Irradiation-Induced Cardiac Connexin-43 and miR-21 Responses Are Hampered by Treatment with Atorvastatin and Aspirin
Abstract
:1. Introduction
2. Results
2.1. Main Characteristics of Experimental Rats
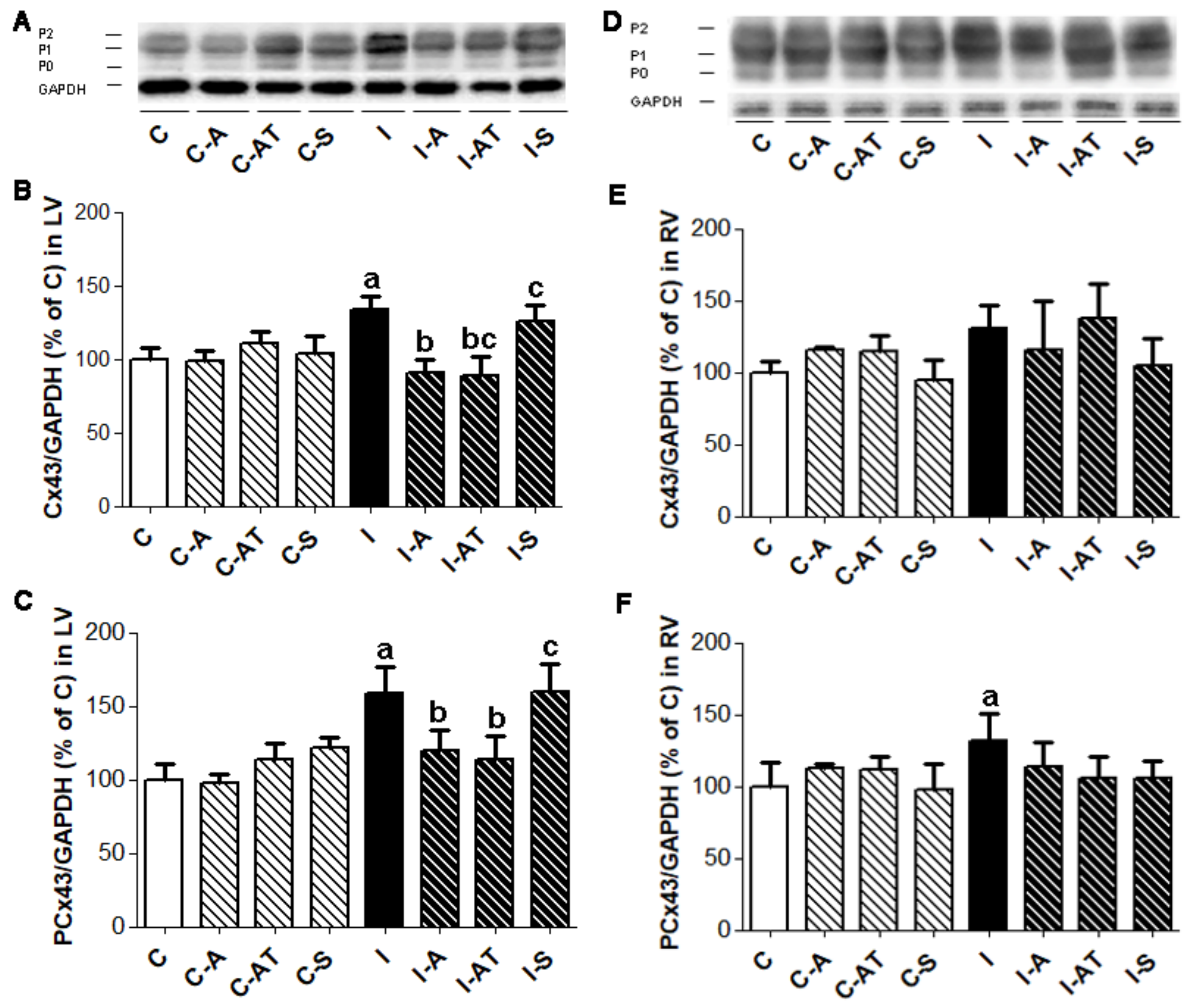
2.2. Protein Expression of Myocardial Cx43 in Control and Irradiated Wistar Rats
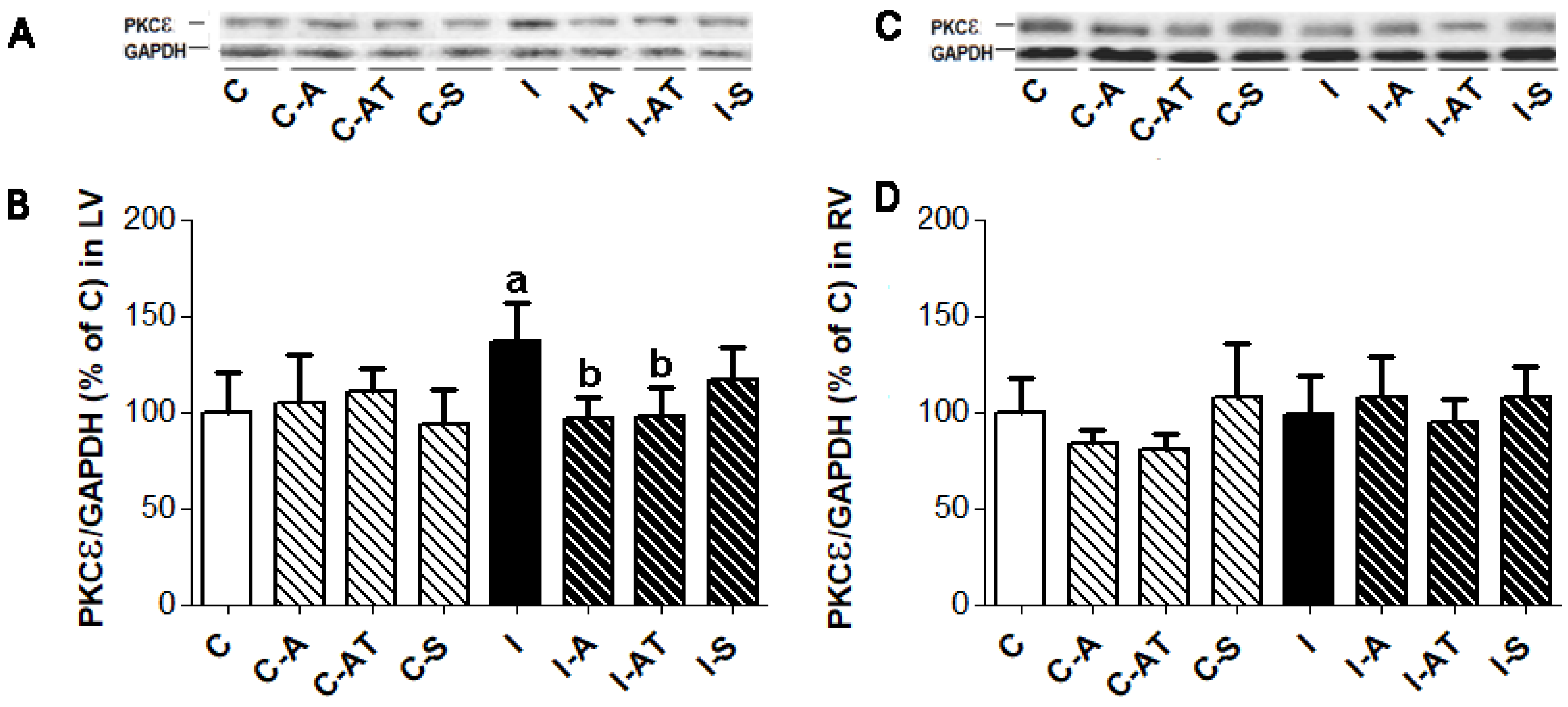
2.3. Expression of Protein Kinase Cε in Control and Irradiated Wistar Rats
2.4. Expression of Protein Kinase C δ in Control and Irradiated Wistar Rats
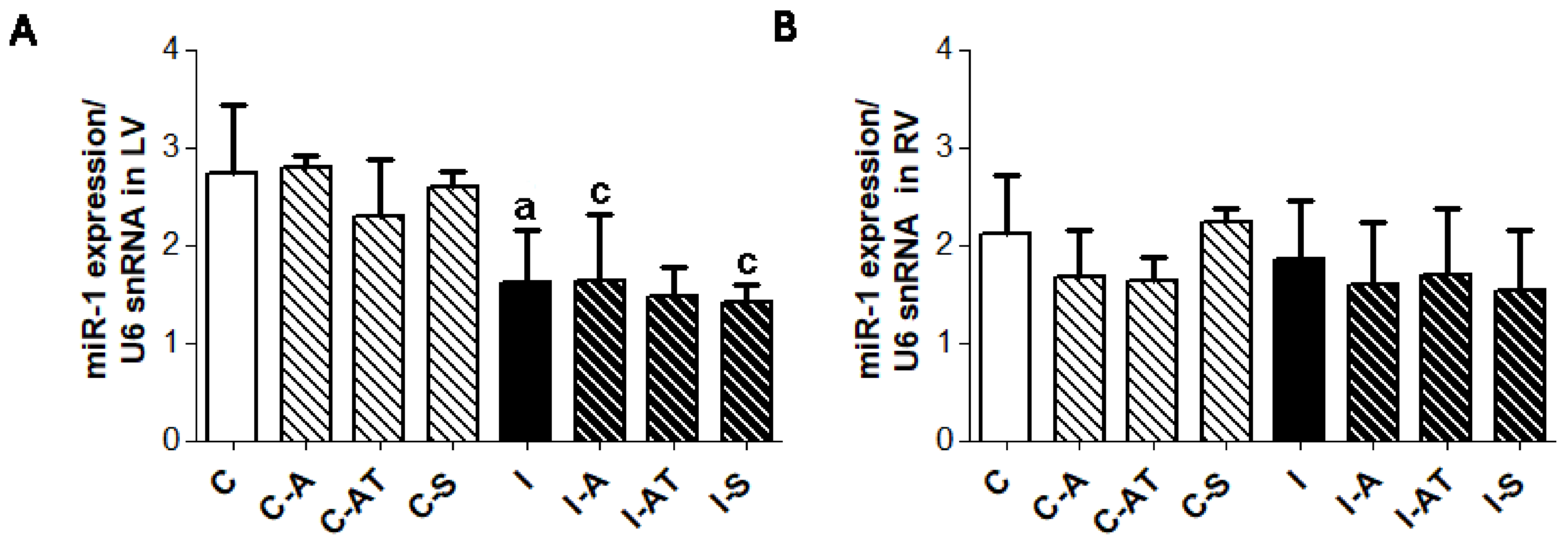
2.5. Myocardial Expression of miR-1 in Control and Irradiated Wistar Rats
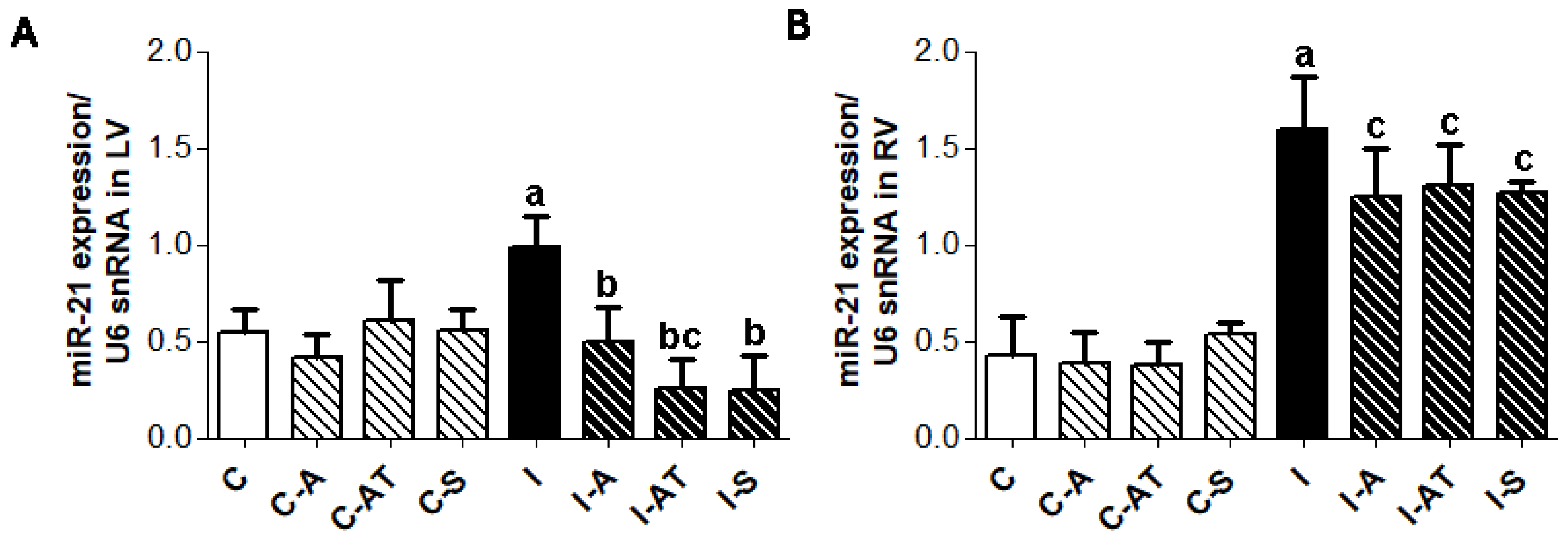
2.6. Expression of miR-21 in Control and Irradiated Wistar Rats
3. Discussion
4. Materials and Methods
4.1. Experimental Model of Irradiation Induced Cardiac Injury
4.2. Determination of Myocardial Cx43, PKCε and PKCδ Protein Expression
4.3. Estimation of Myocardial miR-1 and miR-21 Levels
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cuomo, J.R.; Sharma, G.K.; Conger, P.D.; Weintraub, N.L. Novel concepts in radiation-induced cardiovascular disease. World J. Cardiol. 2016, 8, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Slezak, J.; Kura, B.; Ravingerova, T.; Tribulova, N.; Okruhlicova, L.; Barancik, M. Mechanisms of cardiac radiation injury and potential preventive approaches. Can. J Physiol. Pharmacol. 2015, 27, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Simone, N.L.; Soule, B.P.; Ly, D.; Saleh, A.D.; Savage, J.E.; Degraff, W.; Cook, J.; Harris, C.C.; Gius, D.; Mitchell, J.B. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS ONE 2009, 4, e6377. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Babal, P.; Slezak, J. Implication of microRNAs in the development and potential treatment of radiation—Induced heart disease. Can. J. Physiol. Pharmacol. 2017, 95, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, G.C. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc. Res. 2012, 94, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Viczenczova, C.; Szeiffova Bacova, B.; Egan Benova, T.; Kura, B.; Yin, C.; Weismann, P.; Kukreja, R.; Slezak, J.; Tribulova, N. Myocardial connexin-43 and PKC signalling are involved in adaptation of the heart to irradiation-induced injury: Implication of miR-1 and miR-21. Gen. Physiol. Biophys. 2016, 35, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gürses, I.; Özeren, M.; Serin, M.; Yücel, N.; Erkal, H.S. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol. Res. Pract. 2014, 210, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Amino, M.; Yoshioka, K.; Tanabe, T.; Tanaka, E.; Mori, H.; Furusawa, Y.; Zareba, W.; Yamazaki, M.; Nakagawa, H.; Honjo, H.; et al. Heavy ion radiation up-regulates connexin43 and ameliorates the arrhythmogenic substrates in rabbit hearts after myocardial infarction. Cardiovasc. Res. 2006, 72, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Amino, M.; Yoshioka, K.; Fujibayashi, D.; Hashida, T.; Furusawa, Y.; Zareba, W.; Ikari, Y.; Tanaka, E.; Mori, H.; Inokuchi, S.; et al. Year-long upregulation of connexin43 in rabbit hearts by heavy ion irradiation. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Viczenczova, C.; Kura, B.; Chaudagar, K.K.; Szeiffova Bacova, B.; Egan Benova, T.; Barancik, M.; Knezl, V.; Ravingerova, T.; Tribulova, N.; Slezak, J. Myocardial connexin-43 is upregulated in response to acute cardiac injury in rats. Can. J. Physiol. Pharmacol. 2017, 95, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yan, R.; Wu, Z.; Li, J.; Yan, M.; Hao, X.; Liu, J.; Li, S. 13N-ammonia PET/CT detection of myocardial perfusion abnormalities in beagle dogs after local heart irradiation. J. Nucl. Med. 2017, 58, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Slezak, J.; Kura, B.; Babal, P.; Barancik, M.; Ferko, M.; Frimmel, K.; Kalocayova, B.; Kukreja, R.C.; Lazou, A.; Mezesova, L.; et al. Potential markers and metabolic processes involved in the mechanism of radiation-induced heart injury. Can. J. Physiol. Pharmacol. 2017, 95, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ding, M.; Dong, Z.; Chen, F.; Ye, J.; Wang, S.; Leonard, S.S.; Castranova, V.; Vallyathan, V. Antioxidant properties of aspirin: Characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-kappaB activation, and TNF-alpha production. Mol. Cell. Biochem. 1999, 199, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Carnevale, R.; Pastori, D.; Cangemi, R.; Napoleone, L.; Bartimoccia, S.; Nocella, C.; Basili, S.; Violi, F. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012, 126, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Lefer, A.M.; Scalia, R.; Lefer, D.J. Vascular effects of HMG CoA-reductase inhibitors (statins) unrelated to cholesterol lowering: New concepts for cardiovascular disease. Cardiovasc. Res. 2001, 49, 281–287. [Google Scholar] [CrossRef]
- Webb, D.J.; Freestone, S.; Allen, M.J.; Muirhead, G.J. Sildenafil citrate and blood-pressure-lowering drugs: Results of drug interaction studies with an organic nitrate and a calcium antagonist. Am. J. Cardiol. 1999, 83, 21–28. [Google Scholar] [CrossRef]
- Cihák, R.; Kolár, F.; Pelouch, V.; Procházka, J.; Ostádal, B.; Widimský, J. Functional changes in the right and left ventricle during development of cardiac hypertrophy and after its regression. Cardiovasc. Res. 1992, 26, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Noorman, M.; van Rijen, H.V.; van Veen, T.A.; de Bakker, J.M.; Stein, M. Differences in distribution of fibrosis in the ventricles underlie dominant arrhythmia vulnerability of the right ventricle in senescent mice. Neth. Heart J. 2008, 16, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Szeiffová Bačová, B.; Egan Beňová, T.; Viczenczová, C.; Soukup, T.; Rauchová, H.; Pavelka, S.; Knezl, V.; Barančík, M.; Tribulová, N. Cardiac connexin-43 and PKC signaling in rats with altered thyroid status without and with omega-3 fatty acids intake. Physiol. Res. 2016, 65, 77–90. [Google Scholar]
- Liu, J.; Zhu, H.; Yang, X.; Ge, Y.; Zhang, C.; Qin, Q.; Lu, J.; Zhan, L.; Cheng, H.; Sun, X. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumour Biol. 2014, 35, 3975–3979. [Google Scholar] [CrossRef] [PubMed]
- Rezaeyan, A.; Haddadi, G.H.; Hosseinzadeh, M.; Moradi, M.; Najafi, M. Radioprotective effects of hesperidin on oxidative damages and histopathological changes induced by X-irradiation in rats heart tissue. J. Med. Phys. 2016, 41, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Sieber, F.; Muir, S.A.; Cohen, E.P.; Fish, B.L.; Mäder, M.; Schock, A.M.; Althouse, B.J.; Moulder, J.E. Dietary selenium for the mitigation of radiation injury: Effects of selenium dose escalation and timing of supplementation. Radiat. Res. 2011, 176, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Bagchi, A.S.; Akolkar, G.; Singal, P.K.; Slezak, J. Myocardial changes after mediastinal irradiation in rats: Molecular mechamisms and potential targets to minimize the adverse effects. In Adaptation Biology and Medicine; Kawai, Y., Hargens, A.R., Singal, P.K., Eds.; Narosa Publishing House Ltd.: New Delhi, India, 2017; pp. 93–122. [Google Scholar]
- Smyth, J.W.; Hong, T.T.; Gao, D.; Vogan, J.M.; Jensen, B.C.; Fong, T.S.; Simpson, P.C.; Stainier, D.Y.; Chi, N.C.; Shaw, R.M. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J. Clin. Investig. 2010, 120, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Egan Benova, T.; Szeiffova Bacova, B.; Viczenczova, C.; Diez, E.; Barancik, M.; Tribulova, N. Protection of cardiac cell-to-cell coupling attenuate myocardial remodeling and proarrhythmia induced by hypertension. Physiol. Res. 2016, 65, 29–42. [Google Scholar]
- Tribulova, N.; Szeiffova Bacova, B.; Benova, T.; Viczenczova, C. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J. Electrocardiol. 2015, 48, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Zhao, L.; Zhang, S.; Wang, J.; Chen, N.; Gong, Q.; Tang, J.; Wang, H.; Yao, J.; Wang, Q.; et al. Cardioprotection by PI3K-mediated signaling is required for anti-arrhythmia and myocardial repair in response to ischemic preconditioning in infarcted pig hearts. Lab. Investig. 2015, 95, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.M.; Srisakuldee, W.; Nickel, B.E.; Kardami, E. Connexin43 phosphorylation and cytoprotection in the heart. Biochim. Biophys. Acta 2012, 1818, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Qiang, B.; Toma, J.; Fujii, H.; Osherov, A.B.; Nili, N.; Sparkes, J.D.; Fefer, P.; Samuel, M.; Butany, J.; Leong-Poi, H.; et al. Statin therapy prevents expansive remodeling in venous bypass grafts. Atherosclerosis 2012, 223, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef] [PubMed]

| GROUP | BW (g) | HW (g) | LVW (g) | RVW (g) |
|---|---|---|---|---|
| C | 344.22 ± 28.40 | 0.89 ± 0.11 | 0.36 ± 0.07 | 0.09 ± 0.02 |
| C-A | 382.61 ± 32.19 | 0.92 ± 0.09 | 0.39 ± 0.04 | 0.12 ± 0.01 |
| C-AT | 371.01 ± 20.45 | 0.87 ± 0.07 | 0.37 ± 0.04 | 0.10 ± 0.02 |
| C-S | 363.63 ± 23.93 | 0.90 ± 0.10 | 0.39 ± 0.04 | 0.11 ± 0.02 |
| I | 252.24 ± 14.31 a | 0.83 ± 0.05 | 0.25 ± 0.04 a | 0.10 ± 0.04 |
| I-A | 227.41 ± 26.47 c | 0.73 ± 0.09 c | 0.24 ± 0.02 c | 0.11 ± 0.03 |
| I-AT | 253.23 ± 31.82 c | 0.77 ± 0.08 | 0.27 ± 0.03 c | 0.13 ± 0.03 |
| I-S | 233.62 ± 14.50 c | 0.67 ± 0.05 b,c | 0.23 ± 0.02 c | 0.12 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viczenczova, C.; Kura, B.; Egan Benova, T.; Yin, C.; Kukreja, R.C.; Slezak, J.; Tribulova, N.; Szeiffova Bacova, B. Irradiation-Induced Cardiac Connexin-43 and miR-21 Responses Are Hampered by Treatment with Atorvastatin and Aspirin. Int. J. Mol. Sci. 2018, 19, 1128. https://doi.org/10.3390/ijms19041128
Viczenczova C, Kura B, Egan Benova T, Yin C, Kukreja RC, Slezak J, Tribulova N, Szeiffova Bacova B. Irradiation-Induced Cardiac Connexin-43 and miR-21 Responses Are Hampered by Treatment with Atorvastatin and Aspirin. International Journal of Molecular Sciences. 2018; 19(4):1128. https://doi.org/10.3390/ijms19041128
Chicago/Turabian StyleViczenczova, Csilla, Branislav Kura, Tamara Egan Benova, Chang Yin, Rakesh C. Kukreja, Jan Slezak, Narcis Tribulova, and Barbara Szeiffova Bacova. 2018. "Irradiation-Induced Cardiac Connexin-43 and miR-21 Responses Are Hampered by Treatment with Atorvastatin and Aspirin" International Journal of Molecular Sciences 19, no. 4: 1128. https://doi.org/10.3390/ijms19041128




