CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization
Abstract
:1. Introduction
2. Results
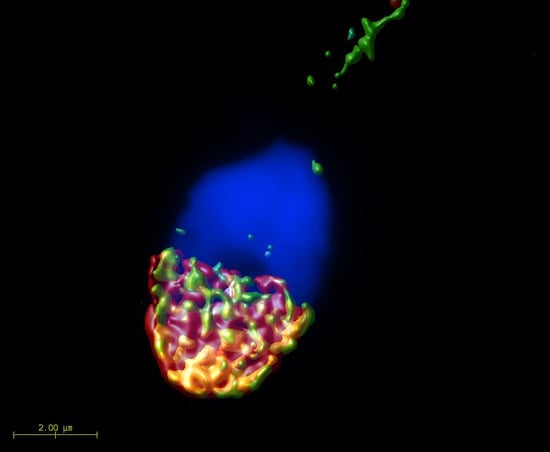
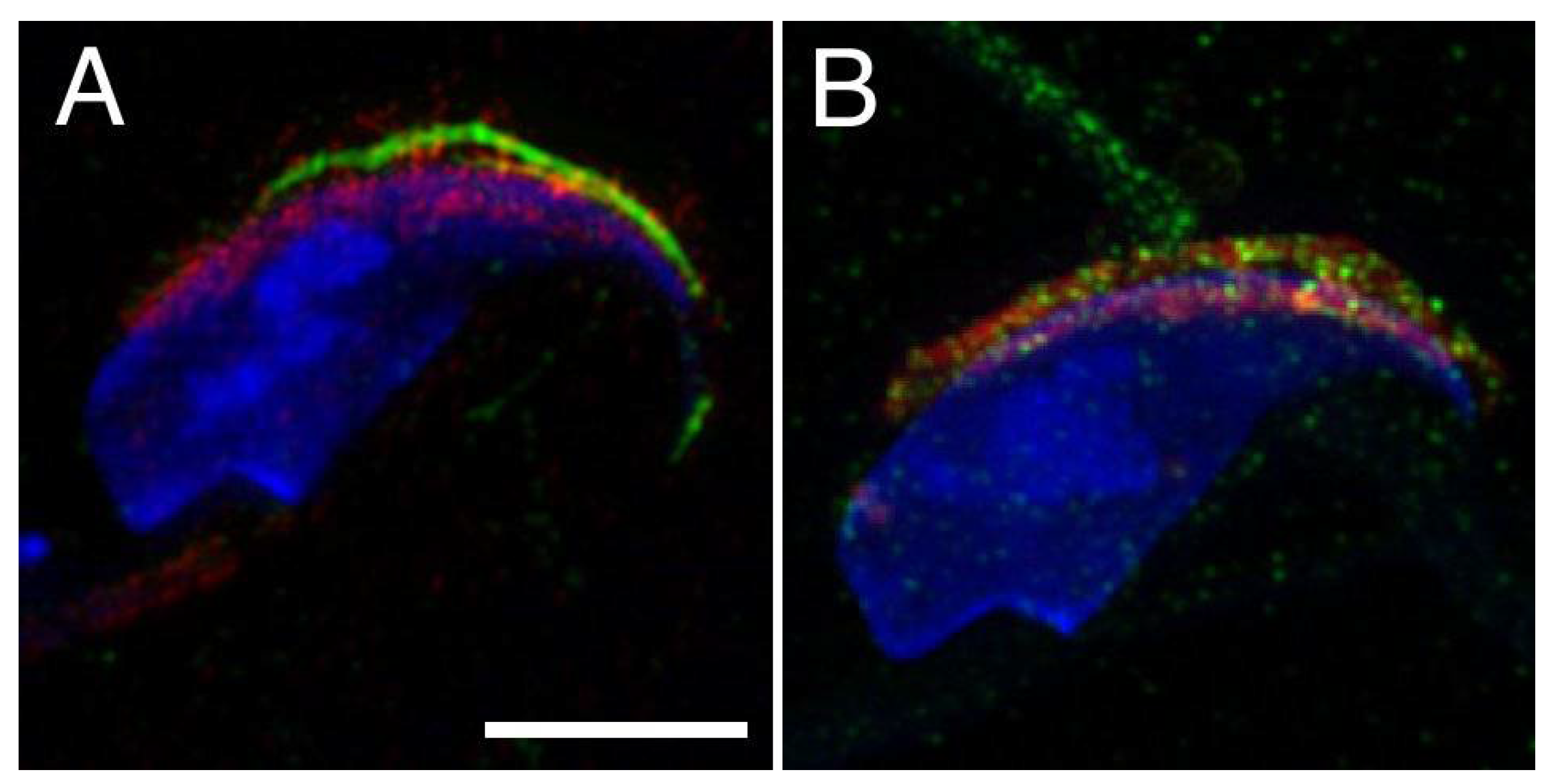
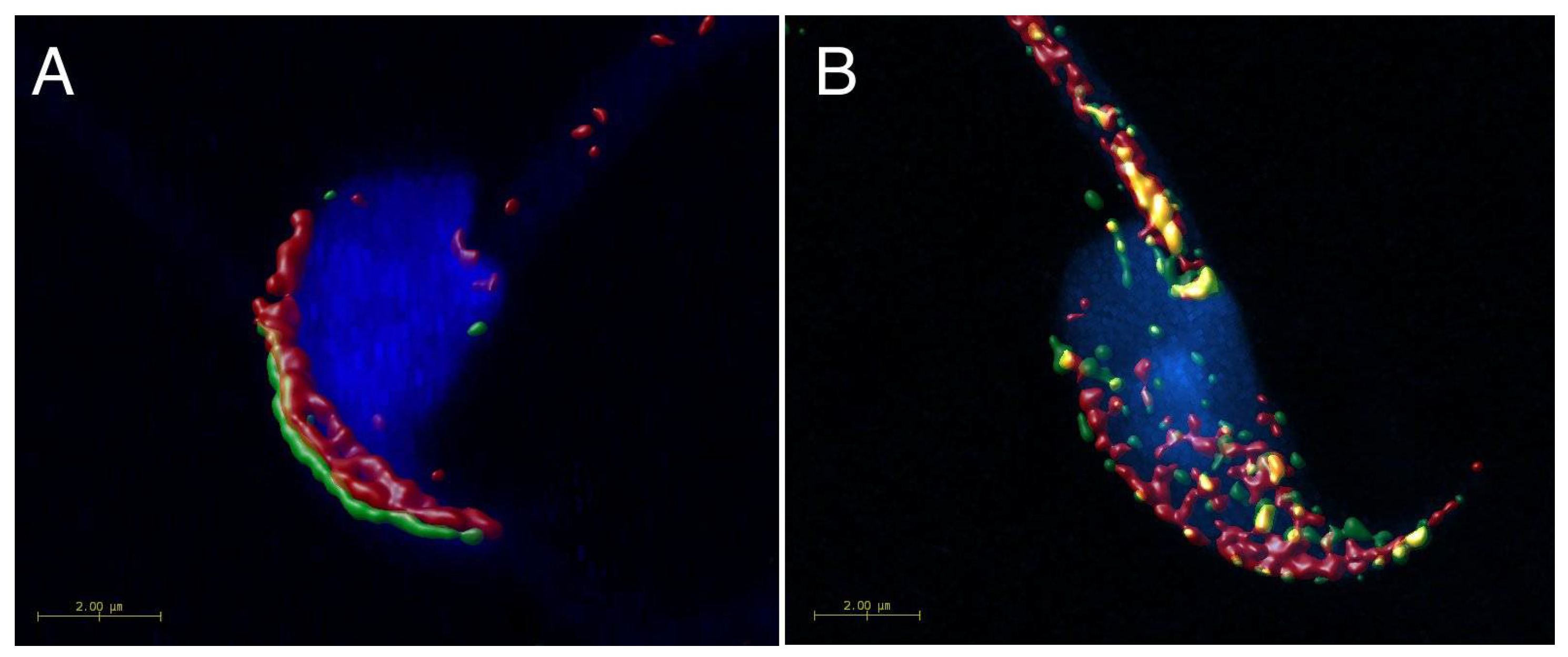
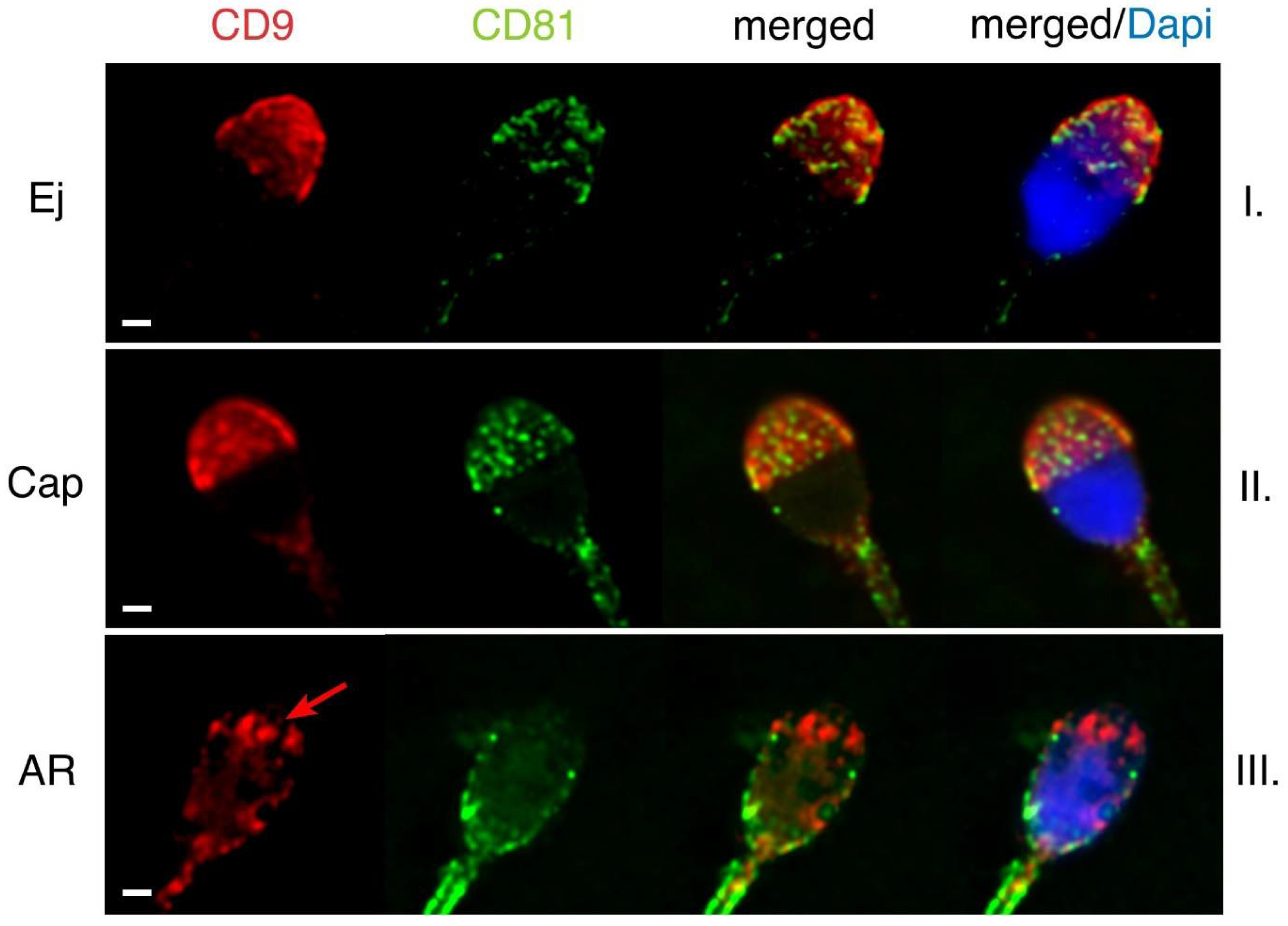
2.1. Mutual Position of CD9 and CD81 on Mouse Sperm Head
2.2. Mutual Localization of CD9 and CD81 in Human Sperm Head
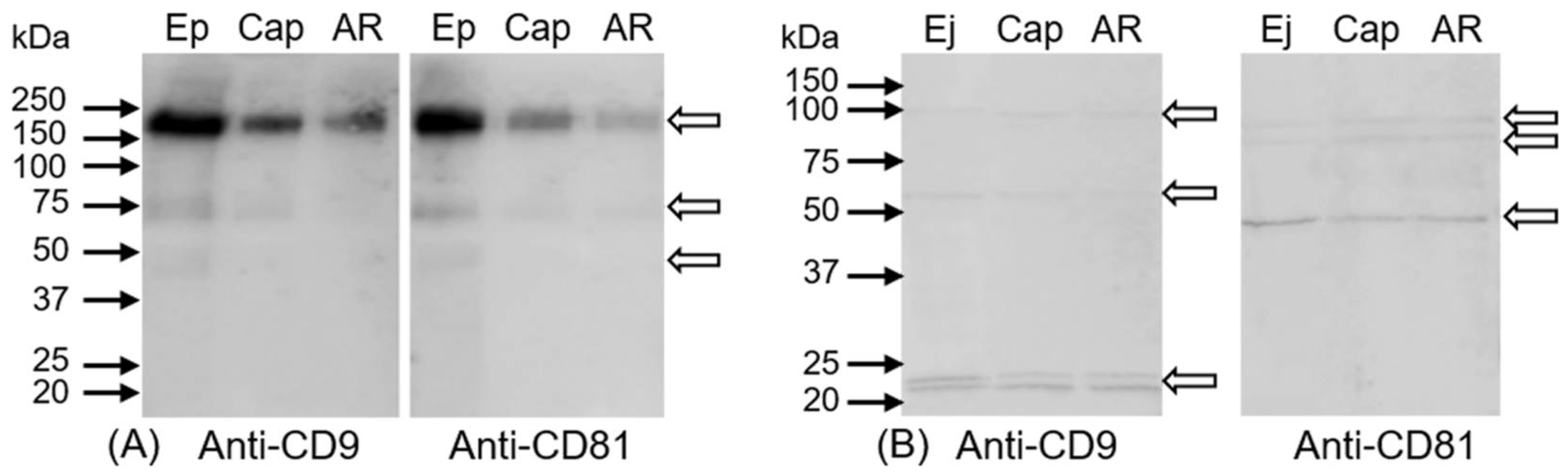
2.3. Immunoprecipitation of CD9 and CD81 Complex from Spermatozoa
2.4. Determination of Intra-Molecular Disulphide Bonds in CD9 and CD81 Tetraspanins
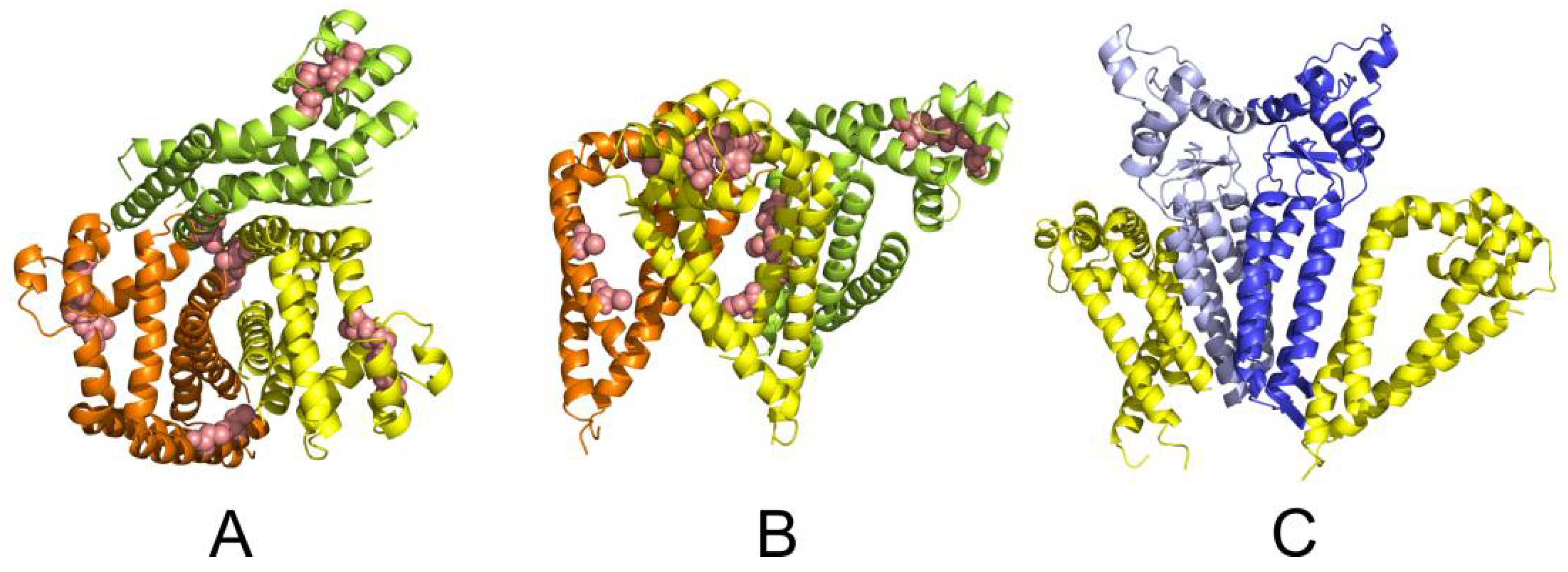
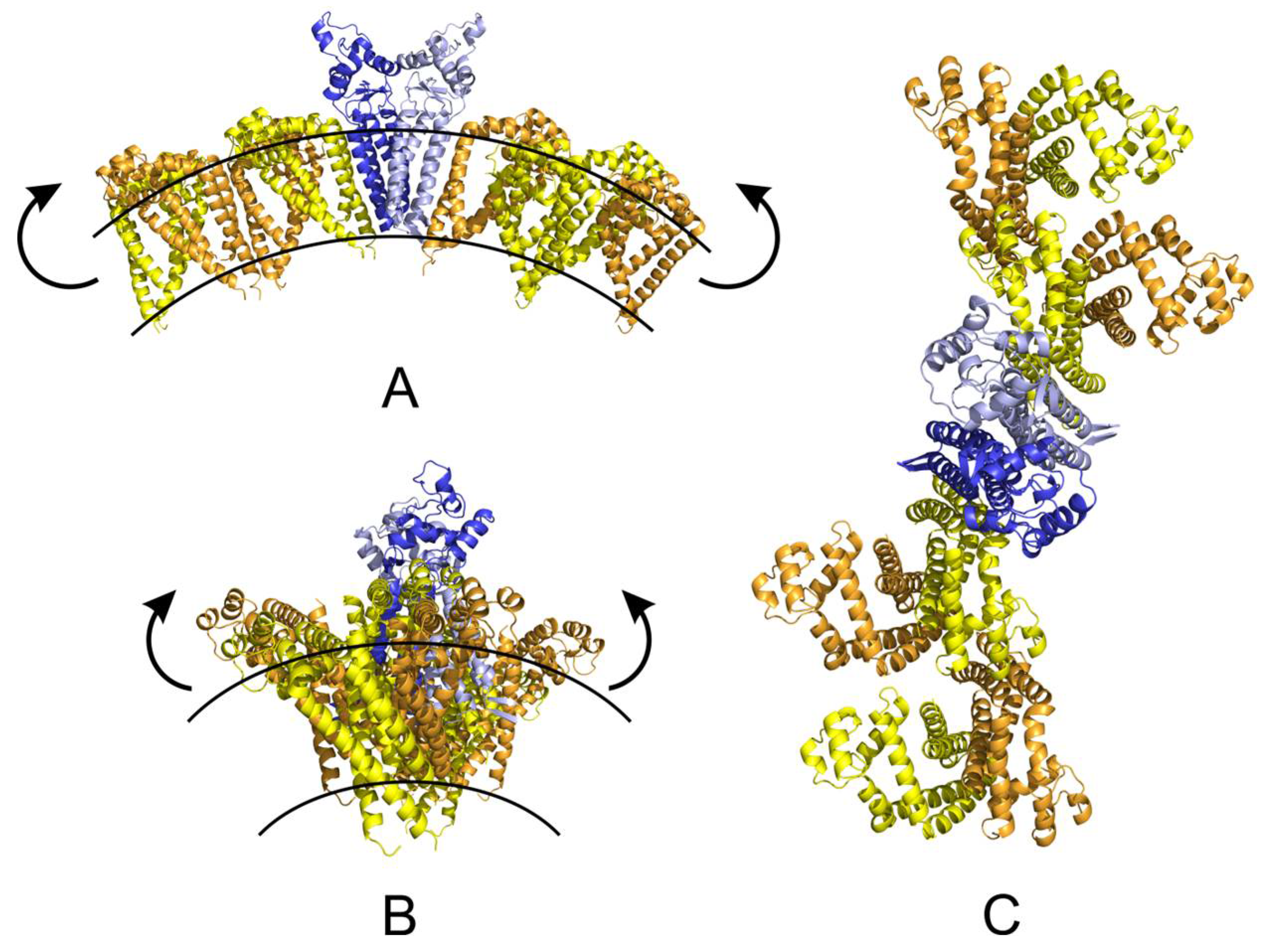
2.5. Molecular Modelling
3. Discussion
4. Materials and Methods
4.1. Primary Antibodies
4.2. Animals
4.3. Human Samples
4.4. Capacitation and Acrosome Reaction
4.4.1. Mouse Sperm
4.4.2. Human Sperm
4.5. Dual Immunofluorescent Staining of CD81 and CD9
4.6. Super-Resolution Microscopy
4.7. Protein Extraction
4.8. Co-Immunoprecipitation of CD9 and CD81 Molecules
4.9. SDS-PAGE with Immunoblotting
4.10. Molecular Modelling of CD9 and CD81 Tetraspanin Web
4.11. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stipp, C.S.; Orlicky, D.; Hemler, M. FPRP, a major, highly stoichiometric, highly specific CD81-and CD9-associated protein. J. Biol. Chem. 2001, 276, 4853–4862. [Google Scholar] [CrossRef] [PubMed]
- Van Spriel, A.B.; Figdor, C.G. The role of tetraspanins in the pathogenesis of infectious diseases. Microbes Infect. 2010, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Boucheix, C.; Rubinstein, E. Tetraspanins. Cell. Mol. Life Sci. 1997, 58, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; le Naour, F.; Silvie, O.; Milhiet, P.E.; Boucheix, C.; Rubinstein, E. Lateral organization of membrane proteins: Tetraspanins spin their web. Biochem. J. 2009, 420, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Shoham, T. The tetraspanin web modulates immune-signaling complexes. Nat. Rev. Immunol. 2005, 5, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspains at a glance. J. Cell Sci. 2014, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F.; Odintsova, E. Characterization of Integrin–Tetraspanin Adhesion Complexes: Role of Tetraspanins in Integrin Signaling. J. Cell Biol. 1999, 146, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Hemler, M.E. Transmembrane-4-superfamily proteins CD151 and CD81 associate with alpha 3 beta 1 integrin, and selectively contribute to alpha 3 beta 1-dependent neurite outgrowth. J. Cell Sci. 2000, 113 Pt 11, 1871–1882. [Google Scholar] [PubMed]
- Bassani, S.; Cingolani, L.A. Tetraspanins: Interactions and interplay with integrins. Int. J. Biochem. Cell Biol. 2012, 44, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Miyado, K.; Yamada, G.; Yamada, S.; Hasuwa, H.; Nakamura, Y.; Ryu, F.; Suzuki, K.; Kosai, K.; Inoue, K.; Ogura, A.; et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 2000, 287, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Ziyyat, A.; Rubinstein, E.; Monier-Gavelle, F.; Barraud, V.; Kulski, O.; Prenant, M.; Boucheix, C.; Bomsel, M.; Wolf, J.P. CD9 controls the formation of clusters that contain tetraspanins and the integrin alpha 6 beta 1, which are involved in human and mouse gamete fusion. J. Cell Sci. 2006, 119 Pt 3, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Frolikova, M.; Sebkova, N.; Ded, L.; Dvorakova-Hortova, K. Characterization of CD46 and beta1 integrin dynamics during sperm acrosome reaction. Sci. Rep. 2016, 6, 33714. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Tejedor, R.; Rouselle, P.; Sanches-Madrid, F. Tetraspanins in intercellular adhesion of polarized epithelial cells: Spatial and functional relationship to integrins and cadherins. J. Cell Sci. 2001, 144, 577–587. [Google Scholar]
- Jégou, A.; Ziyyat, A.; Barraud-Lange, V.; Perez, E.; Wolf, J.P.; Pincet, F.; Gourier, C. CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Ellerman, D.A.; Ha, C.; Primakoff, P.; Myles, D.G.; Dveksler, G.S. Direct binding of the ligand PSG17 to CD9 requires a CD9 site essential for sperm-egg fusion. Mol. Biol. Cell 2003, 14, 5098–5103. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, O.; Yang, X.; Kolesnikova, T.V.; Hemler, M.E. Evidence for specific tetraspanin homodimers: Inhibition of palmitoylation makes cysteine residues available for cross-linking. Biochem. J. 2004, 377, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, O.; Zamai, M.; Yáñez-Mó, M.; Tejera, E.; López-Romero, P.; Monk, P.N.; Gratton, E.; Caiolfa, V.R.; Sánchez-Madrid, F. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J. Cell Biol. 2008, 183, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Maekawa, M.; Miyado, K.; Toshimori, K. Tetraspanin family protein CD9 in the mouse sperm: Unique localization, appearance, behavior and fate during fertilization. Cell Tissue Res. 2010, 340, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Jankovicova, J.; Frolikova, M.; Sebkova, N.; Simon, M.; Cupperova, P.; Lipcseyova, D.; Michalkova, K.; Horovska, L.; Sedlacek, R.; Stopka, P.; et al. Characterization of tetraspanin protein CD81 in mouse spermatozoa and bovine gametes. Reproduction 2000, 152, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Ziyyat, A.; Prenant, M.; Wrobel, E.; Wolf, J.P.; Levy, S.; Le Naour, F.; Boucheix, C. Reduced fertility of female mice lacking CD81. Dev. Biol. 2006, 290, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, F.; Rubinstein, E.; Jasmin, C.; Prenant, M.; Boucheix, C. Severely reduced female fertility in CD9-deficient mice. Science 2000, 287, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Messini, C.; Dafopoulos, K.; Sotiriou, S.; Messinis, I. Molecular and cellular mechanisms of sperm-oocyte interactions opinions relative ti in vitro fertilization (IVF). Int. J. Mol. Sci. 2014, 15, 12972–12997. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.; Kelly, B.; McMilla, B.J.; Seegar, T.C.M.; Dror, R.O.; Kruse, A.C.; Blacklow, S.C. Crystal structure of a full-length human tetraspanin reveals a cholesterol-binding pocket. Cell 2016, 167, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.A.; Huang, C. Tetraspanins and cell membrane tubular structures. Cell. Mol. Life Sci. 2012, 69, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Żyłkiewicz, E.; Nowakowska, J.; Maleszewski, M. Decrease in CD9 content and reorganization of microvilli may contribute to the oolemma block to sperm penetration during fertilization of mouse oocyte. Zygote 2010, 18, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.J. Sperm-Egg interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F.; Odintsova, E.; Sawada, S.; Gilbert, E. Expression of the palmitoylation-deficient CD151 weakens the association of α3β1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signalling. J. Biol. Chem. 2002, 277, 36991–37000. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Manié, S.; Oualid, M.; Billard, M.; Boucheix, C.; Rubinstein, E. Differential stability of tetraspanin/tetraspanin inetractions: Role of palmitoylation. FEBS Lett. 2002, 516, 139–144. [Google Scholar] [CrossRef]
- Charrin, S.; Maniá, S.; Thiele, C.; Billard, M.; Gerlier, D.; Boucheix, C.; Rubinstein, E. A physical and functional link between cholesterol and tetraspanins. Eur. J. Immunol. 2003, 33, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, E.; Butters, T.D.; Monti, E.; Sprong, H.; van Meer, G.; Berditchevski, F. Gangliosides play an important role in the organization of CD82-enriched microdomains. Biochem. J. 2006, 400, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Claas, C.; Kraeft, S.K.; Chen, J.B.; Wang, Z.; Kreidberg, J.A.; Hemler, M.E. Palmitoylation of tetraspanin proteins: Modulation of CD151 latera interactions, Subcellular distribution, and integrin-dependent cell morphology. Mol. Biol. Cell 2002, 13, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. The Physiology of Reprodution; Knobil, J.D.N., Ed.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Sleight, S.B.; Miranda, P.V.; Plaskett, N.W.; Maier, B.; Lysiak, J.; Scrable, H.; Herr, J.; Visconti, P.E. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: Evidence for dissociation of lipid rafts during capacitation. Biol. Reprod. 2005, 73, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Hogue, I.B.; Grover, J.R.; Soheilian, F.; Nagashima, K.; Ono, A. Gag induces the coalescence of clustered lipid rafts and tetraspanin-enriched domains at HIV-1 assembly sites on plasma membrane. J. Virol. 2011, 85, 9749–9766. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, N.; Carmona, E.; Khalil, M.B.; Xu, H.; Berger, T.; Gerton, G. New insights into sperm-zona pellucida interaction: Involvement of sperm lipid rafts. Front. Biosci. 2007, 12, 1748–1766. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, J.; Buffone, M.G.; Visconti, P.E. Analysis of CAPAZA3 localization reveals temporally discrete events during the acrosome reaction. J. Cell. Phys. 2010, 224, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Satouh, Y.; Ikawa, M.; Okabe, M.; Yanagimachi, R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 2011, 108, 20008–20011. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. EWI-2 is a major CD9 and CD81 partner and membber of a novel Ig protein subfamily. J. Biol. Chem. 2001, 276, 40545–40554. [Google Scholar] [CrossRef] [PubMed]
- Sala-Valdés, M.; Ursa, A.; Charrin, S.; Rubinstein, E.; Hemler, M.E.; Sánchez-Madrid, F.; Yáñez-Mó, M. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J. Biol. Chem. 2006, 281, 19665–19675. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Serru, V.; Clay, D.; Billard, M.; Boucheix, C.; Rubinstein, E. CD19 is linked to the integrin-associated tetraspans CD9, CD81, and CD82. J. Biol. Chem. 1998, 273, 30537–30543. [Google Scholar] [CrossRef] [PubMed]
- Sebkova, N.; Cerna, M.; Ded, L.; Peknicova, J.; Dvorakova-Hortova, K. The slower the better: How sperm capacitation and acrosome reaction is modified in the presence of estrogens. Reproduction 2012, 143, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ivankin, A.; Kuzmenko, I.; Gidalevitz, D. Cholesterol mediates membrane curvature during fusion events. Phys. Rev. Lett. 2012, 108, 238103. [Google Scholar] [CrossRef] [PubMed]
- Buschiazzo, J.; Ialy-Radio, C.; Auer, J.; Wolf, J.P.; Serres, C.; Lefevre, B.; Ziyyat, A. Cholesterol depletion disorganizes oocyte membrane rafts altering mouse fertilization. PLoS ONE 2013, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some application. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2014, 47. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, T. Effective energy function for proteins in lipid membranes. Proteins 2003, 52, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L., 3rd; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, L.L.C. The PyMOL Molecular Graphics System, Version 1.8; 2015. Available online: https://pymol.org/2/support.html?#citing (accessed on 4 December 2015).





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolikova, M.; Manaskova-Postlerova, P.; Cerny, J.; Jankovicova, J.; Simonik, O.; Pohlova, A.; Secova, P.; Antalikova, J.; Dvorakova-Hortova, K. CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization. Int. J. Mol. Sci. 2018, 19, 1236. https://doi.org/10.3390/ijms19041236
Frolikova M, Manaskova-Postlerova P, Cerny J, Jankovicova J, Simonik O, Pohlova A, Secova P, Antalikova J, Dvorakova-Hortova K. CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization. International Journal of Molecular Sciences. 2018; 19(4):1236. https://doi.org/10.3390/ijms19041236
Chicago/Turabian StyleFrolikova, Michaela, Pavla Manaskova-Postlerova, Jiri Cerny, Jana Jankovicova, Ondrej Simonik, Alzbeta Pohlova, Petra Secova, Jana Antalikova, and Katerina Dvorakova-Hortova. 2018. "CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization" International Journal of Molecular Sciences 19, no. 4: 1236. https://doi.org/10.3390/ijms19041236