Molecular Characterization and the Function of Argonaute3 in RNAi Pathway of Plutella xylostella
Abstract
:1. Introduction
2. Results
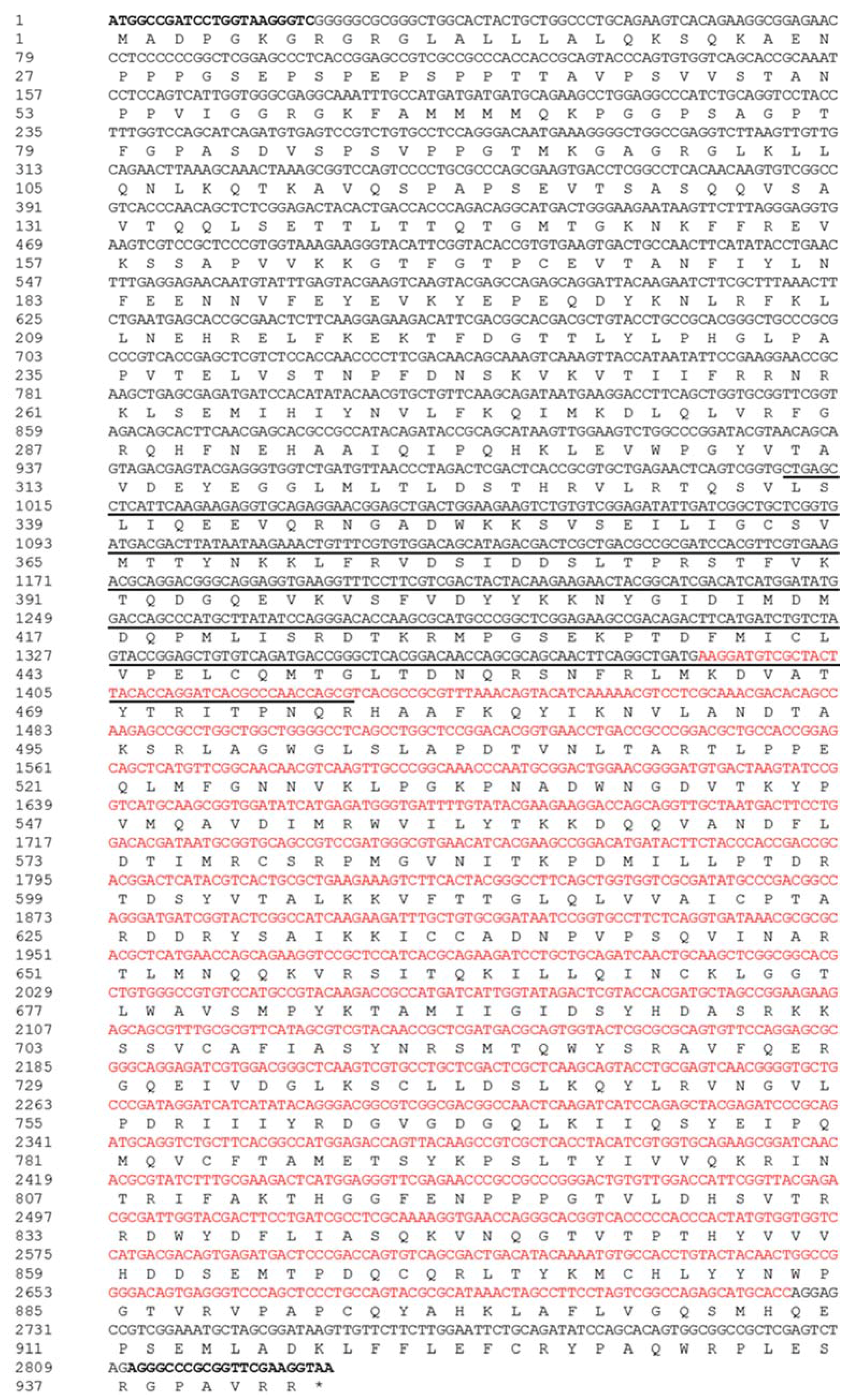
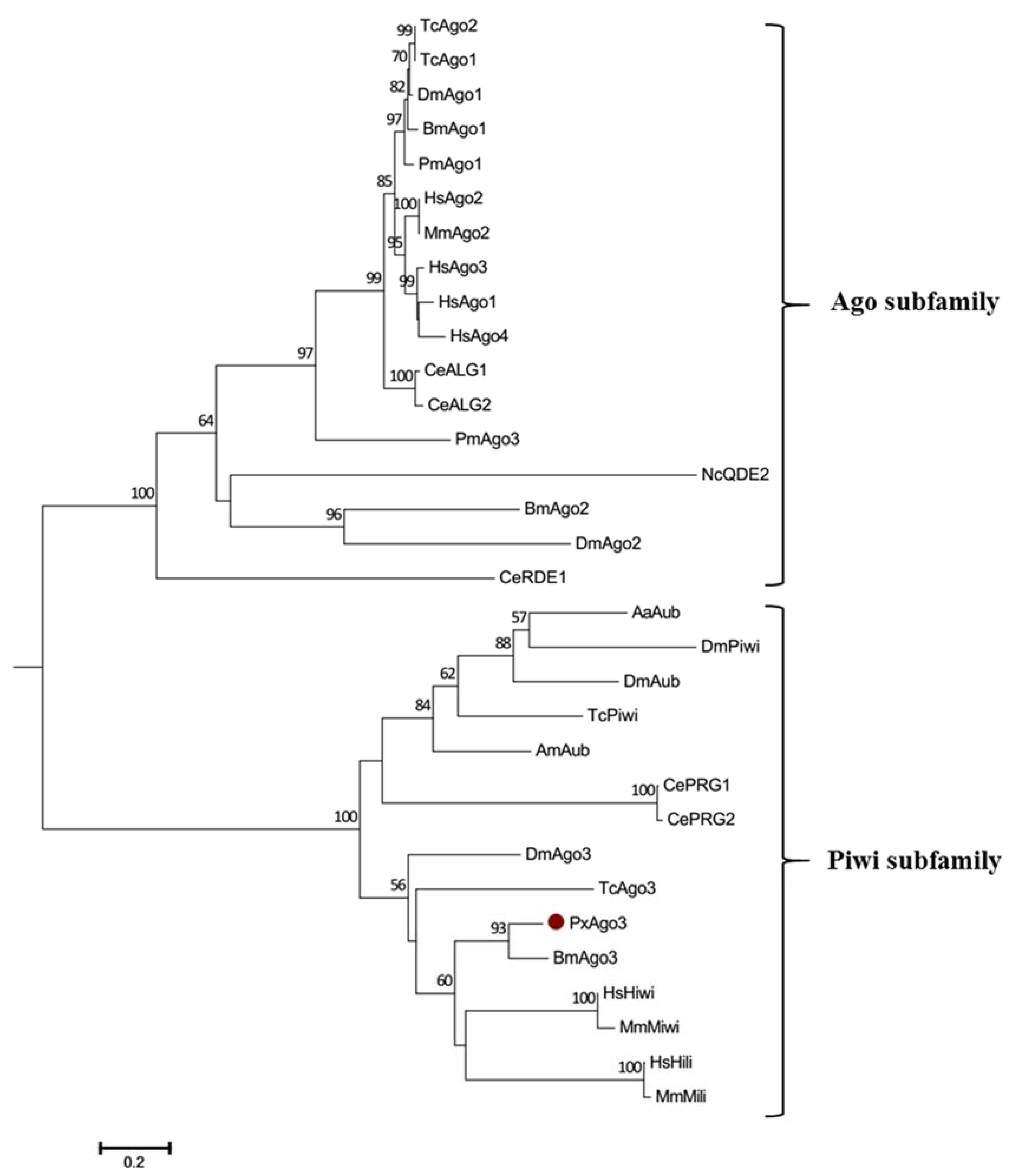
2.1. Identification and Sequence Analysis of PxAgo3 Gene
2.2. PxAgo3 Expression in Response to dsRNA in P. xylostella Larvae
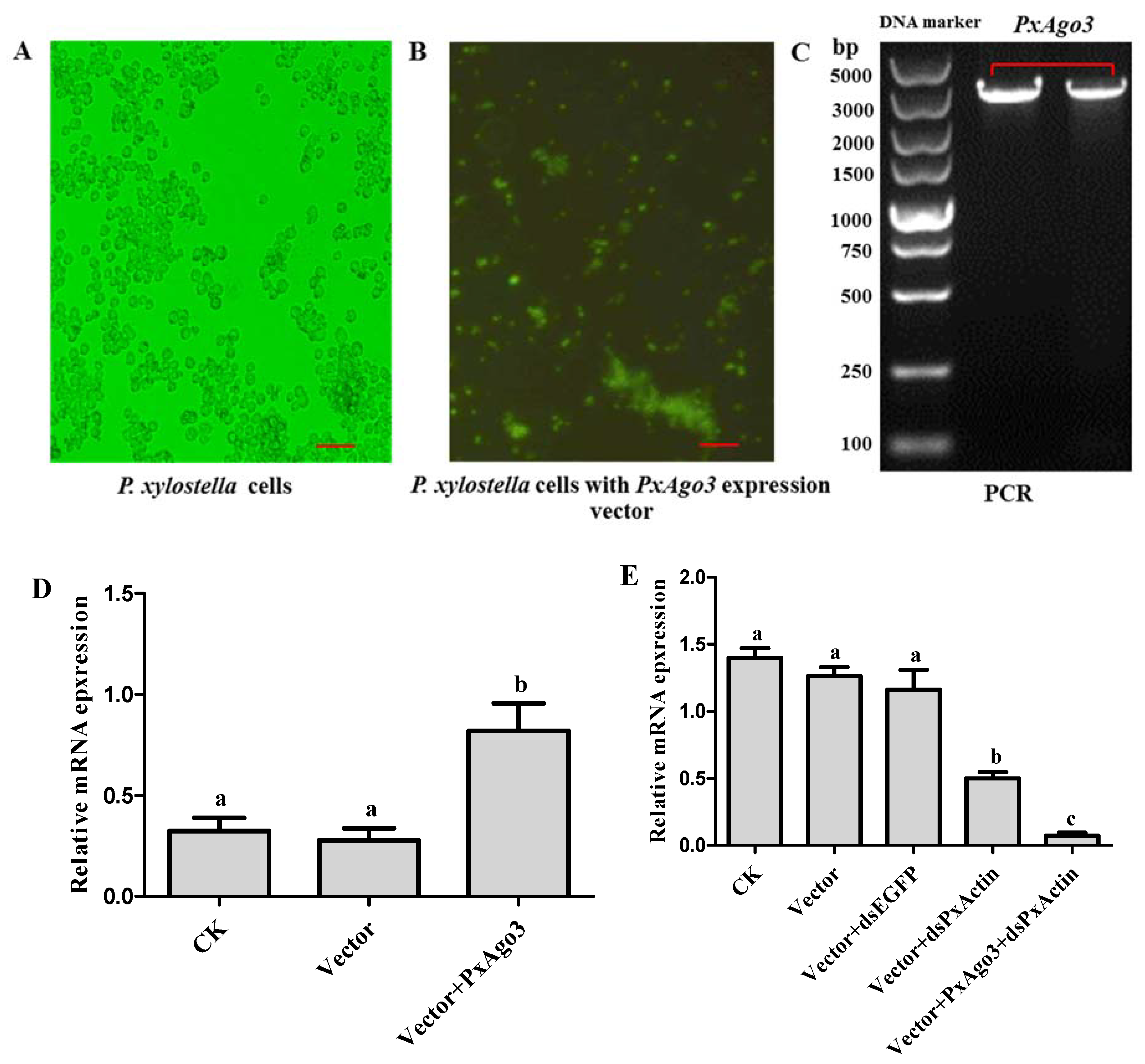
2.3. PxAgo3 Suppression Decreased the RNAi Efficiency in P. xylostella Cells
2.4. PxAgo3 Overexpression Increased the RNAi Efficiency in P. xylostella Cells
3. Discussion
4. Materials and Methods
4.1. P. xylostella Fuzhou-S Rearing and Cell Culture
4.2. Total RNAs Purification and cDNA Preparation
4.3. Molecular Cloning and Expression Vector Construction of PxAgo3
4.4. Phylogenetic Tree and Sequence Analysis
4.5. Double-Stranded RNA (dsRNA) Preparation
4.6. DsRNA Injection and Transfection into P. xylostella Larvae and Cells
4.7. RNAi of RNAi Assay in P. xylostella Cells
4.8. RT-qPCR Analysis
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; Simard, M.J. Argonaute proteins: Key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Dueck, A.; Ziegler, C.; Eichner, A.; Berezikov, E.; Meister, G. microRNAs associated with the different human Argonaute proteins. Nucleic Acids Res. 2012, 40, 9850–9862. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-J.; Liu, J.; Tolia, N.H.; Schneiderman, J.; Smith, S.K.; Martienssen, R.A.; Hannon, G.J.; Joshua-Tor, L. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat. Struct. Mol. Biol. 2003, 10, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-J.; Smith, S.K.; Hannon, G.J.; Joshua-Tor, L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 2004, 305, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Kwak, P.B.; Tomari, Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat. Struct. Mol. Biol. 2012, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lingel, A.; Simon, B.; Izaurralde, E.; Sattler, M. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature 2003, 426, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Boland, A.; Tritschler, F.; Heimstädt, S.; Izaurralde, E.; Weichenrieder, O. Crystal structure and ligand binding of the MID domain of a eukaryotic Argonaute protein. EMBO Rep. 2010, 11, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Phetrungnapha, A.; Ho, T.; Udomkit, A.; Panyim, S.; Ongvarrasopone, C. Molecular cloning and functional characterization of Argonaute-3 gene from Penaeus monodon. Fish Shellfish Immunol. 2013, 35, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Dimopoulos, G.; Zhu, J. Association of microRNAs with Argonaute proteins in the malaria mosquito Anopheles gambiae after blood ingestion. Sci. Rep. 2017, 7, 6493–6506. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-S.; Shukla, J.N.; Gong, Z.J.; Mogilicherla, K.; Palli, S.R. RNA interference in the Colorado potato beetle, Leptinotarsa decemlineata: Identification of key contributors. Insect Biochem. Mol. Biol. 2016, 78, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Masaki, Y.; Tatsuke, T.; Li, Z.; Mon, H.; Xu, J.; Lee, J.; Kusakabe, T. A MC motif in silkworm Argonaute 1 is indispensible for translation repression. Insect Mol. Biol. 2013, 22, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004, 18, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Yigit, E.; Batista, P.J.; Bei, Y.; Pang, K.M.; Chen, C.-C.G.; Tolia, N.H.; Joshua-Tor, L.; Mitani, S.; Simard, M.J.; Mello, C.C. Analysis of the Caenorhabditis elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell 2006, 127, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.W.; Rubin, G.M. ARGONAUTE1 is required for efficient RNA interference in Drosophila embryos. Proc. Natl. Acad. Sci. USA 2002, 99, 6889–6894. [Google Scholar] [CrossRef] [PubMed]
- Kawaoka, S.; Minami, K.; Katsuma, S.; Mita, K.; Shimada, T. Developmentally synchronized expression of two Bombyx mori Piwi subfamily genes, SIWI and BmAGO3 in germ-line cells. Biochem. Biophys. Res. Commun. 2008, 367, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Mituyama, T.; Huang, H.; Chen, D.; Siomi, M.C.; Siomi, H. Biogenesis pathways of piRNAs loaded onto AGO3 in the Drosophila testis. RNA 2010, 16, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Gunawardane, L.S.; Saito, K.; Nishida, K.M.; Miyoshi, K.; Kawamura, Y.; Nagami, T.; Siomi, H.; Siomi, M.C. A slicer-mediated mechanism for repeat-associated siRNA 5′end formation in Drosophila. Science 2007, 315, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Rozhkov, N.V.; Hammell, M.; Hannon, G.J. Multiple roles for Piwi in silencing Drosophila transposons. Genes Dev. 2013, 27, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Phan, H.-D.; Busch, F.; Hinckley, S.H.; Brackbill, J.A.; Wysocki, V.H.; Nakanishi, K. Human Argonaute3 has slicer activity. Nucleic Acids Res. 2017, 45, 11867–11877. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Diederichs, S. Argonaute-3 activates the let-7a passenger strand microRNA. RNA Biol. 2013, 10, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 2007, 128, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, H.; Siomi, H.; Siomi, M.C. Biology of PIWI-interacting RNAs: New insights into biogenesis and function inside and outside of germlines. Genes Dev. 2012, 26, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Khurana, J.S.; Xu, J.; Weng, Z.; Theurkauf, W.E. Distinct functions for the Drosophila piRNA pathway in genome maintenance and telomere protection. PLoS Genet. 2010, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Vagin, V.V.; Lee, S.; Xu, J.; Ma, S.; Xi, H.; Seitz, H.; Horwich, M.D.; Syrzycka, M.; Honda, B.M. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell 2009, 137, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Mingels, L.; Cordeiro dos Santos, D.; Wynant, N.; Vanden Broeck, J.; Swevers, L. RNAi-based interactions: A latent viral infection in a lepidopteran cell line. In Proceeding of the 28th Conference of European Comparative Endocrinologists, Leuven, Belgium, 21–25 August 2016. [Google Scholar]
- Phillips, A. Diamondback Moth: A Key Pest of Cruciferous Crops; Cooperative Extension Report; Cornell University Publishers: Ithaca, NY, USA, 1983; pp. 4–12. [Google Scholar]
- Wang, Y.; Xu, T.; He, W.; Shen, X.; Zhao, Q.; Bai, J.; You, M. Genome-wide identification and characterization of putative lncRNAs in the diamondback moth, Plutella xylostella (L.). Genomics 2018, 110, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, L.; Qi, J.; Hu, M.; Zhong, G. Molecular cloning and characterization of a SID-1-like gene in Plutella xylostella. Arch. Insect Biochem. Physiol. 2014, 87, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Roe, S.M.; Barford, D. Structural insights into mRNA recognition from a PIWI domain–siRNA guide complex. Nature 2005, 434, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Szulwach, K.E.; Zhang, G.; Jin, P.; Chen, D. AGO3 Slicer activity regulates mitochondria–nuage localization of Armitage and piRNA amplification. J. Cell Biol. 2014, 206, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Jiang, L.; Zhu, L.; Cheng, T.C.; Niu, W.H.; Yan, Y.F.; Xia, Q.Y. Characterization of Argonaute family members in the silkworm, Bombyx mori. Insect Sci. 2013, 20, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Tatsuke, T.; Sakashita, K.; Masaki, Y.; Lee, J.M.; Kawaguchi, Y.; Kusakabe, T. The telomere-specific non-LTR retrotransposons SART1 and TRAS1 are suppressed by Piwi subfamily proteins in the silkworm, Bombyx mori. Cell. Mol. Biol. Lett. 2010, 15, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Black, W.C.; Hess, A.M.; Foy, B.D. Comparative genomics of small RNA regulatory pathway components in vector mosquitoes. BMC Genom. 2008, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Rifo, A.; Jannot, G.; Armisen, J.; Labouesse, M.; Bukhari, S.I.A.; Rondeau, E.L.; Miska, E.A.; Simard, M.J. Developmental characterization of the microRNA-specific Caeborhabditis elegans Argonautes alg-1 and alg-2. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.G.; Kingston, R.E.; Lau, N.C. The coming of age for Piwi proteins. Mol. Cell 2007, 26, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ruda, V.M.; Chandwani, R.; Sehgal, A.; Bogorad, R.L.; Akinc, A.; Charisse, K.; Tarakhovsky, A.; Novobrantseva, T.I.; Koteliansky, V. The roles of individual mammalian argonautes in RNA interference in vivo. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Donald, C.L.; Human, S.; Watson, M.; Siu, R.W.; McFarlane, M.; Fazakerley, J.K.; Kohl, A.; Fragkoudis, R. Knockdown of piRNA pathway proteins results in enhanced Semliki Forest virus production in mosquito cells. J. Gen. Virol. 2013, 94, 1680–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, I.; Jansen, S.; Fall, G.; Lorenzen, S.; Rudolf, M.; Huber, K.; Heitmann, A.; Schicht, S.; Watson, M.; Castelli, I. RNA interference restricts Rift Valley Fever virus in multiple insect systems. mSphere 2017, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, D.; Sykora, M.M.; Sachidanandam, R.; Mechtler, K.; Brennecke, J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J. 2010, 29, 3301–3317. [Google Scholar] [CrossRef] [PubMed]
- Kolliopoulou, A.; Swevers, L. Functional analysis of the RNAi response in ovary-derived silkmoth Bm5 cells. Insect Biochem. Mol. Biol. 2013, 43, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zeng, B.; Ling, L.; Xu, J.; You, L.; Aslam, A.F.; Tan, A.; Huang, Y. Enhancement of larval RNAi efficiency by over-expressing Argonaute2 in Bombyx mori. Int. J. Biol. Sci. 2015, 11, 176–185. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.L.; He, W.Y.; Wang, P.; You, M.S. Cell lines from diamondback moth exhibiting differential susceptibility to baculovirus infection and expressing midgut genes. Insect Sci. 2017. [Google Scholar] [CrossRef] [PubMed]



| Primer | Sequence | Use |
|---|---|---|
| PxAgo3-F | 5′ ATGGCCGATCCTGGTAAGGGTC 3′ | PCR |
| PxAgo3-R | 5′ TTACCTTCGAACCGCGGGCCCT 3′ | PCR |
| PxAgo3 KpnI-F1 | 5′ CGGGGTACCATGGCCGATCCTGGTAAGG 3′ | PCR |
| PxAgo3 EcoRI-R1 | 5′ CCGGAATTCCAAGAAGAACAACTTATCCGC 3′ | PCR |
| PIZT-F | 5′ CGCAACGATCTGGTAAACAC 3′ | PCR |
| PIZT-R | 5′ CTGACTAAATCTTAGTTTGTATTGTC 3′ | PCR |
| PxAgo3-F1 | 5′ GCCGCCATACAGATACCG 3′ | RT-qPCR |
| PxAgo3-R1 | 5′ AACATCAGACCACCCTCG 3′ | RT-qPCR |
| PxActin-F2 | 5′ GGAGTGATGGTCGGTATGGGA 3′ | RT-qPCR |
| PxActin-R2 | 5′ CTCGATGGGGTACTTCAGGGT 3′ | RT-qPCR |
| RL32-F | 5′ CAATCAGGCCAATTTACCGC 3′ | RT-qPCR |
| RL32-R | 5′ CTGGGTTTACGCCAGTTACG 3′ | RT-qPCR |
| dsAgo3-F | 5′ GTCCGCTCCATCACGCAGAAA 3′ | dsRNA Preparation |
| dsAgo3-R | 5′ GCGGGATCTCGTAGCTCTGGA 3′ | dsRNA Preparation |
| dsActin-F | 5′ GCCAACCGTGAAAAGATGA 3′ | dsRNA Preparation |
| dsActin-R | 5′ AGGAATGAGGGCTGGAACA 3′ | dsRNA Preparation |
| dsEGFP-F | 5′ GTGTTCAATGCTTTTCCCGTTATCC 3′ | dsRNA Preparation |
| dsEGFP-R | 5′ ACCATGTGGTCACGCTTTTCG 3′ | dsRNA Preparation |
| Organism | Protein | Abbreviation | Acc. Number |
|---|---|---|---|
| Tribolium castaneum | Argonaute-1 | TcAgo1 | KYB26000.1 |
| Argonaute-2 | TcAgo2 | XP_015837988.1 | |
| Argonaute 3 | TcAgo3 | EFA02921.1 | |
| PIWI | TcPIWI | EFA07425.1 | |
| Drosophila melanogaster | Argonaute-1 | DmAgo1 | AAF58313.1 |
| Argonaute-2 | DmAgo2 | NP_730054.1 | |
| Argonaute-3 | DmAgo3 | EAA45981.3 | |
| Aubergine | DmAub | CAA64320.1 | |
| PIWI | DmPIWI | AGL81541.1 | |
| Bombyx mori | Argonaute-1 | BmAgo1 | NP_001095931.1 |
| Argonaute-2 | BmAgo2 | BAD91160.2 | |
| Argonaute-3 | BmAgo3 | A9ZSZ2 | |
| Caenorhabditis elegans | CeALG-1 | CeALG1 | CAR97837.1 |
| CeALG-2 | CeALG2 | CCD73271.1 | |
| CePRG-1 | CePRG1 | CAA98113.1 | |
| CePRG-2 | CePRG2 | CCD62443.1 | |
| CeRDE-1 | CeRDE1 | CAB05546 | |
| Homo sapiens | Argonaute-1 | HsAgo1 | NP_036331.1 |
| Argonaute-2 | HsAgo2 | NP_036286.2 | |
| Argonaute-3 | HsAgo3 | NP_079128.2 | |
| Argonaute-4 | HsAgo4 | NP_060099.2 | |
| HsHiwi | HsHiwi | AAC97371.2 | |
| HsHili | HsHili | NP060538.2 | |
| Penaeus monodon | Argonaute-1 | PmAgo1 | ABG66641.1 |
| Argonaute-3 | PmAgo3 | AGC95229.1 | |
| Apis mellifera | Aubergine | AmAub | NP_001159378.1 |
| Aedes aegypti | Aubergine | AaAub | XP_021707306.1 |
| Neurospora crassa | NcQDE-2 | NcQDE2 | AAF43641 |
| Mus musculus | Argonaute-2 | MmAgo2 | NP_694818 |
| MmMiwi | MmMiwi | BAM37459 | |
| MmMili | MmMili | BAA93706 | |
| Plutella xylostella | Argonaute-3 | PxAgo3 | MG778697 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, M.S.; Wang, Z.; Vasseur, L.; Yang, G. Molecular Characterization and the Function of Argonaute3 in RNAi Pathway of Plutella xylostella. Int. J. Mol. Sci. 2018, 19, 1249. https://doi.org/10.3390/ijms19041249
Hameed MS, Wang Z, Vasseur L, Yang G. Molecular Characterization and the Function of Argonaute3 in RNAi Pathway of Plutella xylostella. International Journal of Molecular Sciences. 2018; 19(4):1249. https://doi.org/10.3390/ijms19041249
Chicago/Turabian StyleHameed, Muhammad Salman, Zhengbing Wang, Liette Vasseur, and Guang Yang. 2018. "Molecular Characterization and the Function of Argonaute3 in RNAi Pathway of Plutella xylostella" International Journal of Molecular Sciences 19, no. 4: 1249. https://doi.org/10.3390/ijms19041249





