Opposing Roles of Calcium and Intracellular ATP on Gating of the Purinergic P2X2 Receptor Channel
Abstract
:1. Introduction
2. Results
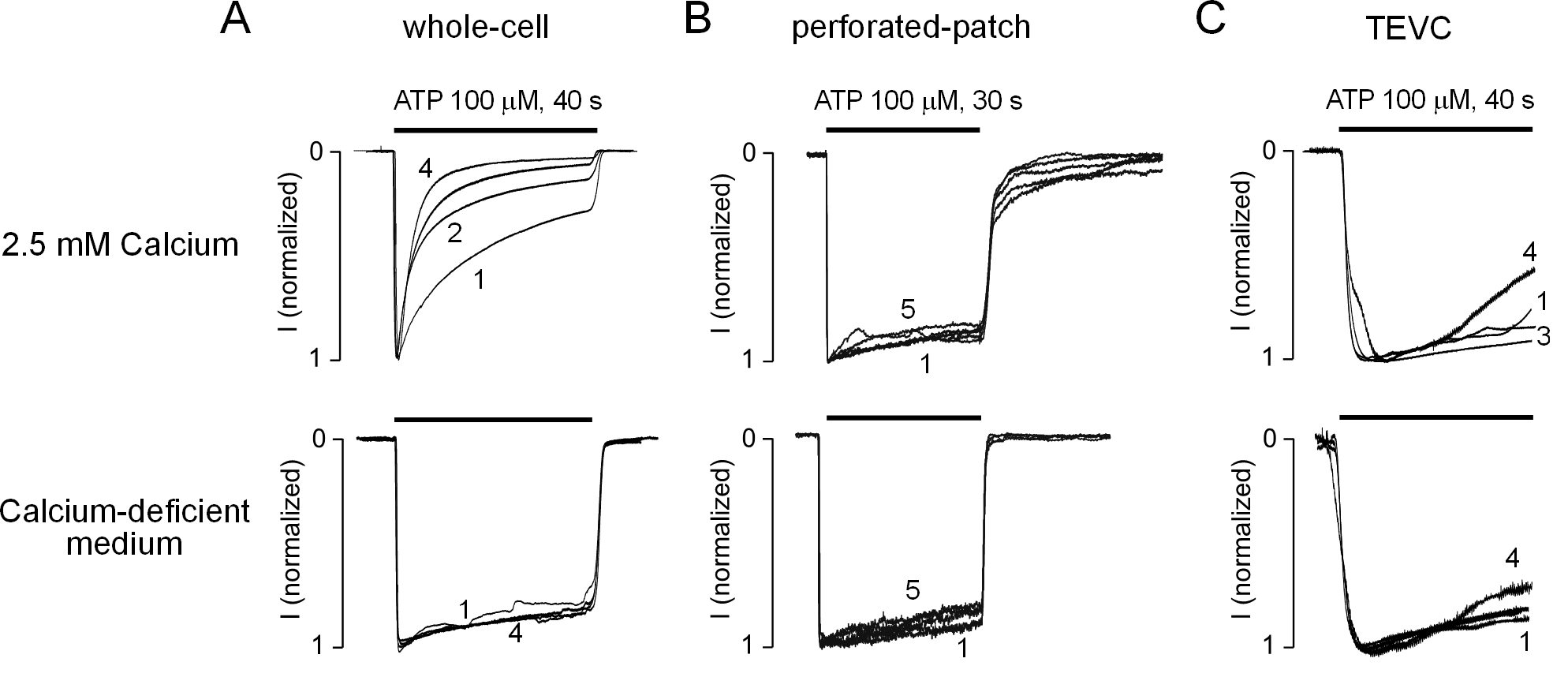
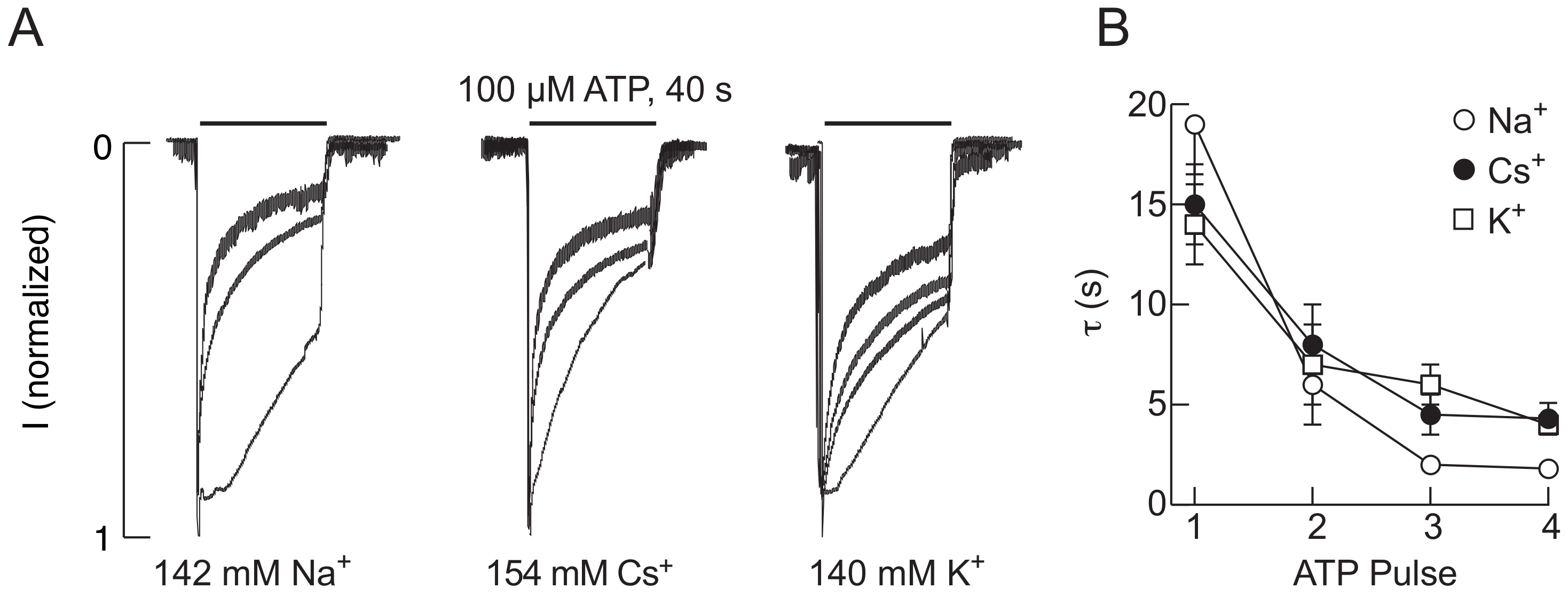
2.1. The P2X2R Current Response Pattern Depends on Recording Conditions
2.2. Endogenous P2X2R-Mediated Currents also Exhibit UDD
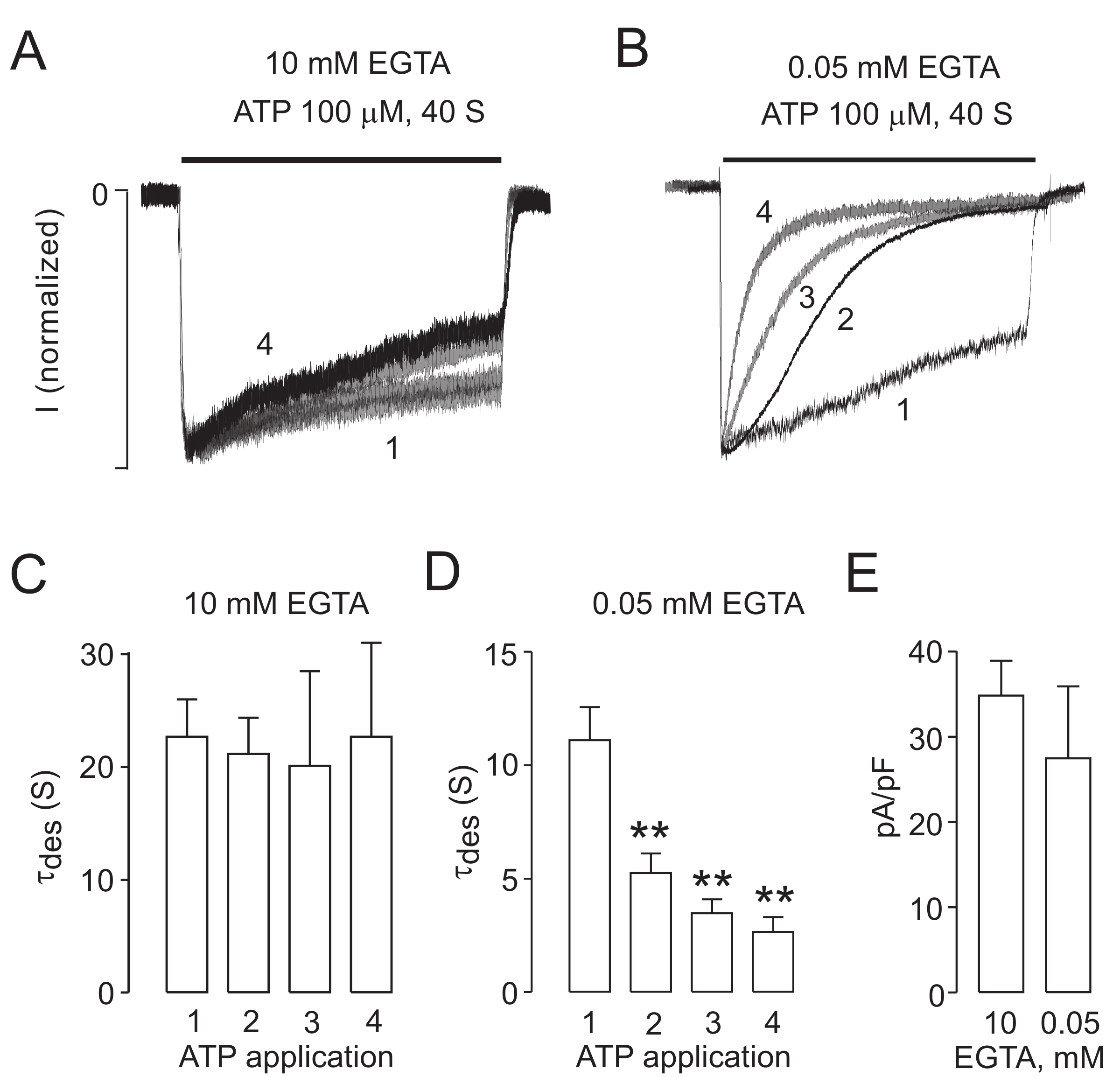
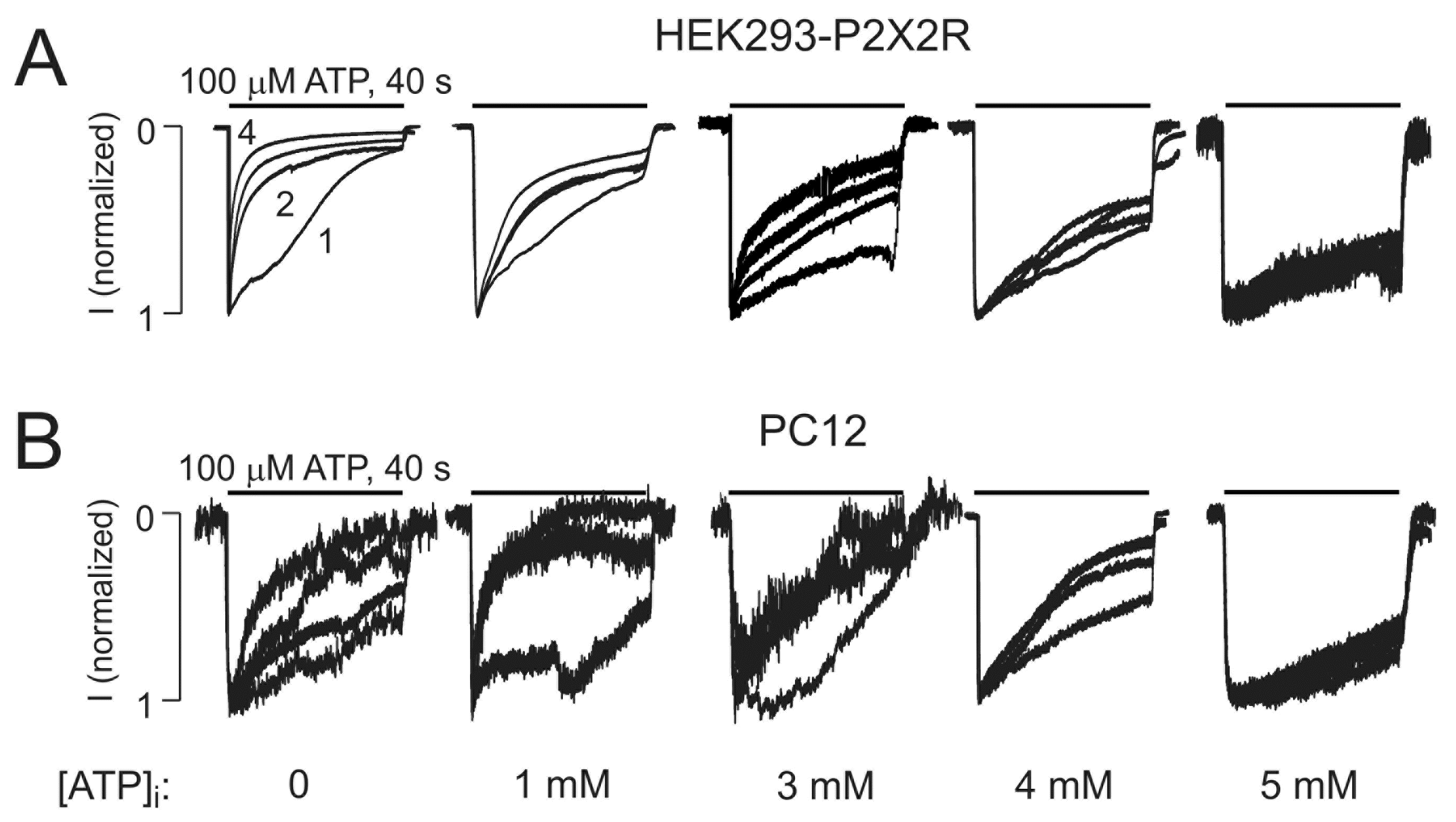
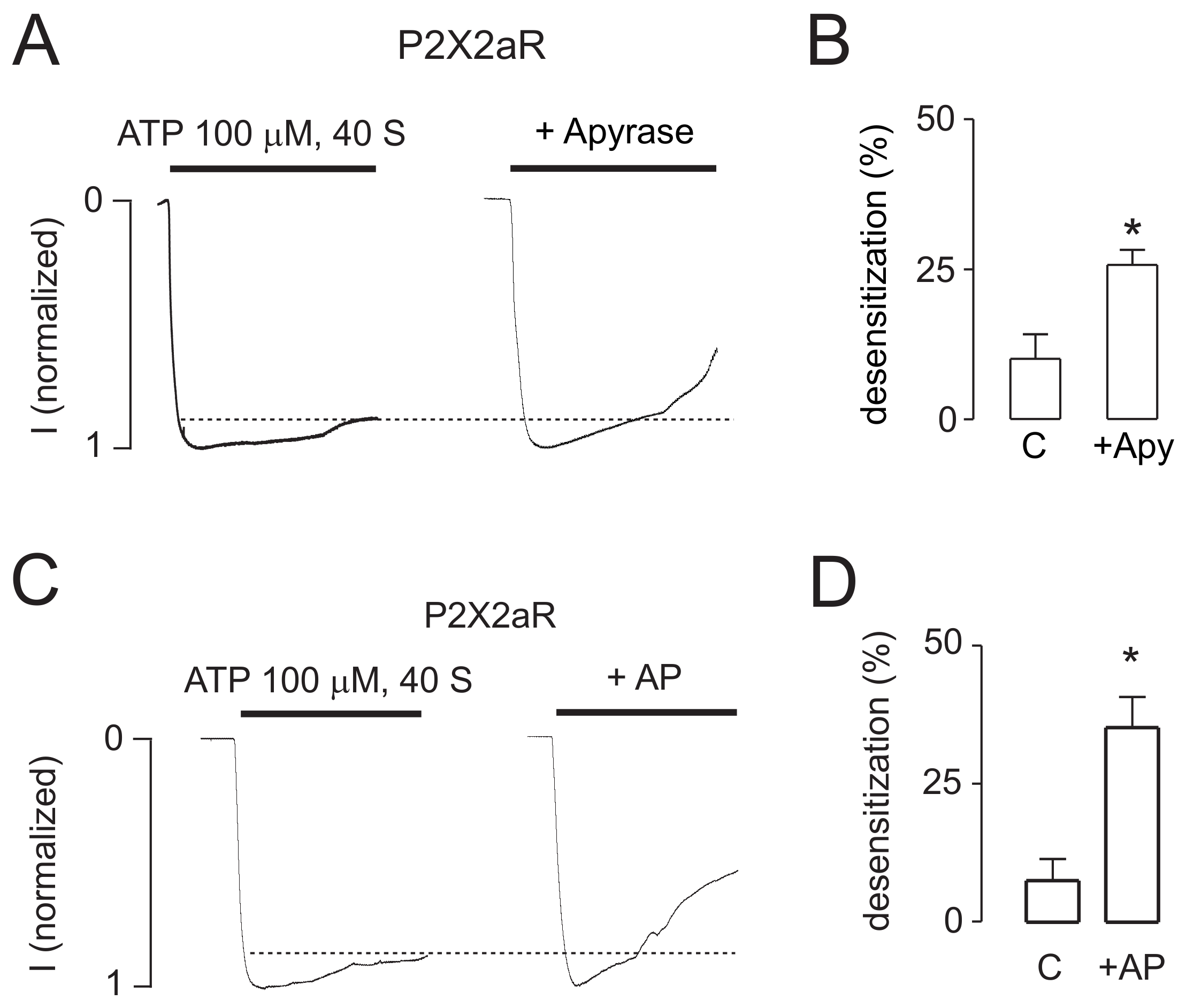
2.3. Intracellularly Applied ATP Abolishes UDD
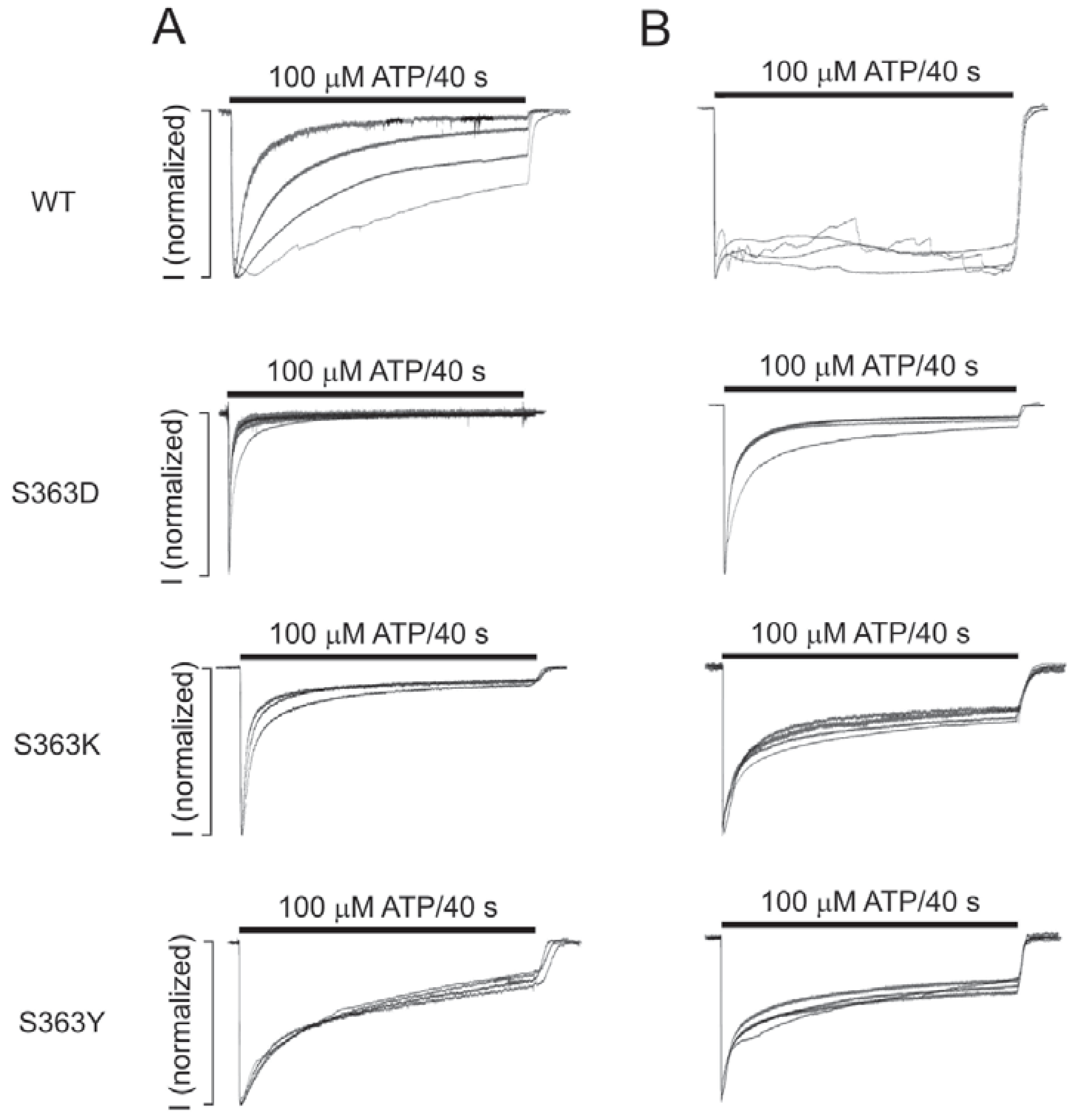
2.4. The S363 Residue is Critical for the Intracellular ATP Effects on the Channel Gating
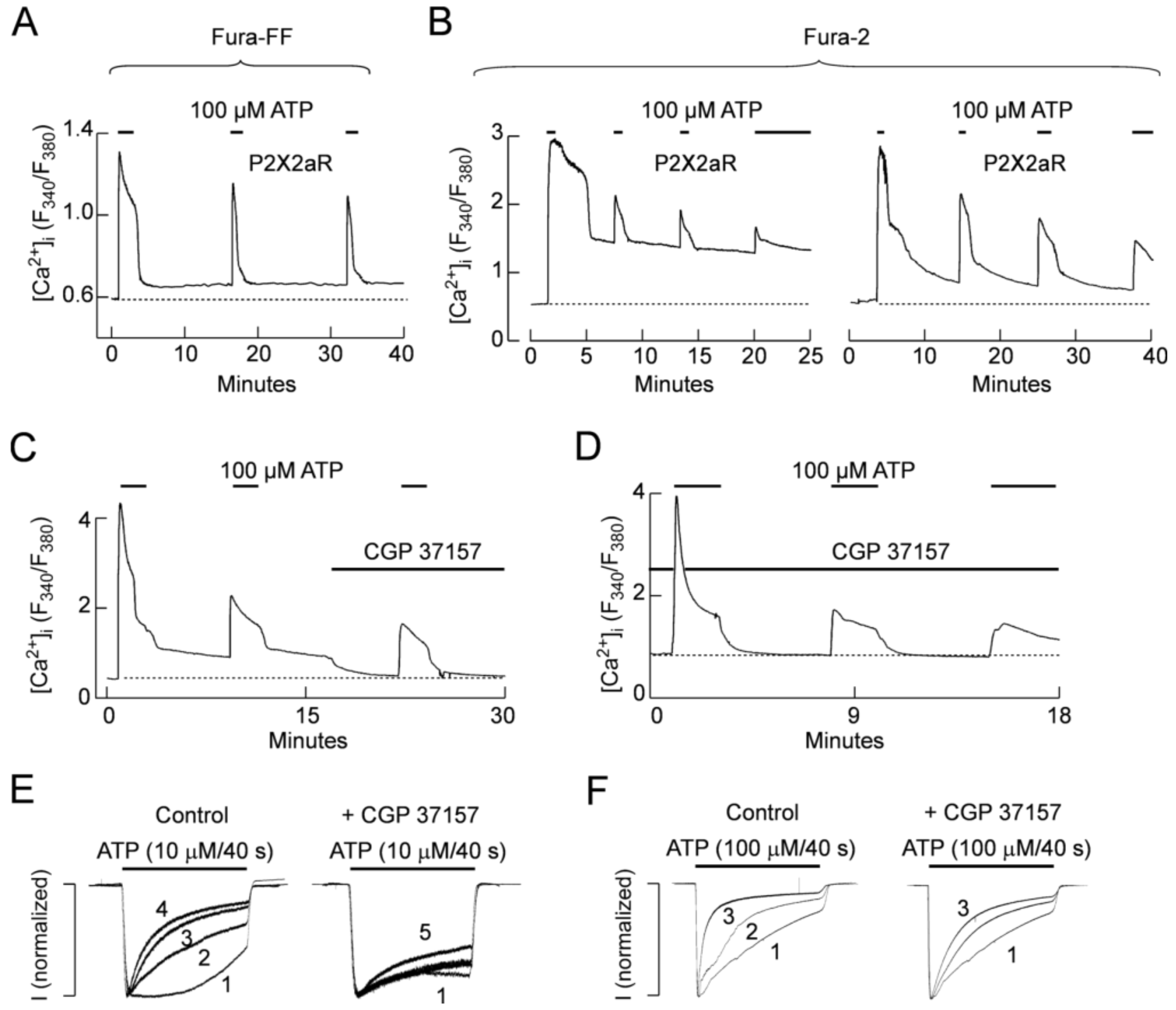
2.5. Role of Mitochondria in P2X2R Desensitization
3. Discussion
4. Material and Methods
4.1. P2XR Constructs Used in Experiment
4.2. Receptor Transfection and Cell Culture
4.3. Patch-Clamp Measurements
4.4. Oocyte Injection and Current Measurements
4.5. Intracellular Calcium Recordings
4.6. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CaM | Calmodulin |
| P2XRs | P2X receptor channels |
| TEVC | two-electrode voltage clamp |
| TRP | Transient receptor potential |
References
- Samways, D.S.; Li, Z.; Egan, T.M. Principles and properties of ion flow in P2X receptors. Front. Cell. Neurosci. 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Coddou, C.; Yan, Z.; Obsil, T.; Huidobro-Toro, J.P.; Stojilkovic, S.S. Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef] [PubMed]
- Lorinczi, E.; Bhargava, Y.; Marino, S.F.; Taly, A.; Kaczmarek-Hajek, K.; Barrantes-Freer, A.; Dutertre, S.; Grutter, T.; Rettinger, J.; Nicke, A. Involvement of the cysteine-rich head domain in activation and desensitization of the P2X1 receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 11396–11401. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, E.; Sokolova, E.; Masten, L.; D’Arco, M.; Fabbro, A.; Nistri, A.; Giniatullin, R. Identification of negative residues in the P2X3 ATP receptor ectodomain as structural determinants for desensitization and the Ca2+-sensing modulatory sites. J. Biol. Chem. 2004, 279, 53109–53115. [Google Scholar] [CrossRef] [PubMed]
- Jindrichova, M.; Khafizov, K.; Skorinkin, A.; Fayuk, D.; Bart, G.; Zemkova, H.; Giniatullin, R. Highly conserved tyrosine 37 stabilizes desensitized states and restricts calcium permeability of ATP-gated P2X3 receptor. J. Neurochem. 2011, 119, 676–685. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Zemkova, H.; Stojilkovic, S.S. Dependence of purinergic P2X receptor activity on ectodomain structure. J. Biol. Chem. 2003, 278, 10182–10188. [Google Scholar] [CrossRef] [PubMed]
- Brandle, U.; Spielmanns, P.; Osteroth, R.; Sim, J.; Surprenant, A.; Buell, G.; Ruppersberg, J.P.; Plinkert, P.K.; Zenner, H.P.; Glowatzki, E. Desensitization of the P2X2 receptor controlled by alternative splicing. FEBS Lett. 1997, 404, 294–298. [Google Scholar] [CrossRef]
- Koshimizu, T.; Tomic, M.; Van Goor, F.; Stojilkovic, S.S. Functional role of alternative splicing in pituitary P2X2 receptor-channel activation and desensitization. Mol. Endocrinol. 1998, 12, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, T.A.; Kretschmannova, K.; He, M.L.; Ueno, S.; Tanoue, A.; Yanagihara, N.; Stojilkovic, S.S.; Tsujimoto, G. Carboxyl-terminal splicing enhances physical interactions between the cytoplasmic tails of purinergic P2X receptors. Mol. Pharmacol. 2006, 69, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, T.; Tomic, M.; Koshimizu, M.; Stojilkovic, S.S. Identification of amino acid residues contributing to desensitization of the P2X2 receptor channel. J. Biol. Chem. 1998, 273, 12853–12857. [Google Scholar] [CrossRef] [PubMed]
- Fountain, S.J.; North, R.A. A C-terminal lysine that controls human P2X4 receptor desensitization. J. Biol. Chem. 2006, 281, 15044–15049. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Evans, R.J. The intracellular amino terminus plays a dominant role in desensitization of ATP-gated P2X receptor ion channels. J. Biol. Chem. 2011, 286, 44691–44701. [Google Scholar] [CrossRef] [PubMed]
- Boue-Grabot, E.; Archambault, V.; Seguela, P. A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X2 ATP-gated channels. J. Biol. Chem. 2000, 275, 10190–10195. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Brockhausen, J.; Bennett, M.R. P2X1 receptor currents after disruption of the PKC site and its surroundings by dominant negative mutations in HEK293 cells. Auton. Neurosci. 2003, 108, 12–16. [Google Scholar] [CrossRef]
- Franklin, C.; Braam, U.; Eisele, T.; Schmalzing, G.; Hausmann, R. Lack of evidence for direct phosphorylation of recombinantly expressed P2X2 and P2X3 receptors by protein kinase C. Purinergic Signal. 2007, 3, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Vial, C.; Tobin, A.B.; Evans, R.J. G-protein-coupled receptor regulation of P2X1 receptors does not involve direct channel phosphorylation. Biochem. J. 2004, 382, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Yule, D.I. Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim. Biophys. Acta. 2007, 1773, 1661–1675. [Google Scholar]
- Yan, Z.; Li, S.; Liang, Z.; Tomic, M.; Stojilkovic, S.S. The P2X7 receptor channel pore dilates under physiological ion conditions. J. Gen. Physiol. 2008, 132, 563–573. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.P.; Rodland, K.D.; McCleskey, E.W. A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J. Neurosci. 1998, 18, 9238–9244. [Google Scholar] [CrossRef] [PubMed]
- Khadra, A.; Yan, Z.; Coddou, C.; Tomic, M.; Sherman, A.; Stojilkovic, S.S. Gating properties of the P2X2a and P2X2b receptor channels: Experiments and mathematical modeling. J. Gen. Physiol. 2012, 139, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Coddou, C.; Yan, Z.; Stojilkovic, S.S. Role of domain calcium in purinergic P2X2 receptor channel desensitization. Am. J. Physiol. Cell Physiol. 2015, 308, C729–C736. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, D.C.; Vergani, P.; Csanady, L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 2006, 440, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.N.; Welsh, M.J. Structure and function of the CFTR chloride channel. Physiol. Rev. 1999, 79 (Suppl. 1), S23–S45. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, C.; Qin, F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- Babenko, A.P.; Aguilar-Bryan, L.; Bryan, J. A view of sur/KIR6.X, KATP channels. Ann. Rev. Physiol. 1998, 60, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Brake, A.J.; Wagenbach, M.J.; Julius, D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 1994, 371, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kubo, Y. Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J. Physiol. 2006, 576, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Mo, G.; Bernier, L.P.; Zhao, Q.; Chabot-Dore, A.J.; Ase, A.R.; Logothetis, D.; Cao, C.Q.; Seguela, P. Subtype-specific regulation of P2X3 and P2X2/3 receptors by phosphoinositides in peripheral nociceptors. Mol. Pain. 2009, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; Ase, A.R.; Tong, X.; Hamel, E.; Blais, D.; Zhao, Q.; Logothetis, D.E.; Seguela, P. Direct modulation of P2X1 receptor-channels by the lipid phosphatidylinositol 4,5-bisphosphate. Mol. Pharmacol. 2008, 74, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; Ase, A.R.; Chevallier, S.; Blais, D.; Zhao, Q.; Boue-Grabot, E.; Logothetis, D.; Seguela, P. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J. Neurosci. 2008, 28, 12938–12945. [Google Scholar] [CrossRef] [PubMed]
- Ase, A.R.; Bernier, L.P.; Blais, D.; Pankratov, Y.; Seguela, P. Modulation of heteromeric P2X1/5 receptors by phosphoinositides in astrocytes depends on the P2X1 subunit. J. Neurochem. 2010, 113, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.; Roux, S.J. Apyrases, extracellular ATP and the regulation of growth. Curr. Opin. Plant Biol. 2011, 14, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.J.; Kidney, B.A. Alkaline phosphatase: Beyond the liver. Vet. Clin. Pathol. Am. Soc. 2007, 36, 223–233. [Google Scholar] [CrossRef]
- Babcock, D.F.; Hille, B. Mitochondrial oversight of cellular Ca2+ signaling. Curr. Opin. Neurobiol. 1998, 8, 398–404. [Google Scholar] [CrossRef]
- Cox, D.A.; Conforti, L.; Sperelakis, N.; Matlib, M.A. Selectivity of inhibition of Na+-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J. Cardiovasc. Pharmacol. 1993, 21, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Czyz, A.; Kiedrowski, L. Inhibition of plasmalemmal Na+/Ca2+ exchange by mitochondrial Na+/Ca2+ exchange inhibitor 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP-37157) in cerebellar granule cells. Biochem. Pharmacol. 2003, 66, 2409–2411. [Google Scholar] [CrossRef] [PubMed]
- Virginio, C.; Church, D.; North, R.A.; Surprenant, A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 1997, 36, 1285–1294. [Google Scholar] [CrossRef]
- Evans, R.J.; Lewis, C.; Virginio, C.; Lundstrom, K.; Buell, G.; Surprenant, A.; North, R.A. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J. Physiol. 1996, 497, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Sachs, F. Ion permeation and block of P2X2 purinoceptors: Single channel recordings. J. Membr. Biol. 1999, 172, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, S.; Compan, V.; Toulme, E.; Richler, E.; Housley, G.D.; Rassendren, F.; Khakh, B.S. Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci. Signal. 2008, 1, ra8. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Khadra, A.; Sherman, A.; Stojilkovic, S.S. Calcium-dependent block of P2X7 receptor channel function is allosteric. J. Gen. Physiol. 2011, 138, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Pelegrin, P.; Surprenant, A. Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J. Neurosci. 2008, 28, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhu, J.X.; Wang, Y.; Zhang, X.; Bao, L. CaMKIIalpha and caveolin-1 cooperate to drive ATP-induced membrane delivery of the P2X3 receptor. J. Mol. Cell Biol. 2014, 6, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.W.; Wang, H.L. Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J. Neurochem. 1998, 70, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovic, S.S.; Tomic, M.; Van Goor, F.; Koshimizu, T. Expression of purinergic P2X2 receptor-channels and their role in calcium signaling in pituitary cells. Biochem. Cell. Biol. 2000, 78, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Penington, N.J.; Williams, K. Inhibition of TRPC5 channels by intracellular ATP. Mol. Pharmacol. 2008, 73, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mercado, J.; Gordon-Shaag, A.; Zagotta, W.N.; Gordon, S.E. Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. Off. J. Soc. 2010, 30, 13338–13347. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry. 5th Edition. Section 14.1: Metabolism is Composed of Many Coupled, Interconnecting Reactions. 2002. Available online: http://www.ncbi.nlm.nih.gov/books/NBK22439/ (accessed on 26 February 2018).
- Sun, J.H.; Cai, G.J.; Xiang, Z.H. Expression of P2X purinoceptors in PC12 phaeochromocytoma cells. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1282–1386. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.D.; Grahames, C.B.; Humphrey, P.P. Functional characterisation of P2 purinoceptors in PC12 cells by measurement of radiolabelled calcium influx. Naunyn Schmiedebergs Arch. Pharmacol. 1996, 354, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Sicheritz-Ponten, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Babcock, D.F.; Herrington, J.; Goodwin, P.C.; Park, Y.B.; Hille, B. Mitochondrial participation in the intracellular Ca2+ network. J. Cell Biol. 1997, 136, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castillo, C.; Morales, B.; Huidobro-Toro, J.P. Zinc and copper modulate differentially the P2X4 receptor. J. Neurochem. 2000, 74, 1529–1537. [Google Scholar] [CrossRef] [PubMed]



| Receptor | τ1 (s) | τ2 (s) | τ3 (s) | τ4 (s) | I1 (nA) | I2 (nA) | I3 (nA) | I4 (nA) |
|---|---|---|---|---|---|---|---|---|
| P2X2a | 14.6 ± 1.7 | 7.4 ± 1.8 | 6.0 ± 1.6 | 4.3 ± 0.1 | 2.6 ± 0.5 | 2.1 ± 0.3 | 1.4 ± 0.3 | 0.7 ± 0.1 |
| D15N | 14.1 ± 3.2 | 17.5 ± 1.4 * | 6.8 ± 2.5 | 2.8 ± 0.5 | 1.4 ± 0.3 * | 1.2 ± 0.1 * | 0.8 ± 0.1 * | 0.56 ± 0.04 |
| Y16D | 13.2 ± 2.6 | 3.6 ± 1.04 * | 1.7 ± 0.1 * | 1.2 ± 0.4 * | 2.1 ± 0.3 | 0.4 ± 0.1 * | 0.2 ± 0.1 * | 0.2 ± 0.1 * |
| E17A | 15.2 ± 2.4 | 5.3 ± 3.0 | 1.9 ± 0.1 * | n.d. | 9.1 ± 0.6 | 5.7 ± 1.4 * | 2.9 ± 0.2 * | n.d. |
| T18V | 0.7 ± 0.1 * | n.d. | n.d. | n.d. | 0.2 ± 0.1 * | n.d. | n.d. | n.d. |
| T354A | 12.03 ± 4.6 | 10.5 ± 1.3 | 9.7 ± 2.2 * | 5.9 ± 1.9 | 2.2 ± 0.4 | 0.9 ± 0.3 * | 0.8 ± 0.2 * | 0.4 ± 0.1 * |
| Y362A | 4.6 ± 1.2 | 3.9 ± 0.8 * | 2.6 ± 0.5 * | 0.9 ± 0.4 * | 2.1 ± 0.3 | 0.9 ± 0.1 * | 0.6 ± 0.1 * | 0.1 ± 0.03 * |
| S363A | 8.4 ± 2.6 * | 5.3 ± 1.1 | 5.1 ± 1.2 | 4.5 ± 0.9 | 3.8 ± 0.8 | 2.8 ± 0.3 | 2.2 ± 0.6 * | 2.4 ± 0.4 * |
| S363C | 3.8 ± 0.5 * | 3.1 ± 0.6 * | 3.9 ± 0.6 | 4.8 ± 0.8 | 1.4 ± 0.3 * | 0.4 ± 0.01 * | 0.2 ± 0.02 * | 0.1 ± 0.01 * |
| S363K | 8.1 ± 2.2 * | 7.2 ± 1.8 | 7.0 ± 1.6 | 6.9 ± 2.2 | 1.9 ± 0.6 | 1.0 ± 0.3 * | 0.8 ± 0.3 | 0.6 ± 0.2 |
| S363D | 5.2 ± 1.1 * | 4.2 ± 0.9 * | 3.4 ± 0.8 * | 2.3 ± 0.5 | 1.9 ± 0.2 | 1.0 ± 0.2 * | 0.9 ± 0.2 | 0.6 ± 0.2 |
| S363G | 7.6 ± 0.9 * | 9.6 ± 1.7 | 9.5 ± 2.9 * | 8.2 ± 1.3 * | 1.2 ± 0.3 * | 1.0 ± 0.2 * | 0.7 ± 0.1 * | 0.5 ± 0.1 |
| S363Y | 8.2 ± 2.8 * | 8.7 ± 2.8 | 6.9 ± 1.7 | 5.3 ± 1.4 | 0.7 ± 0.2 * | 0.5 ± 0.2 * | 0.3 ± 0.1 * | 0.2 ± 0.1 * |
| P2X2b | 6.8 ± 1.1 | 3.5 ± 1.3 | 2.6 ± 0.5 | n.d. | 4.8 ± 0.6 | 4.1 ± 0.5 | 3.3 ± 0.8 | n.d. |
| ΔCR | 22.3 ± 6.9 * | 14.5 ± 5.4 * | 8.3 ± 0.5 | 1.5 ± 0.4 * | 1.9 ± 0.4 | 1.5 ± 0.4 | 0.5 ± 0.1 | 0.17 ± 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokic, M.B.; Castro, P.; Leiva-Salcedo, E.; Tomic, M.; Stojilkovic, S.S.; Coddou, C. Opposing Roles of Calcium and Intracellular ATP on Gating of the Purinergic P2X2 Receptor Channel. Int. J. Mol. Sci. 2018, 19, 1161. https://doi.org/10.3390/ijms19041161
Rokic MB, Castro P, Leiva-Salcedo E, Tomic M, Stojilkovic SS, Coddou C. Opposing Roles of Calcium and Intracellular ATP on Gating of the Purinergic P2X2 Receptor Channel. International Journal of Molecular Sciences. 2018; 19(4):1161. https://doi.org/10.3390/ijms19041161
Chicago/Turabian StyleRokic, Milos B., Patricio Castro, Elias Leiva-Salcedo, Melanija Tomic, Stanko S. Stojilkovic, and Claudio Coddou. 2018. "Opposing Roles of Calcium and Intracellular ATP on Gating of the Purinergic P2X2 Receptor Channel" International Journal of Molecular Sciences 19, no. 4: 1161. https://doi.org/10.3390/ijms19041161





