Structural Requirements of Alkylglyceryl-l-Ascorbic Acid Derivatives for Melanogenesis Inhibitory Activity
Abstract
:1. Introduction
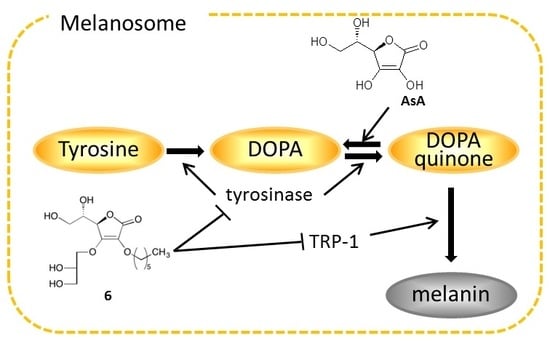
2. Results and Discussion
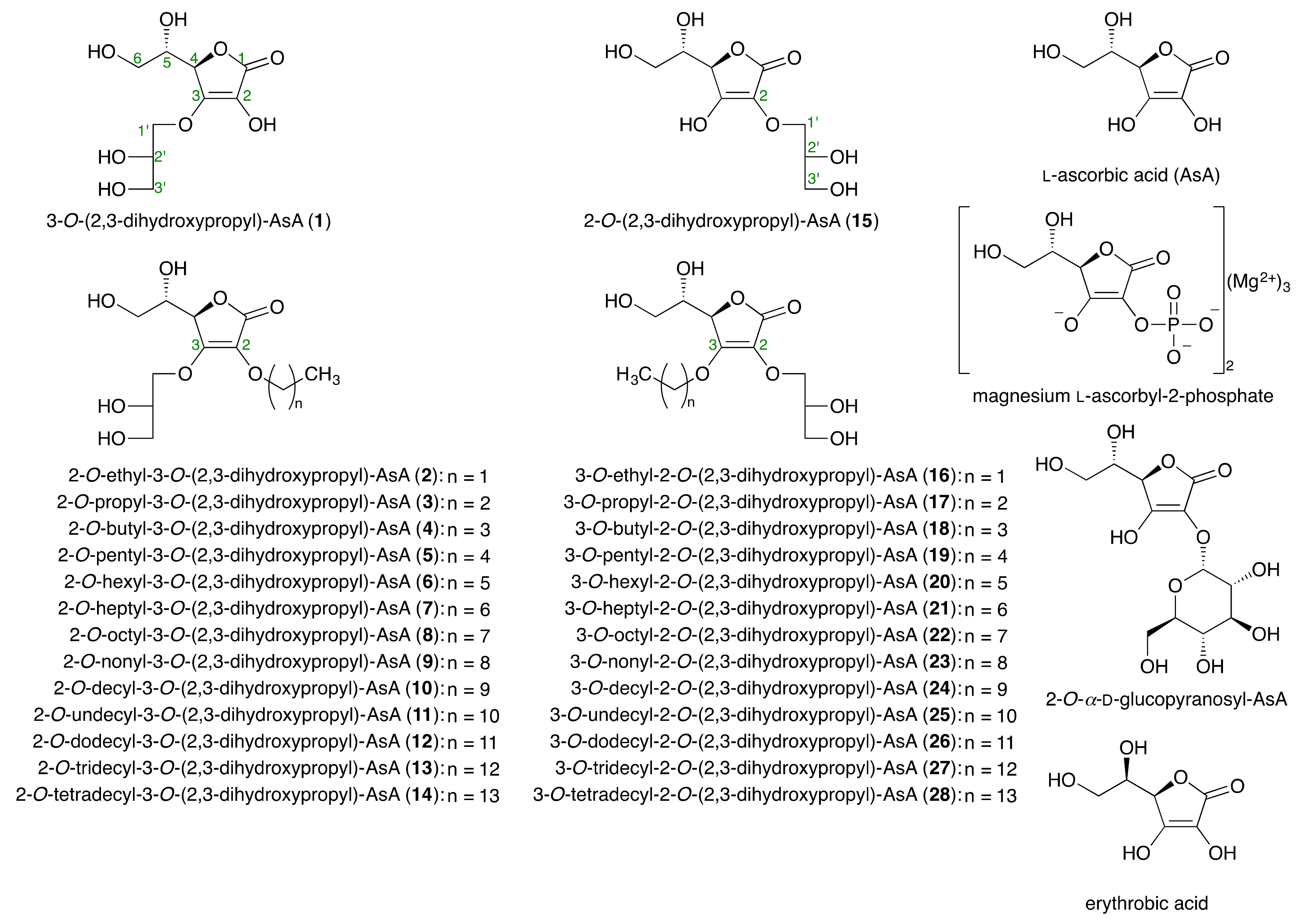
2.1. Syntheses of Alkylglyceryl AsA Derivatives (1–28)
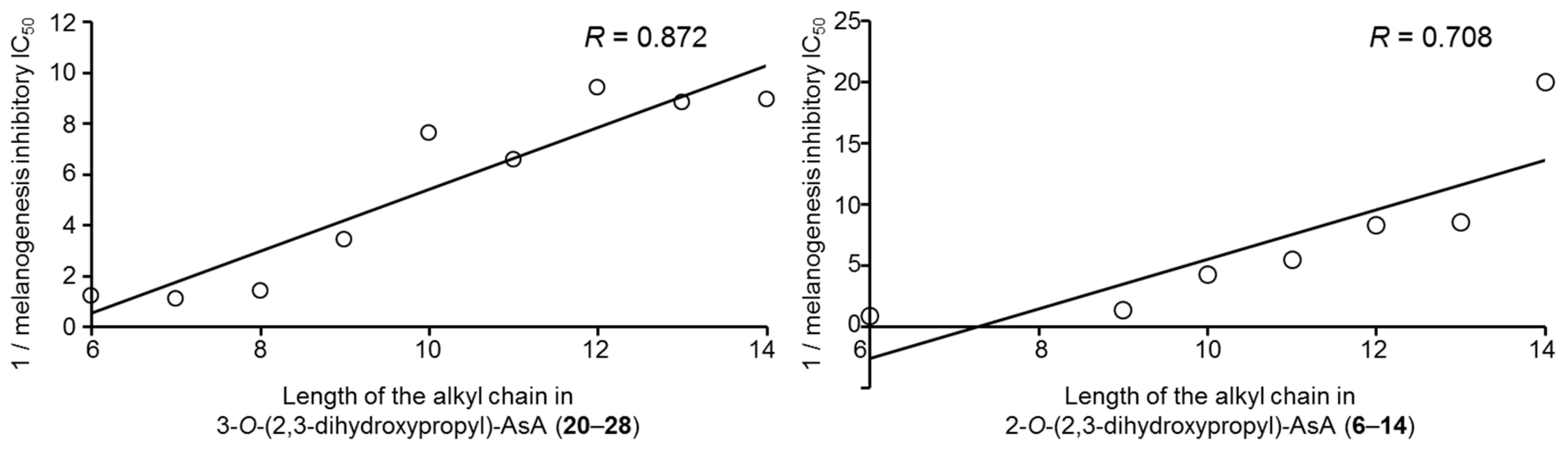
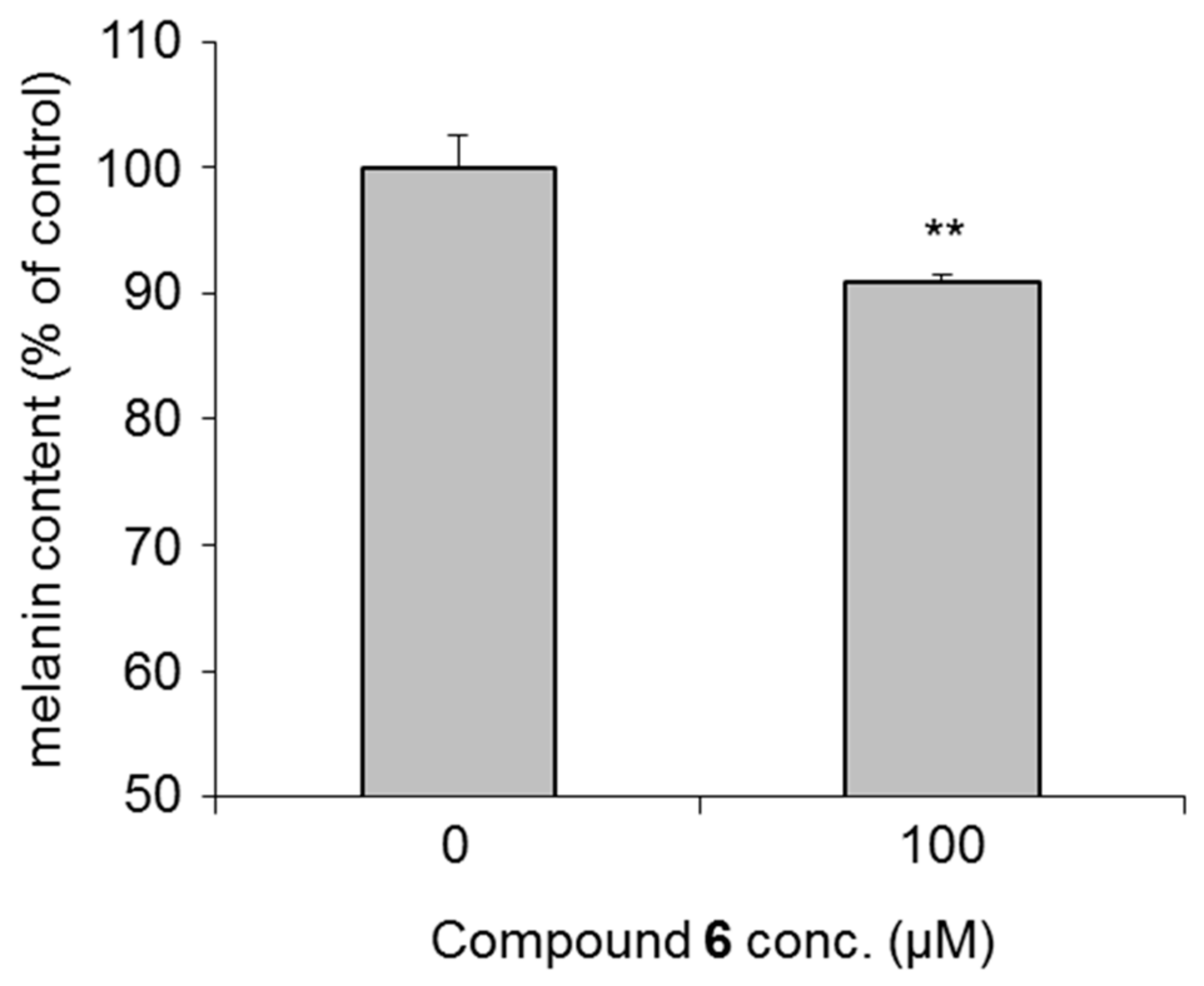
2.2. Effects of the Alkylglyceryl AsA Derivatives (1–28) and Commercially Available AsA Derivatives on Theophylline-Stimulated Melanogenesis Inhibitory Activity
2.3. Stability in Aqueous Solution
2.4. Effects on Tyrosinase
2.5. Effects on Expression of Tyrosinase, TRP-1, and TRP-2 mRNA
2.6. Effects on Expression of Tyrosinase Protein
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Syntheses of Alkylglyceryl Ascorbic Acid Derivatives
3.3. Reagents for Bioassays
3.4. Cell Culture
3.5. Melanogenesis and Cell Viability
3.6. Melanogenesis in Normal Melanocytes
3.7. AsA Derivative Stability
3.8. Mushroom Tyrosinase
3.9. Mammalian Tyrosinase
3.10. Expression of Tyrosinase, TRP-1, and TRP-2 mRNA
3.11. Expression of Tyrosinase Protein
3.12. Tyrosinase Activity in B16 Cells
3.13. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Land, E.J.; Ramsden, C.A.; Riley, P.A. Tyrosinase autoactivation and the chemistry of ortho-quinone amines. Acc. Chem. Res. 2003, 36, 300–308. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Land, E.J.; Ramsden, C.A.; Riley, P.A. 5,6-Dihydroxyindoles and indole-5,6-diones. Adv. Heterocycl. Chem. 2005, 89, 1–63. [Google Scholar]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty Shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigm. Cell Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Prota, G. Progress in the chemistry of melanins and related metabolites. Med. Res. Rev. 1988, 8, 525–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Korner, A.M.; Pawelek, J.M. New regulators of melanogenesis are associated with purified tyrosinase isozymes. J. Investig. Dermatol. 1982, 79, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Jiménez, M. Mammalian tyrosinase-the critical regulatory control point in melanocyte pigmentation. Int. J. Biochem. 1987, 19, 1141–1147. [Google Scholar] [CrossRef]
- Kuzumaki, T.; Matsuda, A.; Wakamatsu, K.; Ito, S.; Ishikawa, K. Eumelanin biosynthesis is regulated by coordinate expression of tyrosinase and tyrosinase-related protein-1 genes. Exp. Cell Res. 1993, 207, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, P.S.; Gilchrest, B.A. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J. Cell. Physiol. 1987, 133, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Slomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to Stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Todd, C.; Cresswell, J.E.; Thody, A.J. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J. Cell Sci. 1994, 107, 205–211. [Google Scholar] [PubMed]
- Steinberg, M.L.; Whittaker, J.R. Stimulation of melanotic expression in a melanoma cell line by theophylline. J. Cell. Physiol. 1976, 87, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Nakanishi, Y.; Ninomiya, K.; Matsuda, H.; Nakashima, S.; Miki, H.; Miyashita, Y.; Yoshikawa, M.; Hayakawa, T.; Muraoka, O. Dimeric pyrrolidinoindoline-type alkaloids with melanogenesis inhibitory activity in flower buds of Chimonanthus praecox. J. Nat. Med. 2014, 68, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, G.; Sugano, Y.; Shirato, M.; Sonoda, N.; Tsutsui, N.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Muraoka, O. Total synthesis of 4,5-didehydroguadiscine: A potent melanogenesis inhibitor from the Brazilian medicinal herb, Hornschuchia obliqua. J. Nat. Prod. 2015, 78, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Kitagawa, N.; Tanabe, G.; Ninomiya, K.; Okugawa, S.; Motai, C.; Yoshikawa, M.; Lee, I.-J.; Muraoka, O. Quantitative determination of alkaloids in lotus flower (flower buds of Nelumbo nucifera) and their melanogenesis inhibitory activity. Molecules 2016, 21, 930. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Ninomiya, K.; Kuramoto, H.; Kamei, I.; Yoshikawa, M.; Muraoka, O. Phenylethanoid and phenylpropanoid glycosides with melanogenesis inhibitory activity from the flowers of Nercissus tazetta var. chinensis. J. Nat. Med. 2016, 70, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Matsumoto, T.; Chaipech, S.; Miyake, S.; Katsuyama, Y.; Tsuboyama, A.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O.; Morikawa, T. Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J. Nat. Med. 2016, 70, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Kamei, I.; Katsuyama, Y.; Imagawa, T.; Chaipech, S.; Muraoka, O.; Morikawa, T. Melanogenesis inhibitory activity of a 7-O-9′-linked neolignan from Alpinia galanga. Bioorg. Med. Chem. 2016, 24, 6215–6224. [Google Scholar] [CrossRef] [PubMed]
- Manse, Y.; Ninomiya, K.; Okazaki, A.; Okada-Nishida, E.; Imagawa, T.; Imamura-Mizushima, M.; Yamano, Y.; Kaname, K.; Nakamura, S.; Morikawa, T. Melanogenesis inhibitory activity of diterpenoid and triterpenoid constituents from the aerial part of Isodon trichocarpus. Nat. Prod. Commun. 2017, 12, 1185–1188. [Google Scholar]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Hashimoto, Y.; Chaipech, S.; Muraoka, O.; Morikawa, T. Labdane-type diterpenes, galangalditerpenes A-C, with melanogenesis inhibitory activity from the fruit of Alpinia galanga. Molecules 2017, 22, 2279. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Murata, T.; Onuma, T.; Kameyama, K.; Sakai, C.; Kondo, S.; Yonemoto, K.; Quigley, J.; Dorsky, A.; Bucks, D.; et al. Inhibitory effects of magnesium ascorbyl phosphate on melanogenesis. J. Soc. Cosmet. Chem. Jpn. 1993, 27, 409–414. [Google Scholar] [CrossRef]
- Kumano, Y.; Sakamoto, T.; Egawa, M.; Iwai, I.; Tanaka, M.; Yamamoto, I. In vitro and in vivo prolonged biological activities of novel vitamin C derivative, 2-O-α-d-glucopyranosyl-l-acsorbic acid (AA-2G), in cosmetic fields. J. Nutr. Sci. Vitaminol. 1998, 44, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; Campos, P.M.M. Histopathological, morphometric and stereological studies of ascorbic acid and magnesium ascorbyl phosphate in a skin formulation. Int. J. Cosmet. Sci. 2000, 22, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Tangsupa-a-nan, V.; Onkoksiing, T.; Kongtaphan, K.; Kasetsinsombat, K.; Akarasereenont, P.; Wongkajornsilp, A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defence and nitric oxide system. Arch. Pharm. Res. 2011, 34, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-A.; Son, Y.-O.; Kook, S.-H.; Choi, K.-C.; Lee, J.-C. Ascorbic acid increases the activity and synthesis of tyrosinase in B16F10 cells through activation of p38 mitogen-activated protein kinase. Arch. Dermatol. Res. 2011, 303, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Sakai, C.; Kondoh, S.; Yonemoto, K.; Nishiyama, S.; Tagawa, M.; Murata, T.; Ohnuma, T.; Dorsky, A.; Bucks, D.; et al. Inhibitory effect of magnesium l-ascorbyl-2-phosphate (VC-PMG) on melanogenesis in vitro and in vivo. J. Am. Acad. Dermatol. 1996, 34, 29–33. [Google Scholar] [CrossRef]
- Matsuda, S.; Shibayama, H.; Hisama, M.; Ohtsuki, M.; Iwaki, M. Inhibitory effects of a novel ascorbic derivative, disodium isostearyl 2-O-l-ascorbyl phosphate on melanogenesis. Chem. Pharm. Bull. 2008, 56, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Park, S.; Seok, J.K.; Liu, K.-H.; Boo, Y.C. Ascorbyl coumarates as multifunctional cosmeceutical agents that inhibit melanogenesis and enhance collagen synthesis. Arch. Dermatol. Res. 2015, 307, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; An, H.S.; Bae, J.-S.; Kim, J.Y.; Choi, C.H.; Kim, J.Y.; Lim, J.K.; Choi, J.-H.; Song, H.; Moon, S.H.; et al. Effects of palmitoyl-KVK-l-ascorbic acid on skin wrinkles and pigmentation. Arch. Dermatol. Res. 2017, 309, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Taira, N.; Kamiyama, A.; Uehara, K.; Hashimoto, N. Ascorbic Acid Derivative or Salt Thereof, Production Method Thereof, and Cosmetic. U.S. Patent US 8163939B2, 24 April 2012. [Google Scholar]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton role for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.W.S.; Maibach, H.I. Skin whitening agents. In Handbook of Cosmetic Science and Technology, 4th ed.; Barel, A.O., Paye, M., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 423–438. ISBN 978-1-84214-564-7. [Google Scholar]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Chiku, K.; Dohi, H.; Saito, A.; Ebise, H.; Kouzai, Y.; Shinoyama, H.; Nishida, Y.; Ando, A. Enzymatic synthesis of 4-hydroxyphenyl-d-oligoxylosides and their notable tyrosinase inhibitory activity. Biosci. Biotechnol. Biochem. 2009, 73, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.-S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Dai, Y.; Zhu, Z.-B.; Tu, F.-J.; Zhang, W.-G.; Yao, X.-S. Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorg. Med. Chem. 2010, 18, 6708–6714. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.-S.; Cho, C.; Hong, M.-C.; Shin, M.-K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, N.; Eldik, R. A mechanistic study of the reduction of quinones by ascorbic acid. J. Chem. Soc. Perkin Trans. 2 1997, 1465–1468. [Google Scholar] [CrossRef]
- Ros, J.R.; Rodríguez-López, J.N.; García-Cánovas, F. Effect of l-ascorbic acid on the monophenolase activity of tyrosinase. Biochem. J. 1993, 295, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; García-Borrón, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18001. [Google Scholar] [PubMed]
- Taira, N.; Katsuyama, Y.; Yoshioka, M.; Okano, Y.; Masaki, H. 3-O-Glycery-2-O-hexyl ascorbate suppresses melanogenesis by interfering with intracellular melanosome transport and suppressing tyrosinase protein synthesis. J. Cosmet. Dermatol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Buscà, R.; Abbe, P.; Bille, K.; Aberdam, E.; Ortonne, J.-P.; Ballotti, R. Different cis-acting elements are lnvolved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell. Biol. 1998, 18, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Mitsuzumi, H. Properties of a novel vitamin C supplement, l-ascorbic acid 2-glucoside, and its uses in the field of foods. Foods Food Ingred. J. Jpn. 2006, 211, 435–444. [Google Scholar]
- Shibayama, H.; Ueda, K.; Yoshio, K.; Matsuda, S.; Hisama, M.; Miyazawa, M. Synthesis and characterization of new ascorbic derivative:sodium isostearyl 2-O-l-ascorbyl phosphate. J. Oleo Sci. 2005, 54, 601–608. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Taira, N.; Yoshioka, M.; Okano, Y.; Masaki, H. Disruption of melanosome transport in melanocytes treated with theophylline causes their degradation by autophagy. Biochem. Biophys. Res. Commun. 2017, 485, 126–130. [Google Scholar] [CrossRef] [PubMed]




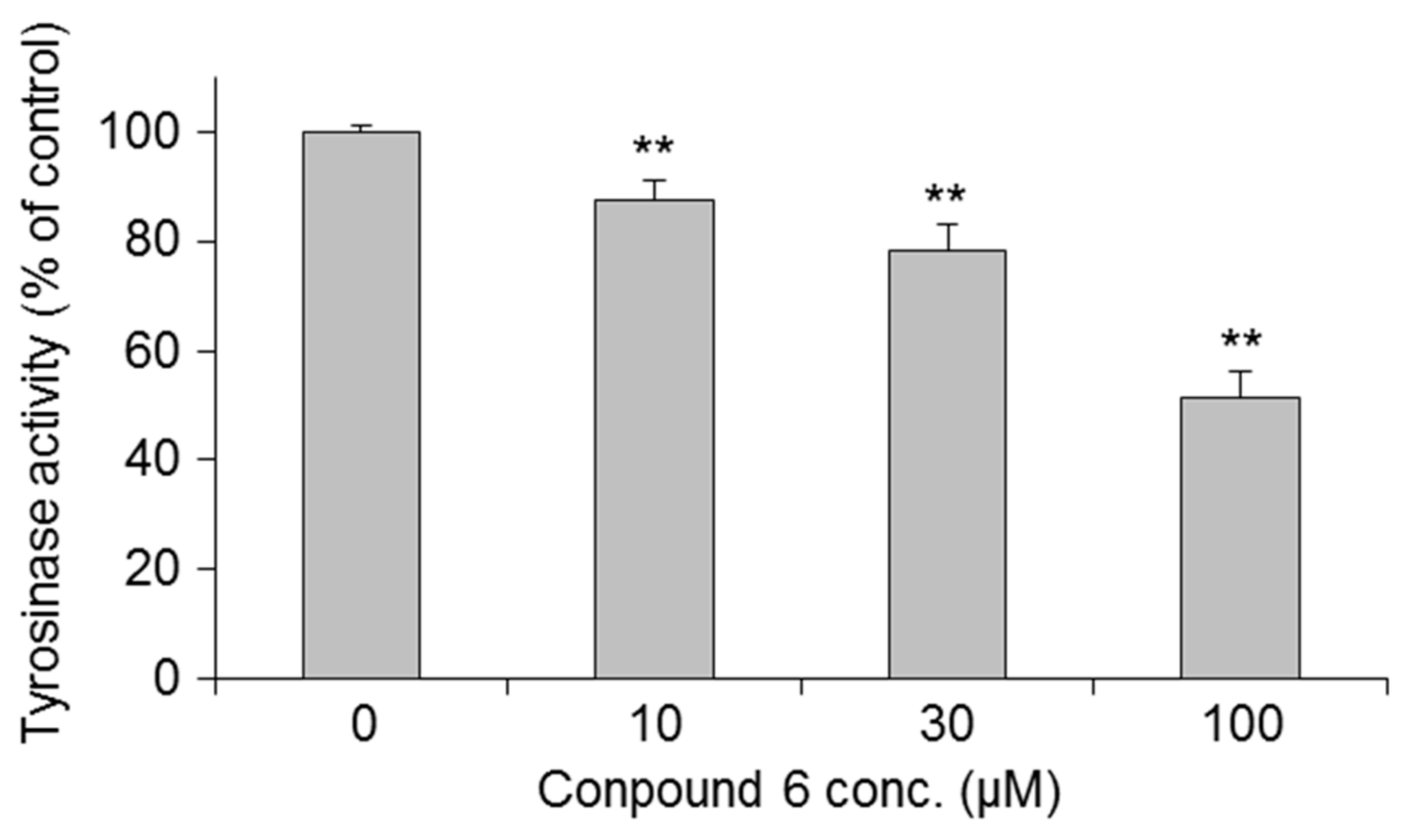
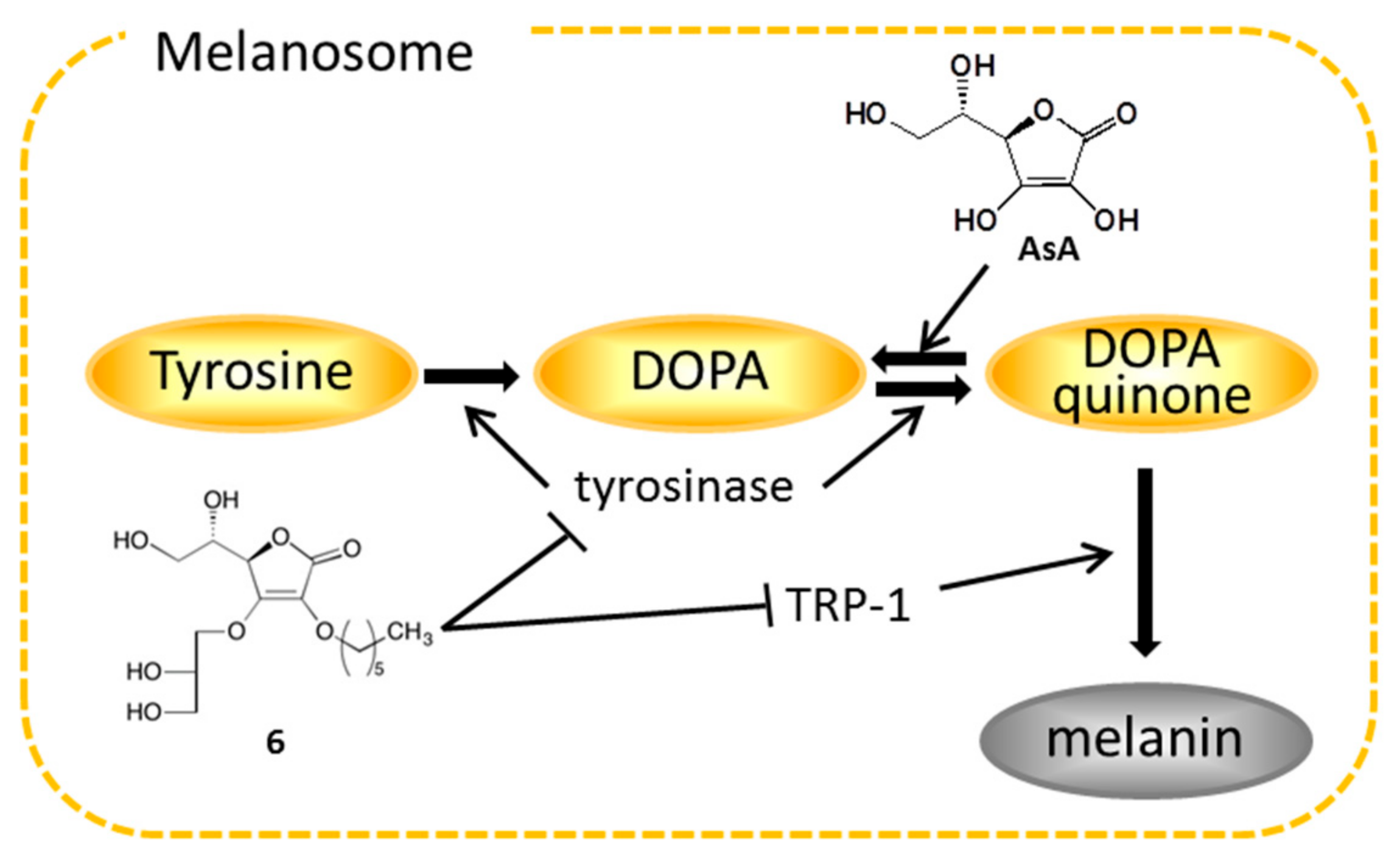
| Treatment | Inhibition (%) | IC50 | ||||
| 0 µM | 100 µM | 300 µM | 1000 µM | 3000 µM | (µM) | |
| 3-O-(2,3-Dihydroxypropyl)-AsA (1) | 0.0 ± 4.1 | −11.8 ± 1.9 | −10.7 ± 1.1 | −5.0 ± 2.9 | 0.5 ± 2.5 | >3000 |
| (100.0 ± 6.4) | (100.0 ± 1.0) | (96.8 ± 2.3) | (100.4 ± 0.4) | (107.9 ± 1.0) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-ethyl-AsA (2) | 0.0 ± 5.9 | −28.1 ± 3.2 | −2.8 ± 3.6 | −8.9 ± 2.0 | 40.4 ± 8.6 ** | >3000 |
| (100.0 ± 3.3) | (101.3 ± 3.3) | (99.7 ± 1.8) | (101.3 ± 1.5) | (103.8 ± 4.1) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-propyl-AsA (3) | 0.0 ± 9.2 | −2.1 ± 5.3 | 8.2 ± 3.2 | 2.5 ± 7.9 | 35.9 ± 3.4 * | >3000 |
| (100.0 ± 1.3) | (101.6 ± 1.9) | (98.2 ± 1.7) | (94.5 ± 2.0) | (95.0 ± 1.5) | ||
| 2-O-Butyl-3-O-(2,3-dihydroxy-propyl)-AsA (4) | 0.0 ± 6.0 | −1.2 ± 0.8 | 20.8 ± 10.6 | 30.2 ± 5.1 ** | 58.9 ± 2.7 ** | 2220 |
| (100.0 ± 2.1) | (94.5 ± 2.3) | (97.4 ± 0.8) | (93.9 ± 0.8) | (88.7 ± 1.1) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-pentyl-AsA (5) | 0.0 ± 11.4 | −14.0 ± 9.7 | 29.8 ± 8.1 | 45.9 ± 1.2 ** | 81.9 ± 3.3 ** | 931 |
| (100.0 ± 2.1) | (118.1 ± 2.9) | (107.3 ± 2.3) | (105.1 ± 1.2) | (78.7 ± 1.1 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-AsA (15) | 0.0 ± 4.4 | −9.7 ± 1.2 | −4.8 ± 1.0 | −2.3 ± 1.7 | −6.1 ± 2.7 | >3000 |
| (100.0 ± 8.5) | (101.2 ± 2.2) | (102.0 ± 3.6) | (101.6 ± 3.2) | (106.7 ± 1.8) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-ethyl-AsA (16) | 0.0 ± 3.0 | −4.2 ± 2.3 | −5.4 ± 1.5 | 12.1 ± 2.2 ** | 42.0 ± 1.4 ** | >3000 |
| (100.0 ± 6.2) | (104.9 ± 1.4) | (95.9 ± 1.2) | (95.9 ± 3.1) | (84.0 ± 1.1) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-propyl-AsA (17) | 0.0 ± 7.4 | −5.9 ± 3.3 | −3.9 ± 4.1 | 1.3 ± 4.2 | 23.2 ± 5.6 ** | >3000 |
| (100.0 ± 4.3) | (98.4 ± 4.7) | (95.6 ± 3.5) | (92.0 ± 2.3) | (82.9 ± 2.3) | ||
| 3-O-Butyl-2-O-(2,3-dihydroxy-propyl)-AsA (18) | 0.0 ± 2.7 | 0.2 ± 4.4 | 36.3 ± 2.8 ** | 68.0 ± 2.1 ** | 84.1 ± 1.4 ** | 473 |
| (100.0 ± 1.0) | (99.8 ± 1.1) | (89.1 ± 1.6) | (78.1 ± 0.5 #) | (70.6 ± 1.0 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-pentyl-AsA (19) | 0.0 ± 5.2 | 14.0 ± 4.4 * | 53.0 ± 1.1 ** | 83.1 ± 1.4 ** | 97.1 ± 0.9 ** | 283 |
| (100.0 ± 0.4) | (86.7 ± 1.5) | (73.4 ± 0.7 #) | (54.7 ± 0.8 #) | (22.5 ± 0.5 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-hexyl-AsA (20) | 0.0 ± 2.9 | 43.5 ± 2.6 ** | 77.0 ± 2.1 ** | 94.9 ± 1.5 ** | 80.9 ± 4.1 ** | 117 |
| (100.0 ± 1.0) | (95.3 ± 0.6) | (83.9 ± 0.4) | (60.8 ± 0.6 #) | (44.7 ± 1.9 #) | ||
| AsA | 0.0 ± 1.7 | — | — | −17.4 ± 4.0 | 3.8 ± 4.7 | >3000 |
| (100.0 ± 2.2) | (103.8 ± 0.8) | (89.2 ± 0.8) | ||||
| Magnesium l-ascorbyl-2-phosphate | 0.0 ± 5.5 | — | — | 2.1 ± 4.1 | 14.5 ± 1.3 * | >3000 |
| (100.0 ± 0.5) | (125.6 ± 3.6) | (92.8 ± 3.0) | ||||
| 2-O-α-d-Glucopyranosyl-AsA | 0.0 ± 3.0 | — | — | −8.9 ± 2.7 | 15.0 ± 3.5 * | >3000 |
| (100.0 ± 4.3) | (106.8 ± 2.2) | (108.4 ± 5.4) | ||||
| Erythrobic acid | 0.0 ± 6.7 | −22.9 ± 6.5 | −10.6 ± 4.8 | 75.8 ± 4.2 ** | 92.6 ± 15.3 ** | — |
| (100.0 ± 5.7) | (98.7 ± 4.3) | (94.8 ± 3.6) | (44.5 ± 2.0 #) | (29.8 ± 2.2 #) | ||
| Arbutin | 0.0 ± 10.0 | 32.2 ± 3.4 ** | 22.3 ± 4.3 ** | 63.0 ± 2.3 ** | 94.0 ± 2.8 ** | 830 |
| (100.0 ± 3.7) | (92.2 ± 0.6) | (96.0 ± 2.0) | (96.2 ± 2.7) | (105.8 ± 2.9) | ||
| Treatment | Inhibition (%) | IC50 | ||||
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | (µM) | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-hexyl-AsA (6) | 0.0 ± 5.7 | −1.8 ± 4.9 | 5.6 ± 2.0 | 26.2 ± 6.0 ** | 53.1 ± 3.1 ** | 81.4 |
| (100.0 ± 6.0) | (102.1 ± 4.9 #) | (96.1 ± 4.9) | (88.4 ± 6.6) | (77.1 ± 5.8 #) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-heptyl-AsA (7) | 0.0 ± 9.5 | −4.2 ± 6.3 | 3.4 ± 5.2 | 20.0 ± 6.4 * | 52.2 ± 5.0 ** | 89.1 |
| (100.0 ± 0.7) | (100.6 ± 3.0) | (95.4 ± 3.6) | (91.9 ± 4.7) | (79.7 ± 2.9 #) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-octyl-AsA (8) | 0.0 ± 8.3 | 5.7 ± 6.9 | 10.6 ± 4.6 * | 22.4 ± 4.4 ** | 64.1 ± 8.2 ** | 68.8 |
| (100.0 ± 5.1) | (108.4 ± 6.2) | (102.8 ± 4.8) | (92.5 ± 4.6) | (80.0 ± 4.4) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-nonyl-AsA (9) | 0.0 ± 5.9 | 0.2 ± 4.4 | 19.1 ± 1.3 ** | 51.9 ± 4.6 ** | 91.6 ± 1.2 ** | 28.8 |
| (100.0 ± 2.3) | (99.2 ± 0.5) | (88.9 ± 4.2) | (77.6 ± 3.6 #) | (58.4 ± 2.3 #) | ||
| 2-O-Decyl-3-O-(2,3-dihydroxy-propyl)-AsA (10) | 0.0 ± 6.7 | 3.7 ± 7.8 | 39.2 ± 4.1 ** | 78.1 ± 4.8 ** | 98.0 ± 3.7 ** | 13.0 |
| (100.0 ± 4.3) | (98.6 ± 4.3) | (85.9 ± 2.3) | (73.5 ± 5.8 #) | (27.9 ± 2.8 #) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-undecyl-AsA (11) | 0.0 ± 2.9 | 12.4 ± 3.0 ** | 35.4 ± 2.8 ** | 89.3 ± 1.5 ** | 100.3 ± 4.7 ** | 15.1 |
| (100.0 ± 4.8) | (106.2 ± 2.5) | (90.8 ± 7.1) | (69.0 ± 1.9 #) | (25.8 ± 1.9 #) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-dodecyl-AsA (12) | 0.0 ± 5.3 | 20.4 ± 10.2 * | 50.0 ± 5.7 ** | 96.0 ± 2.9 ** | 97.9 ± 4.2 ** | 10.6 |
| (100.0 ± 7.3) | (112.1 ± 5.8) | (103.8 ± 1.2) | (73.3 ± 2.1 #) | (24.6 ± 3.4 #) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-tridecyl-AsA (13) | 0.0 ± 5.2 | 0.1 ± 8.6 | 46.0 ± 7.9 ** | 97.1 ± 2.3 ** | 107.8 ± 9.1 ** | 11.3 |
| (100.0 ± 2.7) | (96.3 ± 3.5) | (87.2 ± 1.8) | (63.7 ± 1.1 #) | (23.5 ± 2.1 #) | ||
| 3-O-(2,3-Dihydroxypropyl)-2-O-tetradecyl-AsA (14) | 0.0 ± 7.1 | 6.3 ± 2.6 | 48.4 ± 2.2 ** | 97.6 ± 1.8 ** | 100.0 ± 18.2 ** | 11.1 |
| (100.0 ± 2.3) | (101.2 ± 1.8) | (89.1 ± 4.5) | (56.3 ± 3.2 #) | (21.0 ± 2.4 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-heptyl-AsA (21) | 0.0 ± 2.8 | −6.3 ± 2.7 * | 0.9 ± 8.2 | 21.0 ± 5.0 ** | 44.6 ± 6.2 ** | >100 |
| (100.0 ± 7.1) | (97.9 ± 0.9) | (92.7 ± 3.7) | (92.7 ± 4.2) | (86.3 ± 2.7) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-octyl-AsA (22) | 0.0 ± 8.8 | −10.4 ± 6.0 | 1.0 ± 7.0 | 2.3 ± 5.9 | 34.8 ± 8.2 ** | >100 |
| (100.0 ± 2.0) | (98.8 ± 3.4) | (99.3 ± 5.2) | (91.6 ± 4.0) | (83.0 ± 4.9) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-nonyl-AsA (23) | 0.0 ± 14.8 | −1.3 ± 9.4 | −2.6 ± 8.0 | 14.9 ± 5.6 | 77.1 ± 2.4 ** | 72.9 |
| (100.0 ± 2.4) | (96.6 ± 5.5) | (90.6 ± 2.2) | (81.2 ± 4.7) | (60.7 ± 3.2 #) | ||
| 3-O-Decyl-2-O-(2,3-dihydroxy-propyl)-AsA (24) | 0.0 ± 5.0 | −5.8 ± 6.0 | 20.9 ± 5.4 ** | 64.3 ± 3.8 ** | 102.7 ± 2.4 ** | 23.5 |
| (100.0 ± 3.2) | (92.5 ± 5.7) | (87.1 ± 1.6) | (68.5 ± 2.6 #) | (34.3 ± 1.5 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-undecyl-AsA (25) | 0.0 ± 2.0 | −5.1 ± 2.3 * | 26.6 ± 3.0 ** | 85.0 ± 3.8 ** | 100.8 ± 10.1 ** | 18.1 |
| (100.0 ± 4.8) | (101.0 ± 2.9) | (85.8 ± 3.5) | (53.6 ± 2.7 #) | (24.0 ± 0.5 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-dodecyl-AsA (26) | 0.0 ± 7.9 | 14.1 ± 8.5 * | 46.0 ± 3.0 ** | 96.2 ± 2.8 ** | 89.6 ± 24.4 ** | 12.1 |
| (100.0 ± 7.2) | (99.8 ± 4.2) | (87.3 ± 2.8) | (45.5 ± 1.1 #) | (24.3 ± 12.7 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-tridecyl-AsA (27) | 0.0 ± 8.4 | 20.1 ± 5.7 ** | 44.2 ± 3.9 ** | 96.6 ± 3.0 ** | 112.9 ± 10.1 ** | 11.7 |
| (100.0 ± .5.5) | (104.9 ± 4.1) | (84.3 ± 2.8) | (40.2 ± 2.5 #) | (21.0 ± 1.5 #) | ||
| 2-O-(2,3-Dihydroxypropyl)-3-O-tetradecyl-AsA (28) | 0.0 ± 7.6 | 32.8 ± 7.2 ** | 75.7 ± 3.9 ** | 95.9 ± 8.4 ** | 95.2 ± 20.7 ** | 5.0 |
| (100.0 ± 1.3) | (85.1 ± 4.6) | (62.6 ± 1.6 #) | (23.0 ± 2.1 #) | (18.1 ± 1.9 #) | ||
| Hydroquinone | 0.0 ± 4.4 | 37.4 ± 3.7 ** | 59.5 ± 3.7 ** | 76.3 ± 2.1 ** | — | 8.7 |
| (100.0 ± 1.6) | (94.1 ± 1.7) | (85.2 ± 1.4) | (64.3 ± 0.8 #) | |||
| Treatment | Inhibition (%) | |||||
| Substrate: l-Tyrosine | Substrate: l-DOPA | |||||
| 0 µM | 30 µM | 100 µM | 0 µM | 30 µM | 100 µM | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-hexyl-AsA (6) | 0.0 ± 0.4 | −1.5 ± 6.7 | 0.9 ± 1.4 | 0.0 ± 6.3 | −1.5 ± 1.5 | −1.0 ± 0.7 |
| 3-O-(2,3-Dihydroxypropyl)-2-O-heptyl-AsA (7) | 0.0 ± 0.4 | −1.7 ± 4.0 | 1.0 ± 2.4 | 0.0 ± 6.3 | −0.7 ± 3.1 | 1.1 ± 4.8 |
| 3-O-(2,3-Dihydroxypropyl)-2-O-octyl-AsA (8) | 0.0 ± 0.4 | −0.3 ± 2.3 | −0.6 ± 4.3 | 0.0 ± 2.4 | 2.4 ± 1.1 | 2.3 ± 2.7 |
| 3-O-(2,3-Dihydroxypropyl)-2-O-nonyl-AsA (9) | 0.0 ± 0.4 | 4.0 ± 6.1 | −1.2 ± 4.5 | 0.0 ± 2.4 | 4.8 ± 6.6 | 0.9 ± 6.0 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-hexyl-AsA (20) | 0.0 ± 10.7 | 1.4 ± 4.7 | −6.1 ± 0.6 | 0.0 ± 8.7 | −5.7 ± 5.1 | −2.4 ± 4.2 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-heptyl-AsA (21) | 0.0 ± 10.7 | −9.2 ± 2.1 | −9.8 ± 3.9 | 0.0 ± 8.7 | −10.0 ± 1.7 | −11.3 ± 2.4 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-octyl-AsA (22) | 0.0 ± 10.7 | −11.5 ± 1.6 | −14.4 ± 1.8 | 0.0 ± 11.2 | −13.1 ± 3.3 | −4.5 ± 2.0 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-nonyl-AsA (23) | 0.0 ± 7.0 | −5.0 ± 3.1 | −2.6 ± 2.4 | 0.0 ± 2.2 | −4.1 ± 2.2 | −1.5 ± 2.2 |
| 0 µM | 10 µM | 30 µM | 0 µM | 10 µM | 30 µM | |
| 2-O-Decyl-3-O-(2,3-dihydroxy-propyl)-AsA (10) | 0.0 ± 0.4 | 1.3 ± 3.1 | 0.4 ± 1.7 | 0.0 ± 2.4 | 0.4 ± 1.1 | 2.4 ± 2.9 |
| 3-O-(2,3-Dihydroxypropyl)-2-O-undecyl-AsA (11) | 0.0 ± 0.4 | 2.9 ± 3.9 | 1.0 ± 2.5 | 0.0 ± 3.2 | −4.6 ± 1.4 | −5.1 ± 2.2 |
| 3-O-(2,3-Dihydroxypropyl)-2-O-dodecyl-AsA (12) | 0.0 ± 6.7 | −0.7 ± 4.5 | −7.1 ± 1.4 | 0.0 ± 3.2 | −3.1 ± 3.3 | −4.6 ± 2.2 |
| 3-O-(2,3-Dihydroxypropyl)-2-O-tridecyl-AsA (13) | 0.0 ± 6.7 | −4.7 ± 2.9 | −7.1 ± 1.7 | 0.0 ± 3.2 | −4.7 ± 5.0 | −4.4 ± 1.8 |
| 3-O-(2,3-Dihydroxypropyl)-2-O-tetradecyl-AsA (14) | 0.0 ± 6.7 | −7.0 ± 6.7 | −9.7 ± 2.7 | 0.0 ± 13.8 | −8.9 ± 3.5 | −8.2 ± 4.3 |
| 3-O-Decyl-2-O-(2,3-dihydroxy-propyl)-AsA (24) | 0.0 ± 7.0 | −4.1 ± 5.3 | −3.1 ± 4.1 | 0.0 ± 4.2 | 0.7 ± 3.8 | −5.3 ± 4.2 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-undecyl-AsA (25) | 0.0 ± 7.0 | −8.0 ± 0.6 | −8.7 ± 1.5 | 0.0 ± 4.2 | −10.2 ± 2.3 | −10.7 ± 3.8 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-dodecyl-AsA (26) | 0.0 ± 7.0 | −2.2 ± 6.4 | −0.5 ± 4.3 | 0.0 ± 3.0 | −6.4 ± 3.1 | −6.6 ± 8.7 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-tridecyl-AsA (27) | 0.0 ± 7.0 | −3.3 ± 8.1 | −5.4 ± 1.9 | 0.0 ± 3.0 | −2.0 ± 3.3 | −3.2 ± 2.7 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-tetradecyl-AsA (28) | 0.0 ± 7.0 | −4.4 ± 4.6 | −8.4 ± 1.0 | 0.0 ± 3.0 | −5.7 ± 9.5 | −8.1 ± 5.2 |
| Substrate: l-Tyrosine | Inhibition (%) | |||||
| Treatment | 0 µM | 10 µM | 30 µM | 100 µM | 300 µM | IC50 (µM) |
| Kojic acid [20,22,23,24,25,26,27] | 0.0 ± 2.4 | 12.2 ± 3.3 | 46.4 ± 2.6 ** | 66.5 ± 2.1 ** | 96.8 ± 0.9 ** | 43.6 |
| Substrate: l-DOPA | Inhibition (%) | |||||
| Treatment | 0 µM | 10 µM | 30 µM | 100 µM | 300 µM | IC50 (µM) |
| Kojic acid [20,22,23,24,25,26,27] | 0.0 ± 0.9 | 22.3 ± 2.1 ** | 50.6 ± 0.6 ** | 78.2 ± 0.7 ** | 89.3 ± 0.3 ** | 29.6 |
| Treatment | Tyrosinase mRNA/β-actin mRNA | ||
| 0 µM | 30 µM | 100 µM | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-hexyl-AsA (6) | 1.00 ± 0.05 | 0.60 ± 0.07 ** | 0.42 ± 0.03 ** |
| 2-O-(2,3-Dihydroxypropyl)-3-O-hexyl-AsA (20) | 1.00 ± 0.22 | 0.72 ± 0.10 | 0.59 ± 0.07 * |
| Treatment | TRP-1 mRNA/β-actin mRNA | ||
| 0 µM | 30 µM | 100 µM | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-hexyl-AsA (6) | 1.00 ± 0.21 | 0.48 ± 0.15 * | 0.37 ± 0.05 ** |
| 2-O-(2,3-Dihydroxypropyl)-3-O-hexyl-AsA (20) | 1.00 ± 0.21 | 0.67 ± 0.12 | 0.50 ± 0.15 * |
| Treatment | TRP-2 mRNA/β-actin mRNA | ||
| 0 µM | 30 µM | 100 µM | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-hexyl-AsA (6) | 1.00 ± 0.32 | 0.53 ± 0.18 | 0.70 ± 0.06 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-hexyl-AsA (20) | 1.00 ± 0.10 | 1.07 ± 0.30 | 0.88 ± 0.25 |
| Treatment | Tyrosinase mRNA/β-actin mRNA | ||
| 0 µM | 3 µM | 10 µM | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-tetradecyl-l-ascorbic acid (14) | 1.00 ± 0.12 | 0.78 ± 0.10 | 0.58 ± 0.09 ** |
| 2-O-(2,3-Dihydroxypropyl)-3-O-tetradecyl-l-ascorbic acid (28) | 1.00 ± 0.12 | 0.54 ± 0.03 ** | 0.29 ± 0.08 ** |
| Treatment | TRP-1 mRNA/β-actin mRNA | ||
| 0 µM | 3 µM | 10 µM | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-tetradecyl-l-ascorbic acid (14) | 1.00 ± 0.24 | 1.02 ± 0.27 | 0.92 ± 0.22 |
| 2-O-(2,3-Dihydroxypropyl)-3-O-tetradecyl-l-ascorbic acid (28) | 1.00 ± 0.24 | 0.88 ± 0.20 | 0.83 ± 0.27 |
| Treatment | TRP-2 mRNA/β-actin mRNA | ||
| 0 µM | 3 µM | 10 µM | |
| 3-O-(2,3-Dihydroxypropyl)-2-O-tetradecyl-l-ascorbic acid (14) | 1.00 ± 0.11 | 0.58 ± 0.06 ** | 0.50 ± 0.08 ** |
| 2-O-(2,3-Dihydroxypropyl)-3-O-tetradecyl-l-ascorbic acid (28) | 1.00 ± 0.11 | 0.43 ± 0.11 ** | 0.35 ± 0.05 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, N.; Katsuyama, Y.; Yoshioka, M.; Muraoka, O.; Morikawa, T. Structural Requirements of Alkylglyceryl-l-Ascorbic Acid Derivatives for Melanogenesis Inhibitory Activity. Int. J. Mol. Sci. 2018, 19, 1144. https://doi.org/10.3390/ijms19041144
Taira N, Katsuyama Y, Yoshioka M, Muraoka O, Morikawa T. Structural Requirements of Alkylglyceryl-l-Ascorbic Acid Derivatives for Melanogenesis Inhibitory Activity. International Journal of Molecular Sciences. 2018; 19(4):1144. https://doi.org/10.3390/ijms19041144
Chicago/Turabian StyleTaira, Norihisa, Yushi Katsuyama, Masato Yoshioka, Osamu Muraoka, and Toshio Morikawa. 2018. "Structural Requirements of Alkylglyceryl-l-Ascorbic Acid Derivatives for Melanogenesis Inhibitory Activity" International Journal of Molecular Sciences 19, no. 4: 1144. https://doi.org/10.3390/ijms19041144