Renoprotective Effects of Hypoxylonol C and F Isolated from Hypoxylon truncatum against Cisplatin-Induced Cytotoxicity in LLC-PK1 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protective Effect of H. truncatum Extract against Cisplatin-Induced LLC-PK1 Cell Death
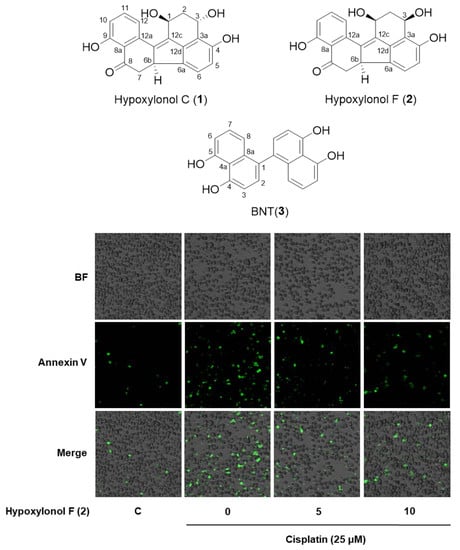
2.2. Effects of Hypoxylonol F (2) on Cisplatin-Induced Apoptosis in LLC-PK1 Cells
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Mushroom Material
3.3. Extraction and Isolation
3.4. Cell Culture and MTT Cell Viability Assay
3.5. Image-Based Cytometric Assay
3.6. Western Blotting Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Camano, S.; Lazaro, A.; Moreno-Gordaliza, E.; Torres, A.M.; de Lucas, C.; Humanes, B.; Lazaro, J.A.; Milagros Gomez-Gomez, M.; Bosca, L.; Tejedor, A. Cilastatin attenuates cisplatin-induced proximal tubular cell damage. J. Pharmacol. Exp. Ther. 2010, 334, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press 2014, 12, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Nagothu, K.K.; Bhatt, R.; Kaushal, G.P.; Portilla, D. Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int. 2005, 68, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Megyesi, J.K.; Kaneto, H.; Price, P.M.; Safirstein, R.L. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am. J. Physiol.-Renal 2004, 287, F543–F549. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, D.J.; Kim, J.H.; Jeong, E.Y.; Jung, M.H.; Kim, T.H.; Yang, J.I.; Lee, G.W.; Chung, H.J.; Chang, S.H. Glutamine protects against cisplatin-induced nephrotoxicity by decreasing cisplatin accumulation. J. Pharmacol. Sci. 2015, 127, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kruidering, M.; Van de Water, B.; de Heer, E.; Mulder, G.J.; Nagelkerke, J.F. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: Mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J. Pharmacol. Exp. Ther. 1997, 280, 638–649. [Google Scholar] [PubMed]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.K.; Cho, W.Y.; Sung, S.A.; Kim, H.K.; Won, N.H. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005, 67, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, T.F.; Choi, M.Y.; Liang, Y.W.; Xue, J.; Wong, Y.S. Cyanidin reverses cisplatin-induced apoptosis in HK-2 proximal tubular cells through inhibition of ROS-mediated DNA damage and modulation of the ERK and AKT pathways. Cancer Lett. 2013, 333, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-P.; Liu, J.-K. Progress in the Chemistry of Organic Natural Products 106; Springer: Berlin, Germany, 2017; pp. 1–201. [Google Scholar]
- Zhong, J.-J.; Xiao, J.-H. Biotechnology in China I; Springer: Berlin, Germany, 2009; pp. 79–150. [Google Scholar]
- Koyama, K.; Kuramochi, D.; Kinoshita, K.; Takahashi, K. Hypoxylonols A and B, novel reduced benzo[j]fluoranthene derivatives from the mushroom Hypoxylon truncatum. J. Nat. Prod. 2002, 65, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Tsukada, M.; Miki, K.; Suzuki, T.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Hypoxylonols C–F, Benzo [j] fluoranthenes from Hypoxylon truncatum. J. Nat. Prod. 2011, 75, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Suzuki, T.; Nagasawa, I.; Kinoshita, K.; Takahashi, K.; Koyama, K. Antiangiogenic activity of hypoxylonol C. J. Nat. Prod. 2014, 77, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Ge, H.; Song, Y.; Ding, H.; Zhu, H.L.; Zhao, X.A.; Tan, R.X. Cytotoxic benzo [j] fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J. Nat. Prod. 2007, 70, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Lockett, H.J. Constituents of the higher fungi. Part XVI. Bulgarhodin and Bulgarein, novel benzofluoranthenequinones from the fungus Bulgaria inquinans (Fries). J. Chem. Soc. Perkin Trans. 1 1976, 2149–2155. [Google Scholar] [CrossRef]
- Xiao, T.; Choudhary, S.; Zhang, W.; Ansari, N.H.; Salahudeen, A. Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. J. Toxicol. Environ. Health Part A 2003, 66, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Pongjit, K.; Ninsontia, C.; Chaotham, C.; Chanvorachote, P. Protective effect of Glycine max and Chrysanthemum indicum extracts against cisplatin-induced renal epithelial cell death. Hum. Exp. Toxicol. 2011, 30, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Kominami, K.; Nakabayashi, J.; Nagai, T.; Tsujimura, Y.; Chiba, K.; Kimura, H.; Miyawaki, A.; Sawasaki, T.; Yokota, H.; Manabe, N.; et al. The molecular mechanism of apoptosis upon caspase-8 activation: Quantitative experimental validation of a mathematical model. BBA-Mol. Cell Res. 2012, 1823, 1825–1840. [Google Scholar] [CrossRef] [PubMed]
- Turk, B.; Stoka, V. Protease signalling in cell death: Caspases versus cysteine cathepsins. FEBS Lett. 2007, 581, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Kaushal, V.; Hong, X.; Shah, S.V. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001, 60, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pabla, N.; Tang, C.; He, L.; Dong, Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch. Toxicol. 2015, 89, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Thongnuanjan, P.; Soodvilai, S.; Chatsudthipong, V.; Soodvilai, S. Fenofibrate reduces cisplatin-induced apoptosis of renal proximal tubular cells via inhibition of JNK and p38 pathways. J. Toxicol. Sci. 2016, 41, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, H.J.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, J.M. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J. Appl. Toxicol. 2005, 25, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, G.; Jiang, M.; Huang, S.; Kumar, M.V.; Messing, R.O.; Dong, Z. Inhibition of PKC delta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J. Clin. Investig. 2011, 121, 2709–2722. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, K.H.; Lee, J.; Hwang, G.S.; Lee, H.L.; Hahm, D.H.; Huh, C.K.; Lee, S.C.; Lee, S.; Kang, K.S. Protective effect of cirsimaritin against streptozotocin-induced apoptosis in pancreatic beta cells. J. Pharm. Pharmacol. 2017, 69, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kang, K.S. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J. Ginseng Res. 2017, 41, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, H.; Nam, K.; Shin, I. Glut1 promotes cell proliferation, migration and invasion by regulating epidermal growth factor receptor and integrin signaling in triple-negative breast cancer cells. BMB Rep. 2017, 50, 132–137. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, B.S.; Lee, D.; Choi, P.; Kim, K.S.; Choi, S.-J.; Song, B.G.; Kim, T.; Song, J.H.; Kang, K.S.; Ham, J. Renoprotective Effects of Hypoxylonol C and F Isolated from Hypoxylon truncatum against Cisplatin-Induced Cytotoxicity in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19, 948. https://doi.org/10.3390/ijms19040948
Hwang BS, Lee D, Choi P, Kim KS, Choi S-J, Song BG, Kim T, Song JH, Kang KS, Ham J. Renoprotective Effects of Hypoxylonol C and F Isolated from Hypoxylon truncatum against Cisplatin-Induced Cytotoxicity in LLC-PK1 Cells. International Journal of Molecular Sciences. 2018; 19(4):948. https://doi.org/10.3390/ijms19040948
Chicago/Turabian StyleHwang, Buyng Su, Dahae Lee, Pilju Choi, Kyu Sun Kim, Seon-Jun Choi, Bong Geun Song, Taejung Kim, Ji Hoon Song, Ki Sung Kang, and Jungyeob Ham. 2018. "Renoprotective Effects of Hypoxylonol C and F Isolated from Hypoxylon truncatum against Cisplatin-Induced Cytotoxicity in LLC-PK1 Cells" International Journal of Molecular Sciences 19, no. 4: 948. https://doi.org/10.3390/ijms19040948