.T.; Yao, Y.; Wang, Y.L.; Wu, X.H.; Sun, C.; Li, R.M.; Fu, S.P.; Duan, R.J.; Liu, J.; Hu, X.W.; et al. Structure, Expression, and Functional Analysis of the Hexokinase Gene Family in Cassava. Int. J. Mol. Sci. 2017, 18, 1041. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shi, H.; Xia, Z.; Tie, W.; Ding, Z.; Yan, Y.; Wang, W.; Hu, W.; Li, K. Genome-wide identification and expression analysis of the WRKY gene family in cassava. Front. Plant Sci. 2016, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, L.; Ding, Z.; Tie, W.; Ding, X.; Zeng, C.; Wei, Y.; Zhao, H.; Peng, M.; Hu, W. Genome-wide identification and expression analysis of the mitogen-activated protein kinase gene family in cassava. Front. Plant Sci. 2016, 7, 1294. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Mutka, A.M.; Hummel, A.W.; Berry, J.; Chauhan, R.D.; Vijayaraghavan, A.; Taylor, N.J.; Voytas, D.F.; Chitwood, D.H.; Bart, R.S. Gene expression atlas for the food security crop cassava. New Phytol. 2017, 213, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Heymann, J.A.; Shi, D.; Sarker, R.; Maloney, P.C.; Subramaniam, S. Three-dimensional structure of a bacterial oxalate transporter. Nat. Struct. Mol. Biol. 2002, 9, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon–intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Starke, J.; Büttner, M.; Sauer, N. AtSTP6, a new pollen-specific H+-monosaccharide symporter from Arabidopsis. Plant Physiol. 2003, 131, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.A.; Bi, Y.M.; Kant, S.; Rothstein, S.J. Over-expression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings. Plant Cell Environ. 2009, 32, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Schneidereit, A.; Scholz-Starke, J.; Sauer, N.; Büttner, M. AtSTP11, a pollen tube-specific monosaccharide transporter in Arabidopsis. Planta 2005, 221, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bredeson, J.V.; Lyons, J.B.; Prochnik, S.E.; Wu, G.A.; Ha, C.M.; Edsinger-Gonzales, E.; Grimwood, J.; Schmutz, J.; Rabbi, I.Y.; Egesi, C. Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat. Biotechnol. 2016, 34, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science. 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Purugganan, M.D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Lemoine, R.; Sauer, N. Sugar transporters in higher plants—A diversity of roles and complex regulation. Trends Plant Sci. 2000, 5, 283–290. [Google Scholar] [CrossRef]
- Gear, M.L.; McPhillips, M.L.; Patrick, J.W.; McCurdy, D.W. Hexose transporters of tomato: Molecular cloning, expression analysis and functional characterization. Plant Mol. Biol. 2000, 44, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Viola, R.; Roberts, A.G.; Haupt, S.; Gazzani, S.; Hancock, R.D.; Marmiroli, N.; Machray, G.C.; Oparka, K.J. Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. Plant Cell 2001, 13, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kanai, M.; Osakabe, Y.; Ohiraki, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Monosaccharide absorption activity of Arabidopsis roots depends on expression profiles of transporter genes under high salinity conditions. J. Biol. Chem. 2011, 286, 43577–43586. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Yang, C.; Tian, Z.; Li, J. AtSWEET4, a hexose facilitator, mediates sugar transport to axial sinks and affects plant development. Sci. Rep. 2016, 6, 24563. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J. A sugar transporter from Medicago truncatula: Altered expression pattern in roots during vesicular-arbuscular (VA) mycorrhizal associations. Plant J. 1996, 9, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wieczorke, R.; Krampe, S.; Weierstall, T.; Freidel, K.; Hollenberg, C.P.; Boles, E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999, 464, 123–128. [Google Scholar] [CrossRef]
- Morita, T.; Takegawa, K. A simple and efficient procedure for transformation of Schizosaccharomyces pombe. Yeast 2004, 21, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Songkumarn, P.; Liu, J.; Wang, G.-L. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009, 150, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Liu, Q.; Geng, X.S.; Li, K.M.; Luo, L.J.; Liu, J.P. Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz). BMC Biotechnol. 2017, 17, 29. [Google Scholar] [CrossRef] [PubMed]







| Gene Name | Gene Locus | CDS Length (bp) | AA a | MW b (kDa) | pI c | TMD d |
|---|---|---|---|---|---|---|
| MeSTP1 | Manes.01G067100 | 1560 | 519 | 57.08 | 8.51 | 11 |
| MeSTP2 | Manes.01G164600 | 1566 | 521 | 57.48 | 10.06 | 12 |
| MeSTP3 | Manes.01G182200 | 1392 | 463 | 51.28 | 9.4 | 10 |
| MeSTP4 | Manes.02G122000 | 1575 | 524 | 57.37 | 9.91 | 12 |
| MeSTP5 | Manes.02G122100 | 1536 | 511 | 56.51 | 9.08 | 11 |
| MeSTP6 | Manes.03G025000 | 1530 | 509 | 55.91 | 8.81 | 10 |
| MeSTP7 | Manes.03G180400 | 1590 | 529 | 58.25 | 9.26 | 12 |
| MeSTP8 | Manes.03G180600 | 1482 | 493 | 54.36 | 9.02 | 11 |
| MeSTP9 | Manes.04G053100 | 1545 | 514 | 56.31 | 7.44 | 12 |
| MeSTP10 | Manes.06G132600 | 1524 | 507 | 55.70 | 9.34 | 10 |
| MeSTP11 | Manes.06G132700 | 1527 | 508 | 55.37 | 9.61 | 10 |
| MeSTP12 | Manes.11G102900 | 1545 | 514 | 56.07 | 8.85 | 12 |
| MeSTP13 | Manes.14G139800 | 1548 | 515 | 55.51 | 9.08 | 11 |
| MeSTP14 | Manes.15G027100 | 1548 | 515 | 56.84 | 6.84 | 12 |
| MeSTP15 | Manes.15G027300 | 1596 | 531 | 58.54 | 8.85 | 12 |
| MeSTP16 | Manes.15G030700 | 1548 | 515 | 56.21 | 8.97 | 12 |
| MeSTP17 | Manes.16G020100 | 1575 | 524 | 57.85 | 9.16 | 12 |
| MeSTP18 | Manes.16G111500 | 1329 | 442 | 48.97 | 9.94 | 8 |
| MeSTP19 | Manes.17G033300 | 1569 | 522 | 57.58 | 8.9 | 12 |
| MeSTP20 | Manes.18G067900 | 1347 | 448 | 48.74 | 10.09 | 11 |
| Motif | Length | Protein Sequences | Pfam Domain |
|---|---|---|---|
| 1 | 80 | WSWGPLGWLVPSEIFPLETRSAGQSITVCVNMLFTFVIAQCFLTMLCHMKYGIFLFFAGWIVIMTIFVYFLLPETKNIPI | Sugar_tr |
| 2 | 82 | NNYCKYDNQYLQLFTSSLYLAALVASFFASYVTRKYGRKPSMQVGSISFCAGAILNAAAQNVWMLIIGRCLLGCGVGFANQA | Sugar_tr |
| 3 | 41 | CMIAAMGGLMFGYDIGISGGVTSMDDFLKKFFPTVYRKKHH | Sugar_tr |
| 4 | 35 | IPFFQQLTGINVIMFYAPVLFQTMGFGDDASLYSA | Sugar_tr |
| 5 | 41 | VPLYLSEMAPPKYRGALNICFQLTITIGILIANLINYGTEK | Sugar_tr |
| 6 | 41 | HPWGWRLSLGLAAVPALIMTVGSLFLPETPNSLIERGHHEE | Sugar_tr |
| 7 | 49 | VITGAVNVISTLVSMYTVDKWGRRVLFLEAGIQMFICQVAVGCCLAAHF | Sugar_tr |
| 8 | 21 | AKQVKHPWRNLMKRKYRPQLV | |
| 9 | 21 | EEMDRVWKNHWFWKRYMDDDD | |
| 10 | 21 | LKKIRGTDNVDEEFDDLVDAS | |
| 11 | 21 | LPKGYAIFVVCMICVYVAGFA | |
| 12 | 15 | AHDYEGKITPYVIVC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Dang, H.; Chen, Z.; Wu, J.; Chen, Y.; Chen, S.; Luo, L. Genome-Wide Identification, Expression, and Functional Analysis of the Sugar Transporter Gene Family in Cassava (Manihot esculenta). Int. J. Mol. Sci. 2018, 19, 987. https://doi.org/10.3390/ijms19040987
Liu Q, Dang H, Chen Z, Wu J, Chen Y, Chen S, Luo L. Genome-Wide Identification, Expression, and Functional Analysis of the Sugar Transporter Gene Family in Cassava (Manihot esculenta). International Journal of Molecular Sciences. 2018; 19(4):987. https://doi.org/10.3390/ijms19040987
Chicago/Turabian StyleLiu, Qin, Huijie Dang, Zhijian Chen, Junzheng Wu, Yinhua Chen, Songbi Chen, and Lijuan Luo. 2018. "Genome-Wide Identification, Expression, and Functional Analysis of the Sugar Transporter Gene Family in Cassava (Manihot esculenta)" International Journal of Molecular Sciences 19, no. 4: 987. https://doi.org/10.3390/ijms19040987