Oncosuppressors and Oncogenes: Role in Haemangioma Genesis and Potential for Therapeutic Targeting
Abstract
:1. Introduction
2. Vascular Endothelial Growth Factor
2.1. Vascular Endothelial Growth Factor Signaling in Angiogenesis
2.2. Vascular Endothelial Growth Factor Signaling in Hemangioma
2.3. Therapeutic Targeting of Vascular Endothelial Growth Factor
3. Oncogenic Pathways Linked to Tumor Angiogenesis
3.1. Altered Expression of Bcl-2 in Angiogenesis and Hemangioma
3.2. STAT3 Overexpression Promotes Tumor Angiogenesis
3.3. Possible Role of K-Ras in Hemangioma Growth
4. Tumor Suppressors in Angiogenesis and Hemangioma Development
4.1. Phosphatase and Tensin Homolog Is Downregulated in Hemangioma
4.2. p53 Modulates Hypoxia Inducible Factor and Angiogenesis in IH
4.3. Deregulation of Kiss1 Metastasis-Suppressor Is Associated with IH
4.4. Cyclin-Dependent Kinase Inhibitor 2A
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| Bcl-2 | B-cell lymphoma 2 |
| bFGF | Basic fibroblast growth factor |
| COX-2 | Cyclooxygenase 2 |
| EC | Endothelial cell |
| GTP-ase | Guanosine triphosphatase |
| HemECs | Hemangioma endothelial cells |
| HemEPCs | Hemangioma endothelial progenitor cells |
| HemSc | Hemangioma stem cells |
| HIF-1α | Hypoxia inducible factor-1α |
| IH | Infantile hemangioma |
| JAK | Janus kinases |
| K-ras | Kirsten rat sarcoma oncogene homologue |
| mTOR | Mammalian target of rapamycin |
| NFAT | Nuclear translocation of nuclear factor of activated T cells |
| PDK1/2 | Protein 3-phosphoinositide-dependent protein kinase-1 and -2 |
| PI3k | Phosphatidylinositide 3-kinases |
| PKB | Protein kinase B |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 1 |
| TSP-1 | Thrombospondin-1 |
| STAT3 | Signaling transducer and activator of transcription |
| VEGF | Vascular endothelial growth factor |
| VEGFR-1 | Vascular endothelial growth factor receptor-1 |
| VEGFR-2 | Vascular endothelial growth factor receptor-2 |
References
- Mulliken, J.B.; Glowacki, J. Classification of pediatric vascular lesions. Plast. Reconstr. Surg. 1982, 70, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Blei, F. Basic science and clinical aspects of vascular anomalies. Curr. Opin. Pediatr. 2005, 17, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Mabeta, P.; Pepper, M.S. Hemangiomas-current therapeutic strategies. Int. J. Dev. Biol. 2011, 55, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, S. Infantile Hemangioma: New Insights on Pathogenesis and β Blockers Mechanisms of Action. In Angiogenesis-Based Dermatology, 1st ed.; Abiser, J.L., Ed.; Springer: Berlin, Germany, 2017; pp. 27–39. ISBN 978-1-4471-7314-4. [Google Scholar]
- Li, P.; Guo, Z.; Gao, Y.; Pan, W. Propranolol represses infantile hemangioma cell growth through the B2-adrenergic receptor in a HIF-1α dependent manner. Oncol. Rep. 2015, 33, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Boye, E.; Yu, Y.; Paranya, G.; Mulliken, J.B.; Olsen, B.R.; Bischoff, J. Clonality and altered behavior of endothelial cells from hemangiomas. J. Clin. Investig. 2001, 107, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, S.; Bischoff, J. Pathogenesis of infantile haemangioma. Br. J. Dermatol. 2013, 169, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Boye, E.; Olsen, B.R. Signaling mechanisms in infantile hemangioma. Curr. Opin. Hematol. 2009, 16, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Dameron, K.M.; Volpert, O.V.; Tainsky, M.A.; Bouck, N. The p53 tumor suppressor gene inhibits angiogenesis by stimulating the production of thrombospondin. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1994; Volume 59, pp. 483–489. [Google Scholar]
- Rodriguez, S.; Huynh-Do, U. The role of PTEN in tumor angiogenesis. J. Oncol. 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Yu, J.; Klement, G.; Kerbel, R. Oncogenes and angiogenesis: Signaling three-dimensional tumor growth. J. Investig. Dermatol. Symp. Proc. 2000, 5, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Bottos, A.; Bardelli, A. Oncogenes and angiogenesis: A way to personalize anti-angiogenic therapy? Cell. Mol. Life Sci. 2013, 70, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Ye, W. The complexity of translating anti-angiogenesis therapy from basic science to the clinic. Dev. Cell 2016, 37, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling? In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Ambler, C.A.; Schmunk, G.M.; Bautch, V.L. Stem cell-derived endothelial cells/progenitors migrate and pattern in the embryo using the VEGF signaling pathway. Dev. Biol. 2003, 257, 205–219. [Google Scholar] [CrossRef]
- Yu, Y.; Flint, A.F.; Mulliken, J.B.; Wu, J.K.; Bischoff, J. Endothelial progenitor cells in infantile hemangioma. Blood 2004, 103, 1373–1375. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Mulliken, J.B.; Kozakewich, H.P.; Rogers, R.A.; Folkman, J.; Ezekowitz, R.A. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J. Clin. Investig. 1994, 93, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, S.; Li, K.; Li, L.; Xu, C.; Xiang, B. Signaling pathways in the development of infantile hemangioma. J. Hematol. Oncol. 2014, 7, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Li, P.; Guo, Z.; Huang, Q.; Gao, Y. Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr. Blood Cancer 2015, 62, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Mabeta, P. Inhibition of phosphoinositide 3-kinase is associated with reduced angiogenesis and an altered expression of angiogenic markers in endothelioma cells. Biomed. Pharmacother. 2014, 68, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Tang, W.; Zhang, Y.; Li, X. Rapamycin inhibits the proliferation of endothelial cells in hemangioma by blocking the mTOR-FABP4 pathway. Biomed. Pharmacother. 2017, 85, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Mabeta, P. PF573, 228 inhibits vascular tumor cell growth, migration as well as angiogenesis, induces apoptosis and abrogates PRAS40 and S6RP phosphorylation. Acta Pharm. 2016, 66, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Kaylani, S.; Theos, A.J.; Pressey, J.G. Treatment of infantile hemangiomas with Sirolimus in a patient with PHACE syndrome. Pediatr. Dermatol. 2013, 30, e194–e197. [Google Scholar] [CrossRef] [PubMed]
- Mabeta, P.; Pepper, M.S. Inhibition of hemangioma development in a syngeneic mouse model correlates with Bcl-2 suppression and the inhibition of Akt kinase activity. Angiogenesis 2012, 15, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jinnin, M.; Medici, D.; Park, L.; Limaye, N.; Liu, Y.; Boscolo, E.; Bischoff, J.; Vikkula, M.; Boye, E.; Olsen, B.R. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat. Med. 2008, 14, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Berard, M.; Sordello, S.; Ortega, N.; Carrier, J.L.; Peyri, N.; Wassef, M.; Bertrand, N.; Enjolras, O.; Drouet, L.; Plouet, J. Vascular endothelial growth factor confers a growth advantage in vitro and in vivo to stromal cells cultured from neonatal hemangiomas. Am. J. Pathol. 1997, 150, 1315–1326. [Google Scholar] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Biroccio, A.; Candiloro, A.; Mottolese, M.; Sapora, O.; Albini, A.; Zupi, G.; Del Bufalo, D. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vivo angiogenesis in a breast carcinoma cell line. FASEB J. 2000, 14, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Fontanini, G.; Boldrini, L.; Vignati, S.; Chinè, S.; Basolo, F.; Silvestri, V.; Lucchi, M.; Mussi, A.; Angeletti, C.A.; Bevilacqua, G. Bcl2 and p53 regulate vascular endothelial growth factor (VEGF)-mediated angiogenesis in non-small cell lung carcinoma. Eur. J. Cancer 1998, 34, 718–723. [Google Scholar] [CrossRef]
- Dituri, F.; Mazzocca, A.; Giannelli, G.; Antonaci, S. PI3K functions in cancer progression, anticancer immunity and immune evasion by tumors. Clin. Dev. Immunol. 2011, 947858–947958. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, S.I.; Zvelebi, M.J.; Shuttleworth, S.J.; Hancox, T.; Saghir, N. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem. J. 2007, 404, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Liu, L.Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2008, 102, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Nesterova, A.; Sable, C.; Heidenreich, K.A.; Boxer, L.M.; Heasley, L.E.; Reusch, J.E. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 2000, 275, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Karl, E.; Zhang, Z.; Dong, Z.; Neiva, K.G.; Soengas, M.S.; Koch, A.E.; Polverini, P.J.; Núñez, G.; Nör, J.E. Unidirectional crosstalk between Bcl-xL and Bcl-2 enhances the angiogenic phenotype of endothelial cells. Cell Death Differ. 2007, 14, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhang, D. Detection of p53 and Bcl-2 expression in cutaneous hemangioma through the quantum dot technique. Oncol. Lett. 2017, 13, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Nor, J.E.; Christensen, J.; Liu, J.; Peters, M.; Mooney, D.J.; Strieter, R.M.; Polverini, P.J. Up-regulation of Bcl-2 in microvascular endothelial cell enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001, 61, 2183–2188. [Google Scholar] [PubMed]
- Zeitlin, B.D.; Joo, E.; Dong, Z.; Warner, K.; Wang, G.; Nikolovska-Coleska, Z.; Wang, S.; Nor, J.E. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 2006, 66, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, B.D.; Zeitlin, I.J.; Nor, J.E. Expanding circle of inhibition: Small-molecule inhibitors of Bcl-2 as anticancer cell and antiangiogenic agents. J. Clinl. Oncol. 2008, 26, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Cang, S.; Iragavarapu, C.; Savooji, J.; Song, Y.; Liu, D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J. Hematol. Oncol. 2015, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Peterson, T.; Horvat, T.; Rodriguez, M.; Tang, L. Venetoclax: A First-in-Class Oral BCL-2 Inhibitor for the Management of Lymphoid Malignancies. Ann. Pharmacother. 2017, 51, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Mordant, P.; Brown, B.D.; Bourhis, J.; Soria, J.C.; Deutsch, E. Inhibition of Bcl-2 in small cell lung cancer cell lines with oblimersen, an antisense Bcl-2 oligodeoxynucleotide (ODN): In vitro and in vivo enhancement of radiation response. Anticancer Res. 2010, 30, 3869–3878. [Google Scholar] [PubMed]
- Barata, P.; Sood, A.K.; Hong, D.S. RNA-targeted therapeutics in cancer clinical trials: Current status and future directions. Cancer Treat. Rev. 2016, 50, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Grandis, J.R.; Bauman, J.E. The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations. Oral Oncol. 2016, 56, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.-Q.; Sethi, G.; Goh, B.-C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ong, C.; Hur, G.; Shen, H. Inhibition of the JAK-STAT3 pathway by andrographolide enhances chemosensitivity of cancer cells to doxorubicin. Biochem. Pharmacol. 2010, 79, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Itinteang, T.; Tan, S.T.; Brasch, H.D.; Steel, R.; Best, H.A.; Vishvanath, A.; Jia, J.; Day, D.J. Infantile haemangioma expresses embryonic stem cell markers. J. Clin. Pathol. 2012, 65, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Tran, D.; Bryan, B.A.; Mitchell, D.C. Meta-analysis of infantile hemangioma endothelial cell microarray expression data reveals significant aberrations of gene networks involved in cell adhesion and extracellular matrix composition. Angiol. Open Access 2013, 1, 107. [Google Scholar] [CrossRef]
- Lim, Y.H.; Douglas, S.R.; Ko, C.J.; Antaya, R.J.; McNiff, J.M.; Zhou, J.; Choate, K.A.; Narayan, D. Somatic Activating RAS Mutations Cause Vascular Tumors Including Pyogenic Granuloma. J. Investig. Dermatol. 2015, 135, 1698–1700. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, O.; Gebbink, M.F.B.G.; Voest, E.E. Stimulation of angiogenesis by Ras proteins. Biochim Biophys. Acta Rev. Cancer 2004, 1654, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Zheng, Q.; Adam, A.; Vincent, P.; Pumiglia, K. Activation of endothelial ras signaling bypasses senescence and causes abnormal vascular morphogenesis. Cancer Res. 2010, 70, 3803–3812. [Google Scholar] [CrossRef] [PubMed]
- Grugel, S.; Finkenzeller, G.; Weindel, K.; Barleon, B.; Marmé, D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J. Biol. Chem. 1995, 270, 25915–25919. [Google Scholar] [CrossRef] [PubMed]
- Meadows, K.N.; Bryant, P.; Pumiglia, K. Vascular endothelial growth factor induction of the angiogenic phenotype requires Ras activation. J. Biol. Chem. 2001, 276, 49289–49298. [Google Scholar] [CrossRef] [PubMed]
- Pollyea, D.A. Bcl-2 Inhibition in Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2017, 17, S112–S114. [Google Scholar] [CrossRef]
- Nakajima, W.; Sharma, K.; Hicks, M.A.; Le, N.; Brown, R.; Krystal, G.W.; Harada, H. Combination with vorinostat overcomes ABT-263 (navitoclax) resistance of small cell lung cancer. Cancer Biol. Ther. 2016, 17, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H.M.; Saglio, G.; Steegmann, J.L.; Shah, N.P.; Boque, C.; Chuah, C.; Pavlovsky, C.; Mayer, J.; Cortes, J.; et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014, 123, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.; Harrison, C.; Cortes, J.E.; Cervantes, F.; Mesa, R.A.; Milligan, D.; Masszi, T.; Mishchenko, E.; Jourdan, E.; Vannucchi, A.M.; et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: A randomized clinical trial. JAMA Oncol. 2015, 1, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, Z.G.; Biau, J.; Kemeny, J.; Morel, L.; Verrelle, P.; Chautard, E. Role of STAT3 in genesis and progression of human malignant gliomas. Mol. Neurobiol. 2017, 54, 5780–5797. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; Zhou, T.; Schmidt, J.; Jo, M.; et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci. Transl. Med. 2015, 7, 314ra185. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Thomas, S.M.; Kim, S.; Yeh, J.I.; Ferris, R.L.; Johnson, J.T.; Duvvuri, U.; Lee, J.; Sahu, N.; Joyce, S.; et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: Implications for cancer therapy. Cancer Discov. 2012, 2, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Calicchio, M.L.; Collins, T.; Kozakewich, H.P. Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am. J. Pathol. 2009, 174, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.S. The molecular biology of cancer. Mol. Asp. Med. 2000, 21, 167–223. [Google Scholar] [CrossRef]
- Jiang, B.H.; Zheng, J.Z.; Aoki, M.; Vogt, P.K. Phosphotidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Manzano, C.; Fueyo, J.; Jiang, H.; Glass, T.L.; Lee, H.; Hu, M.; Liu, J.; Jasti, S.L.; Liu, T.; Conrad, C.A. Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann. Neurol. 2003, 53, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zundel, W.; Schindler, C.; Haas-Kogan, D.; Koong, A.; Kaper, F.; Chen, E.; Gottschalk, A.R.; Ryan, H.E.; Johnson, R.S.; Jefferson, A.B.; et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000, 14, 391–396. [Google Scholar] [PubMed]
- Tan, W.H.; Baris, H.N.; Burrows, P.E.; Robson, C.D.; Alomari, A.I.; Mulliken, J.B.; Fishman, S.J.; Irons, M.B. The spectrum of vascular anomalies in patients with PTEN mutations: Implications for diagnosis and management. J. Med. Genet. 2007, 44, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, I.A.; Kravchenko, J.E.; Chumakov, P.M. Homeostatic functions of the p53 tumor suppressor: Regulation of energy metabolism and antioxidant defense. Semin. Cancer Biol. 2009, 19, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis—The p53 network. J. Cell Sci. 2003, 116, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Green, D.R. Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 2001, 29, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Sermeus, A.; Michiels, C.M. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Mookerjee, B.; Bhujwalla, Z.M.; Sutter, C.H.; Artemov, D.; Zeng, Q.; Dillehay, L.E.; Madan, A.; Semenza, G.L.; Bedi, A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 2000, 14, 34–44. [Google Scholar] [PubMed]
- De Moraes, E.; Dar, N.A.; de Moura Gallo, C.V.; Hainaut, P. Cross-talks between cyclooxygenase-2 and tumor suppressor protein p53: Balancing life and death during inflammatory stress and carcinogenesis. Int. J. Cancer 2007, 121, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Creancier, L.; Zanibellato, C.; Prats, A.; Prats, H. Tumour suppressor p53 inhibits human fibroblast growth factor 2 expression by a post-transcriptional mechanism. Oncogene 2001, 20, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.G.; Parker, A.E.; Zhu, X.; Green, M.R. P53-Mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science 2006, 313, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Antiangiogenesis in cancer therapy—Endostatin and its mechanisms of action. Exp. Cell Res. 2006, 312, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Koukourakis, M.I.; Kakolyris, S.; Turley, H.; O’Byrne, K.; Scott, P.A.E.; Pezzella, F.; Georgoulias, V.; Harris, A.L.; Gatter, K.C. Vascular endothelial growth factor, wild type p53, and angiogenesis in early operable non-small cell lung cancer. Clin. Cancer Res. 1998, 4, 3017–3024. [Google Scholar] [PubMed]
- Perrone, G.; Santini, V.D.; Verzi, A.; Tonini, G.; Vetrani, A.; Rabitti, V.C. Correlation of p53 and Bcl-2 expression with vascular endothelial growth factor (VEGF), microvessel density (MVD) and clinico-pathological features in colon cancer. Cancer Lett. 2004, 208, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tahaney, W.M.; Mazumdar, A.; Savage, M.I.; Brown, P.H. Molecularly targeted therapies for p53-mutant cancers. Cell. Mol. Life Sci. 2017, 74, 4171–4178. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.-B.; Li, Q.-Y.; Jiang, F.; Hu, X.-Y.; Ma, R.-Z.; Dong, Q.; Zhang, H.; Pattar, P.; Li, S.-X. Pingyangymycin stimulates apoptosis in human hemangioma-derived endothelial cells through activation of the p53 pathway. Mol. Med. Rep. 2014, 10, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, K.; Xiao, X.; Zheng, S.; Xu, T.; Chen, S. Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J. Pediatr. Surg. 2012, 47, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.M.; Rowntree, R.K.; Amaya, C.; Diaz, D.; Kokta, V.; Mitchell, D.C.; Bryan, B.A. Gene expression analysis reveals marked differences in the transcriptome of infantile hemangioma endothelial cells compared to normal dermal microvascular endothelial cells. Vasc. Cell 2013, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Al-Odaini, A.A.; Wang, Y.; Korah, J.; Dai, M.; Xiao, L.; Ali, S.; Lebrun, J.J. KiSS1 gene as a novel mediator of TGFβ-mediated cell invasion in triple negative breast cancer. Cell Signal. 2018, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alhejaily, A.; Day, A.G.; Feilotter, H.E.; Baetz, T.; LeBrun, D.P. Inactivation of the CDKN2A tumor-suppressor gene by deletion or methylation is common at diagnosis in follicular lymphoma and associated with poor clinical outcome. Clin. Cancer Res. 2014, 20, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
 inhibit; →, activate.
inhibit; →, activate.
 inhibit; →, activate.
inhibit; →, activate.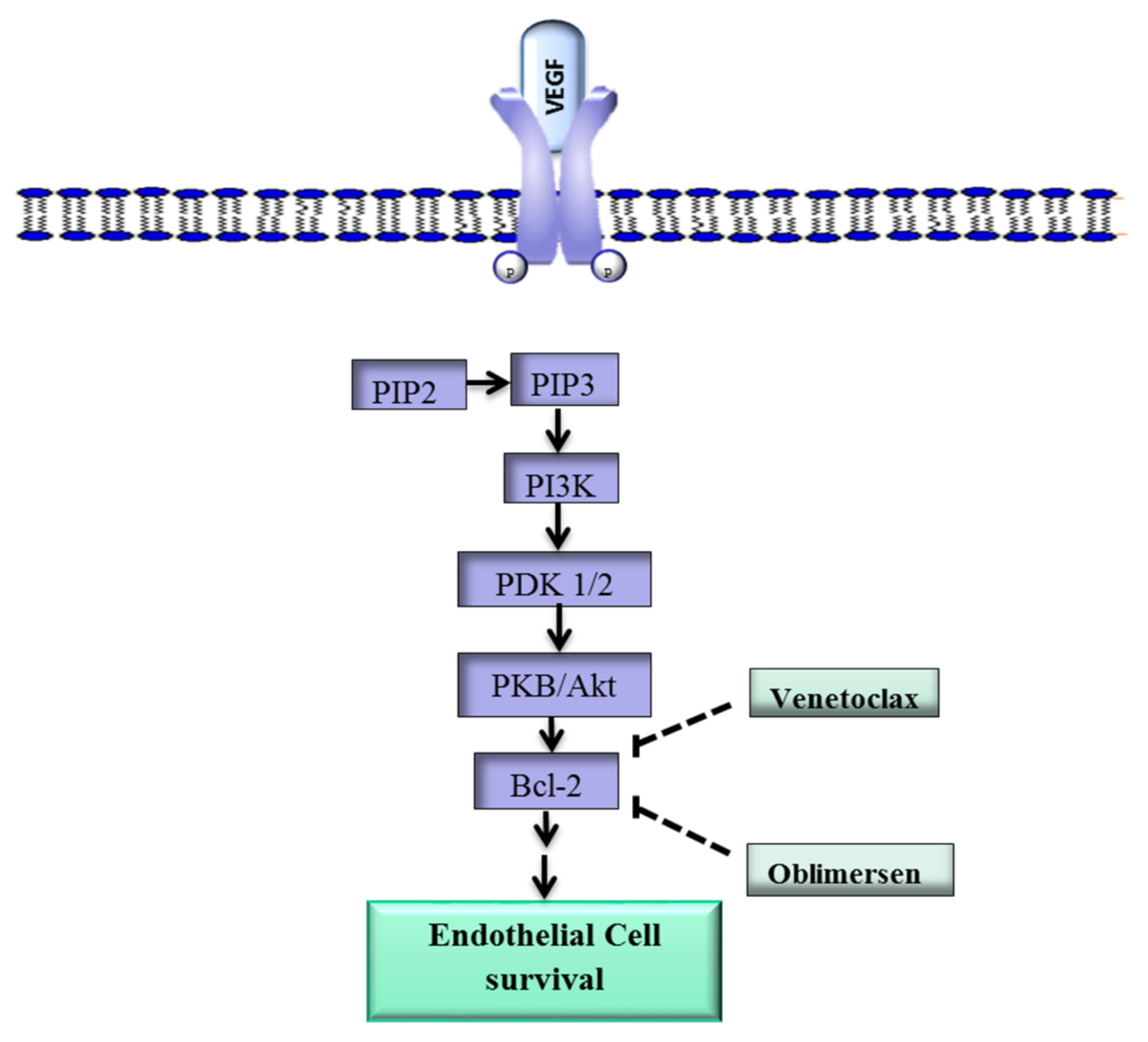
 , translocate into the nucleus.
, translocate into the nucleus.
 , translocate into the nucleus.
, translocate into the nucleus.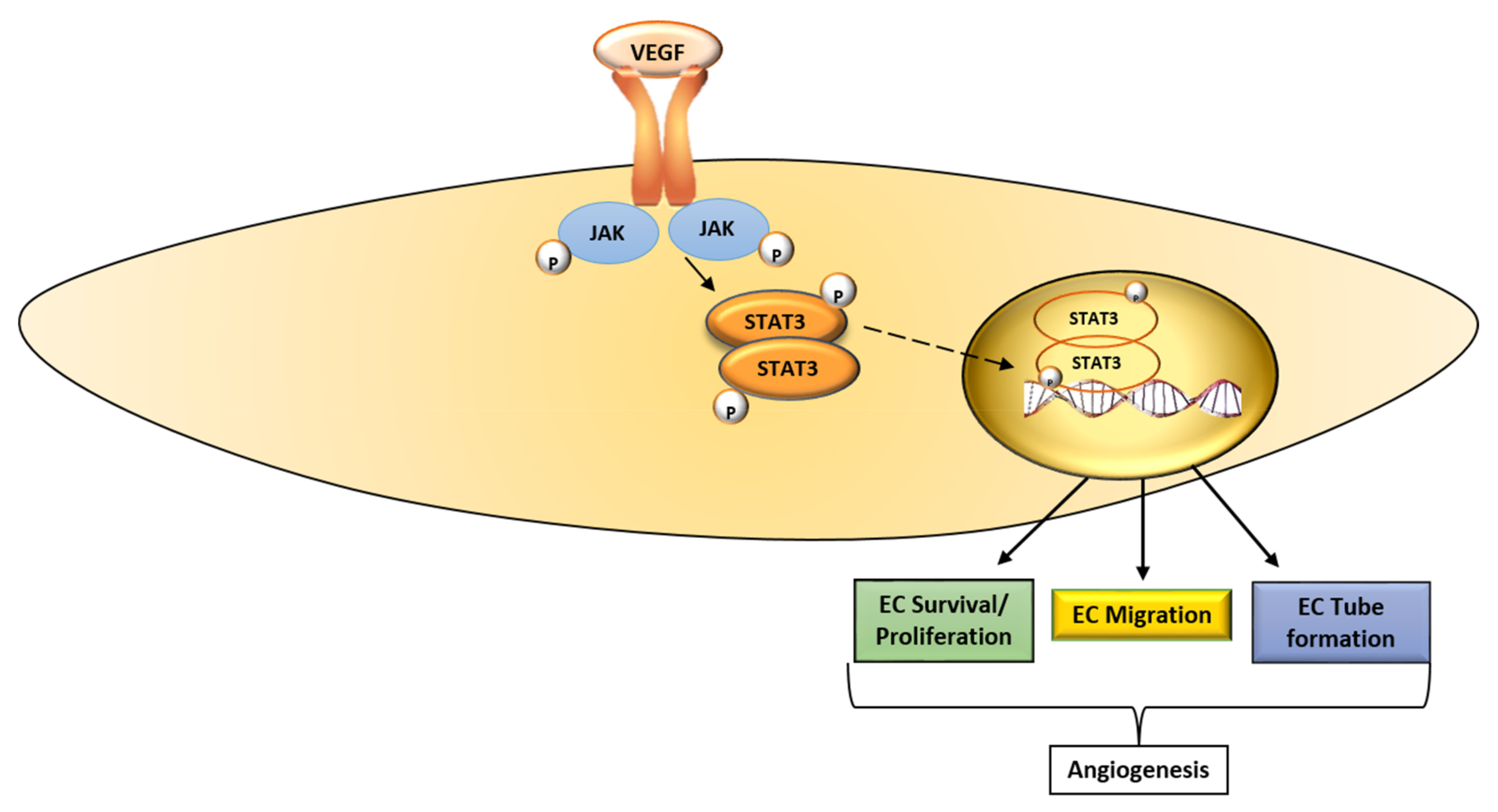
 , drug inhibition;
, drug inhibition;  , suppress; →, activate/stimulate.
, suppress; →, activate/stimulate.
 , drug inhibition;
, drug inhibition;  , suppress; →, activate/stimulate.
, suppress; →, activate/stimulate.
| Drug | Target | Disease Indication | Reference |
|---|---|---|---|
| Aflibercept | VEGF-A, VEGF-B, PlGF | Broad spectrum of malignancies | [13,14] |
| Axitinib | VEGFR-1, VEGFR-2, VEGFR-3 | Renal cell carcinoma | [13] |
| Bevacizumab | VEGF-A | Breast, ovarian, metastatic colorectal and non-small cell lung cancers, recurrent glioblastoma | [13,15] |
| Lenvatinib | VEGF-A | Thyroid cancer | [16] |
| Pegaptanib | VEGF-A | Age-related macular degeneration | [14] |
| Pazopanib | VEGFR1, VEGFR2, VEGFR3 | Advanced soft tissue sarcoma | [13] |
| Sorafenib | VEGFR1, VEGFR2, VEGFR3 | Renal cell cancer, hepatocellular carcinoma | [13,14] |
| Sunitinib | VEGFR1, VEGFR2, VEGFR3 | Renal cell carcinoma, pancreatic neuroendocrine | [16] |
| Regorafenib | Refractory metastatic colorectal cancer | [14] | |
| Ramucirumab | VEGFR-2 | Metastatic colorectal, gastric/gastro-esophageal, non-small-cell lung cancer | [14] |
| Vatalanib | VEGFR1, VEGFR2, VEGFR3 | Solid tumors | [14] |
| Target | Drug | Phase of Development | Reference |
|---|---|---|---|
| Bcl-2 | |||
| S-055746 | I | [55] | |
| PNT-2258 | II | [41] | |
| Navitoclax | I/II and III | [56] | |
| Venetoclax | I/II | [41,42] | |
| Oblimersen | I/II and III | [43,44] | |
| STAT3 | |||
| Upstream tyrosine kinase inhibitors | Ruxolitinib | I–III | [45] |
| Dasatinib | III | [57] | |
| Fedratinib | I/II and III | [58] | |
| Tofacitinib | I/II | [45] | |
| SH2 domain inhibitors | OPB-31121 | I/II | [46] |
| OBP-51602 | I/II | [59] | |
| STAT3 DNA-binding domain inhibitors | CPA-1 | Preclinical | [46] |
| Cyclic STAT3 decoy | Preclinical | [45] | |
| STAT3 transcription inhibitors | AZD9150 | I/II | [60] |
| STAT3 decoy oligonucleotide | 0 | [61] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabeta, P. Oncosuppressors and Oncogenes: Role in Haemangioma Genesis and Potential for Therapeutic Targeting. Int. J. Mol. Sci. 2018, 19, 1192. https://doi.org/10.3390/ijms19041192
Mabeta P. Oncosuppressors and Oncogenes: Role in Haemangioma Genesis and Potential for Therapeutic Targeting. International Journal of Molecular Sciences. 2018; 19(4):1192. https://doi.org/10.3390/ijms19041192
Chicago/Turabian StyleMabeta, Peace. 2018. "Oncosuppressors and Oncogenes: Role in Haemangioma Genesis and Potential for Therapeutic Targeting" International Journal of Molecular Sciences 19, no. 4: 1192. https://doi.org/10.3390/ijms19041192





