Regulation of Bicarbonate Secretion in Marine Fish Intestine by the Calcium-Sensing Receptor
Abstract
:1. Introduction
2. Results
2.1. CaSR Expression
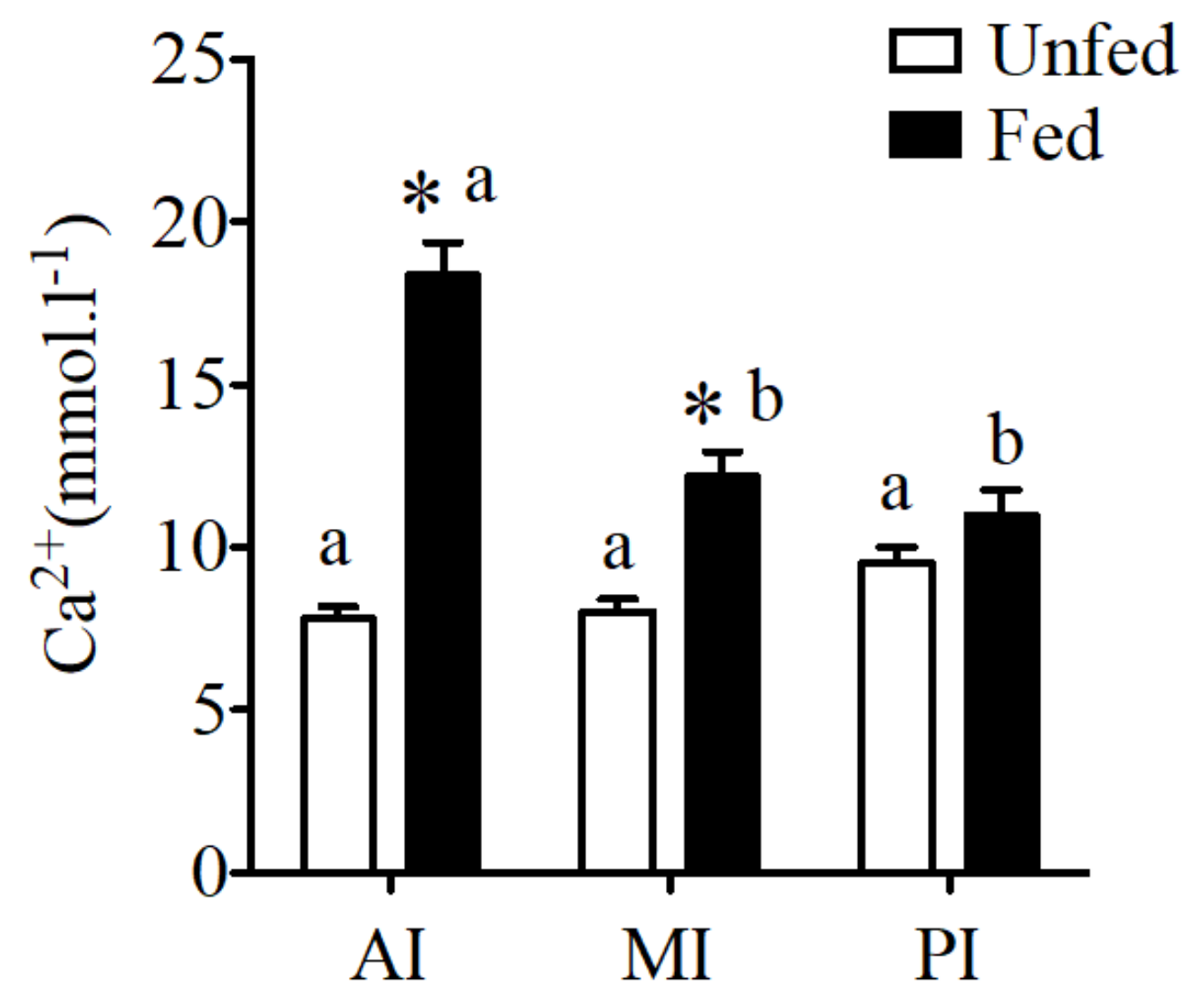
2.2. Effects of Feeding in Plasma and Fluid Calcium
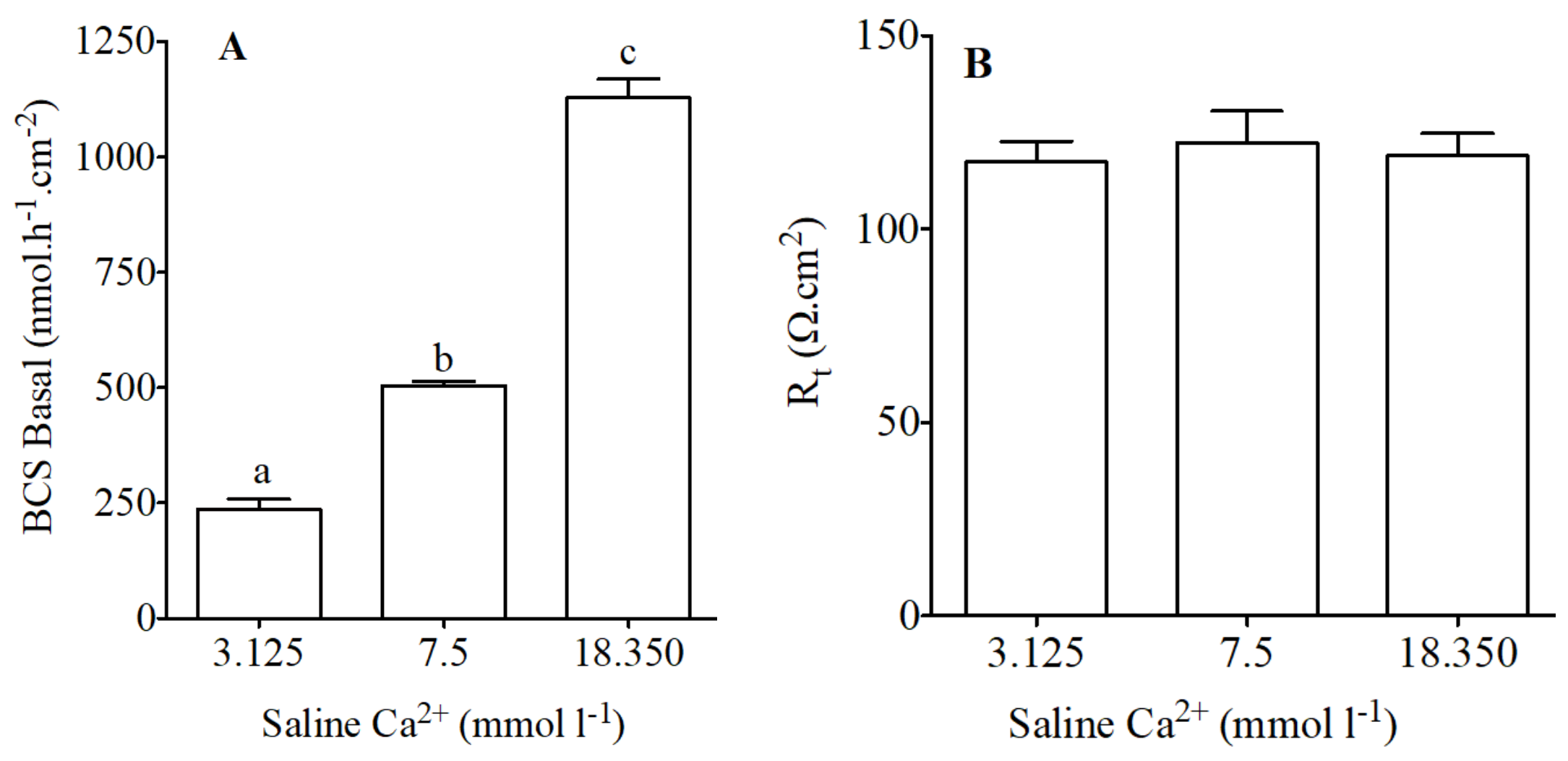
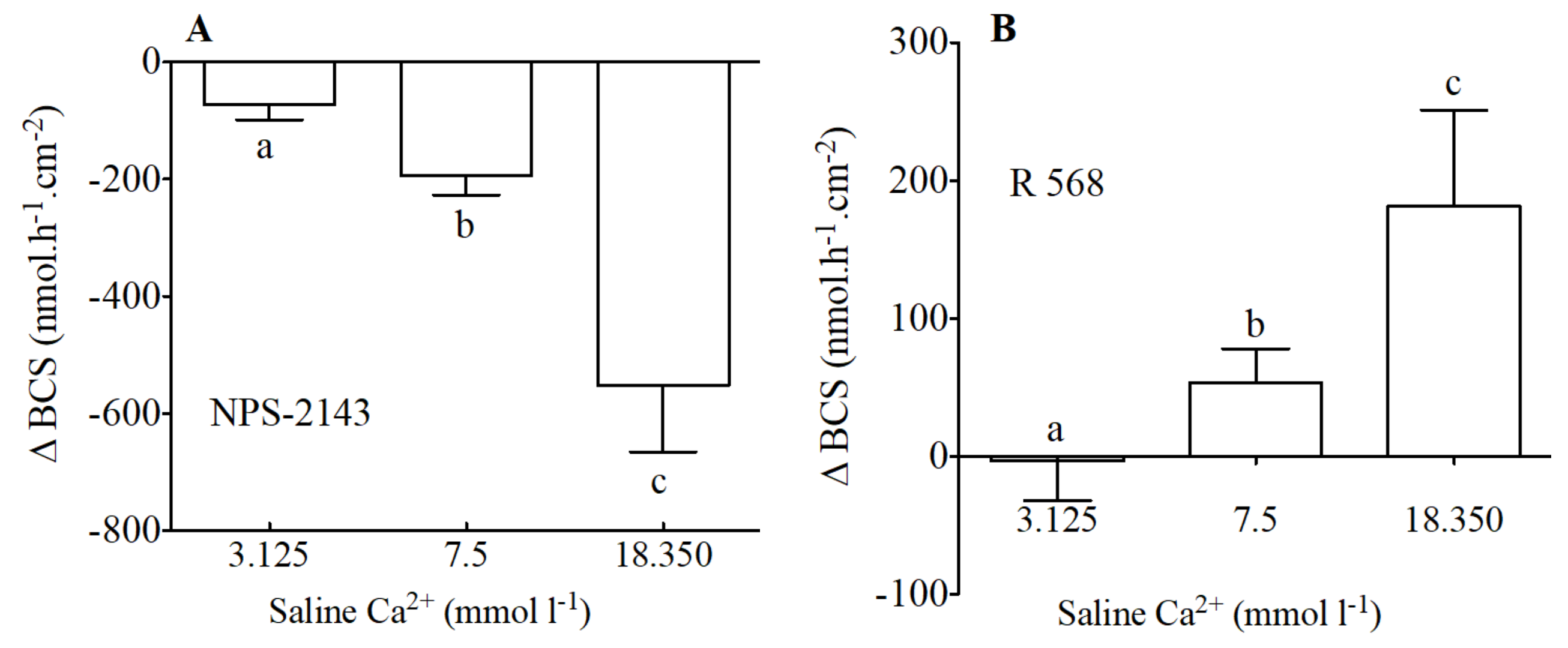
2.3. HCO3− Secretion
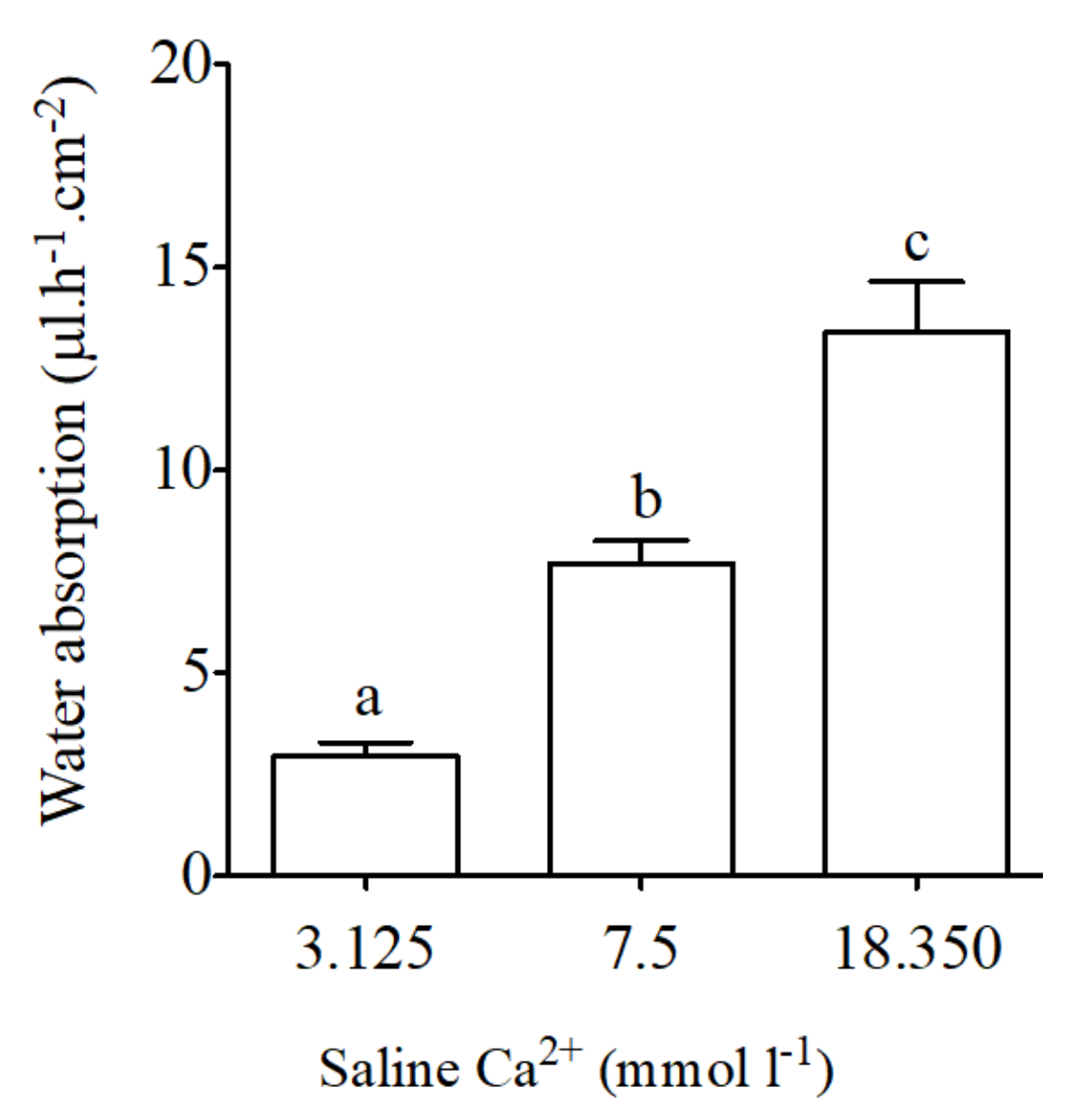
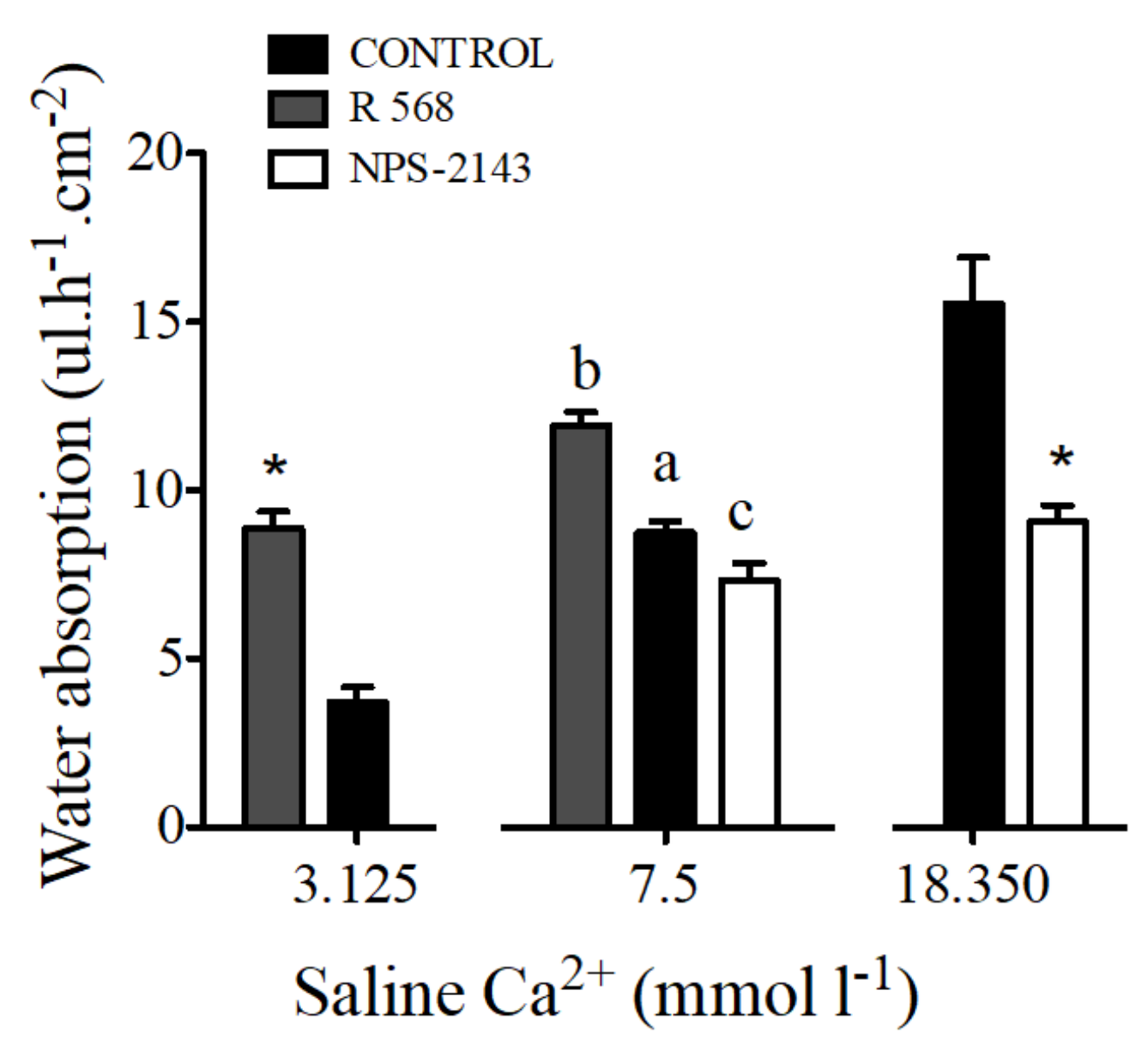
2.4. Gravimetric Water Absorption
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal Maintenance and Experimental Conditions
4.3. Food, Plasma and Fluid Calcium
4.4. Calcium-Sensing Receptor (CaSR) Expression
4.5. Intestinal HCO3− Secretion
4.6. Gravimetric Water Absorption
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fletcher, C.R. Osmotic and ionic regulation in the cod (Gadus callarias L.). J. Comp. Physiol. 1978, 124, 157–168. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Nilsson, S. Renal and extra-renal excretion of calcium in the marine teleost, Gadus morhua. Am. J. Physiol. 1985, 248, R18–R22. [Google Scholar] [CrossRef] [PubMed]
- Sundell, K.; Björnsson, B.T. Kinetics of Calcium Fluxes Across the Intestinal Mucosa of the Marine Teleost, Gadus Morhua, Measured Using an In Vitro Perfusion Method. J. Exp. Biol. 1988, 140, 171–186. [Google Scholar]
- Fuentes, J.; Eddy, F.B. Drinking in Atlantic salmon presmolts and smolts in response to growth hormone and salinity. Comp. Biochem. Physiol. A Physiol. 1997, 117, 487–491. [Google Scholar] [CrossRef]
- Fuentes, J.; Figueiredo, J.; Power, D.M.; Canario, A.V.M. Parathyroid hormone-related protein regulates intestinal calcium transport in sea bream (Sparus auratus). AJP Regul. Integr. Comp. Physiol. 2006, 291, R1499–R1506. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Eddy, F. Drinking in marine, euryhaline and freshwater teleost fish. In In Ionic Regulation in Animals; Hazon, N., Eddy, F.B., Flik, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 135–149. [Google Scholar]
- Guerreiro, P.M.; Fuentes, T.; Power, D.M.; Ingleton, P.M.; Flik, G.; Canario, A.V.M. Parathyroid hormone-related protein: A calcium regulatory factor in sea bream (Sparus aurata L.) larvae. AJP Regul. Integr. Comp. Physiol. 2001, 281, R855–R860. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, P.M.; Fuentes, J.; Flik, G.; Rotllant, J.; Power, D.M.; Canario, A.V.M. Water calcium concentration modifies whole-body calcium uptake in sea bream larvae during short-term adaptation to altered salinities. J. Exp. Biol. 2004, 207, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.S.M.; Gregório, S.F.; Canário, A.V.M.; Power, D.M.; Fuentes, J. PTHrP regulates water absorption and aquaporin expression in the intestine of the marine sea bream (Sparus aurata L.). Gen. Comp. Endocrinol. 2015, 213, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gregório, S.F.; Carvalho, E.S.M.; Campinho, M.A.; Power, D.M.; Canário, A.V.M.; Fuentes, J. Endocrine regulation of carbonate precipitate formation in marine fish intestine by stanniocalcin and PTHrP. J. Exp. Biol. 2014, 217, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Eddy, F.B. Effect of manipulation of the renin-angiotensin system in control of drinking in juvenile Atlantic salmon (Salmo salar L.) in fresh water and after transfer to sea water. J. Comp. Physiol. B 1997, 167, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Mayer-Gostan, N. Eel esophagus as an osmoregulatory organ. Proc. Natl. Acad. Sci. USA 1976, 73, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Parmelee, J.T.; Renfro, J.L. Esophageal desalination of seawater in flounder: Role of active sodium transport. AJP Regul. Integr. Comp. Physiol. 1983, 245, R888–R893. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Farinacci, N.; Breitwieser, A. The absorption and excretion of water and salts by marine teleosts. Am. J. Physiol. 1930, 93, 480–505. [Google Scholar] [CrossRef]
- Hickman, C.P., Jr. Ingestion, intestinal absorption, and elimination of seawater and salts in the southern flounder, Paralichthys lethostigma. Can. J. Zool. 1968, 46, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, Z.H.; Gordon, M.S. The role of the intestine in salinity adaptation of the rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol. 1969, 30, 397–418. [Google Scholar] [CrossRef]
- Wilson, R.; Gilmour, K.; Henry, R.; Wood, C. Intestinal base excretion in the seawater-adapted rainbow trout: A role in acid-base balance? J. Exp. Biol. 1996, 199, 2331–2343. [Google Scholar] [PubMed]
- Wilson, R.W.; Wilson, J.M.; Grosell, M. Intestinal bicarbonate secretion by marine teleost fish—Why and how? Biochim. Biophys. Acta 2002, 1566, 182–193. [Google Scholar] [CrossRef]
- Whittamore, J.M.; Cooper, C.A.; Wilson, R.W. HCO3− secretion and CaCO3 precipitation play major roles in intestinal water absorption in marine teleost fish in vivo. AJP Regul. Integr. Comp. Physiol. 2010, 298, R877–R886. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Wood, C.M.; Wilson, R.W.; Bury, N.R.; Hogstrand, C.; Rankin, C.; Jensen, F.B. Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. AJP Regul. Integr. Comp. Physiol. 2005, 288, R936–R946. [Google Scholar] [CrossRef] [PubMed]
- Kurita, Y.; Nakada, T.; Kato, A.; Doi, H.; Mistry, A.C.; Chang, M.-H.; Romero, M.F.; Hirose, S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. AJP Regul. Integr. Comp. Physiol. 2008, 294, R1402–R1412. [Google Scholar] [CrossRef] [PubMed]
- Ruhr, I.M. The Physiological Effects of the Guanylin Peptides in the Intestine of the Gulf Toadfish (Opsanus beta). Open Access Dissertations, University of Miami, Miami, FL, USA, 2016. [Google Scholar]
- Wilson, R.W.; Grosell, M. Intestinal bicarbonate secretion in marine teleost fish—Source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis. Biochim. Biophys. Acta 2003, 1618, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M. Intestinal anion exchange in marine fish osmoregulation. J. Exp. Biol. 2006, 209, 2813–2827. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Power, D.M.; Canário, A.V.M. Parathyroid hormone-related protein-stanniocalcin antagonism in regulation of bicarbonate secretion and calcium precipitation in a marine fish intestine. AJP Regul. Integr. Comp. Physiol. 2010, 299, R150–R158. [Google Scholar] [CrossRef] [PubMed]
- Bucking, C.; Wood, C.M. Gastrointestinal processing of Na+, Cl−, and K+ during digestion: Implications for homeostatic balance in freshwater rainbow trout. Am. J. Physiol. 2006, 291, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Grosell, M. Feeding and osmoregulation: Dual function of the marine teleost intestine. J. Exp. Biol. 2006, 209. [Google Scholar] [CrossRef] [PubMed]
- Márquez, L.; Fuentes, J. In vitro characterization of acid secretion in the gilthead sea bream (Sparus aurata) stomach. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 167, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, S.F.; Carvalho, E.S.; Encarnacao, S.; Wilson, J.M.; Power, D.M.; Canario, A.V.; Fuentes, J. Adaptation to different salinities exposes functional specialization in the intestine of the sea bream (Sparus aurata L.). J. Exp. Biol. 2013, 216, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, J.C.; Flik, G.; Verbost, P.M. A Passive Immunization Technique against the Teleost Hypocalcemic Hormone Stanniocalcin Provides Evidence for the Cholinergic Control of Stanniocalcin Release and the Conserved Nature of the Molecule. Gen. Comp. Endocrinol. 1995, 98, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Haond, C.; Guerreiro, P.M.; Silva, N.; Power, D.M.; Canário, A.V.M. Regulation of calcium balance in the sturgeon Acipenser naccarii: A role for PTHrP. AJP Regul. Integr. Comp. Physiol. 2007, 293, R884–R893. [Google Scholar] [CrossRef] [PubMed]
- Radman, D.P.; McCudden, C.; James, K.; Nemeth, E.M.; Wagner, G.F. Evidence for calcium-sensing receptor mediated stanniocalcin secretion in fish. Mol. Cell. Endocrinol. 2002, 186, 111–119. [Google Scholar] [CrossRef]
- Takei, Y.; Loretz, C.A. Chapter 8: Endocrinology. In The Physiology of Fishes, 3rd ed.; Evans, D.H., Claiborne, J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 271–318. [Google Scholar]
- Guerreiro, P.M.; Fuentes, J.; Canario, A.V.M.; Power, D.M. Calcium balance in sea bream (Sparus aurata): The effect of oestradiol-17beta. J. Endocrinol. 2002, 173, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Brinca, L.; Guerreiro, P.M.; Power, D.M. PRL and GH synthesis and release from the sea bream (Sparus auratus L.) pituitary gland in vitro in response to osmotic challenge. Gen. Comp. Endocrinol. 2010, 168, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Loretz, C.A. Extracellular calcium-sensing receptors in fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.F.; Jaworski, E. Calcium regulates stanniocalcin mRNA levels in primary cultured rainbow trout corpuscles of Stannius. Mol. Cell. Endocrinol. 1994, 99, 315–322. [Google Scholar] [CrossRef]
- Hebert, S.C.; Cheng, S.; Geibel, J. Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium 2004, 35, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Magno, A.L.; Ward, B.K.; Ratajczak, T. The Calcium-Sensing Receptor: A Molecular Perspective. Endocr. Rev. 2011, 32, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Su, C.-H.; Hwang, P.-P. Calcium-Sensing Receptor Mediates Ca2+ Homeostasis by Modulating Expression of PTH and Stanniocalcin. Endocrinology 2014, 155, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.W.M.; Auprix, D.; Perry, S.F. Involvement of the calcium-sensing receptor in calcium homeostasis in larval zebrafish exposed to low environmental calcium. AJP Regul. Integr. Comp. Physiol. 2014, 306, R211–R221. [Google Scholar] [CrossRef] [PubMed]
- Mailland, M.; Waelchli, R.; Ruat, M.; Boddeke, H.G.W.M.; Seuwen, K. Stimulation of cell proliferation by calcium and a calcimimetic compound. Endocrinology 1997, 138, 3601–3605. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.F.; Steffey, M.E.; Hammerland, L.G.; Hung, B.C.; Van Wagenen, B.C.; DelMar, E.G.; Balandrin, M.F. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; MacLeod, R.J. Extracellular Calcium Sensing and Extracellular Calcium Signaling. Physiol. Rev. 2001, 81, 239–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cavanaugh, A.; Breitwieser, G.E. Regulation of Stability and Trafficking of Calcium-Sensing Receptors by Pharmacologic Chaperones. Adv. Pharmacol. 2011, 62, 143–173. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.S.M.; Gregório, S.F.; Power, D.M.; Canário, A.V.M.; Fuentes, J. Water absorption and bicarbonate secretion in the intestine of the sea bream are regulated by transmembrane and soluble adenylyl cyclase stimulation. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Carvalho, E.S.M.; Gregório, S.F.; Power, D.M.; Canario, A.V.M.; Trischitta, F.; Fuentes, J. Prolactin regulates luminal bicarbonate secretion in the intestine of the sea bream (Sparus aurata L.). J. Exp. Biol. 2012, 215, 3836–3844. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Genz, J. Ouabain-sensitive bicarbonate secretion and acid absorption by the marine teleost fish intestine play a role in osmoregulation. AJP Regul. Integr. Comp. Physiol. 2006, 291, R1145–R1156. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Mager, E.M.; Williams, C.; Taylor, J.R. High rates of HCO3− secretion and Cl− absorption against adverse gradients in the marine teleost intestine: The involvement of an electrogenic anion exchanger and H+-pump metabolon? J. Exp. Biol. 2009, 212, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.A.; Bendell, L.A.; Guerreiro, P.M.; Clark, M.S.; Power, D.M.; Canario, A.V.M.; Brown, B.L.; Ingleton, P.M. Cloning of the cDNA for the putative calcium-sensing receptor and its tissue distribution in sea bream (Sparus aurata). Gen. Comp. Endocrinol. 2002, 127, 117–127. [Google Scholar] [CrossRef]
- Loretz, C.; Pollina, C.; Hyodo, S.; Takei, Y. Tilapia extracellular Ca2+-sensing receptor is expressed in osmoregulatory and endocrine tissues. FASEB J. 2007, 21, A1396. [Google Scholar]
- Nearing, J.; Betka, M.; Quinn, S.; Hentschel, H.; Elger, M.; Baum, M.; Bai, M.; Chattopadyhay, N.; Brown, E.M.; Hebert, S.C.; et al. Polyvalent cation receptor proteins (CaRs) are salinity sensors in fish. Proc. Natl. Acad. Sci. USA 2002, 99, 9231–9236. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E. Ca2+ Receptor-Dependent Regulation of Cellular Functions. News Physiol. Sci. 1995, 10, 1–5. [Google Scholar] [CrossRef]
- Nemeth, E.F. Calcium Receptors as Novel Drug Targets. In Principles of Bone Biology; Bilezikian, J.P., Lawrence, G., Raisz, T.J.M., Eds.; Academic: San Diego, CA, USA, 1996; pp. 1339–1359. ISBN 9780123738844. [Google Scholar]
- Nemeth, E.F.; Delmar, E.G.; Heaton, W.L.; Miller, M.A.; Lambert, L.D.; Conklin, R.L.; Gowen, M.; Gleason, J.G.; Bhatnagar, P.K.; Fox, J. Calcilytic compounds: Potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J. Pharmacol. Exp. Ther. 2001, 299, 323–331. [Google Scholar] [PubMed]
- Greenwood, M.P.; Flik, G.; Wagner, G.F.; Balment, R.J. The corpuscles of stannius, calcium-sensing receptor, and stanniocalcin: Responses to calcimimetics and physiological challenges. Endocrinology 2009, 150, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, M.G. Role of calcium signaling in epithelial bicarbonate secretion. Cell Calcium 2014, 55, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Dong, X.; Wong, C.; Vallon, V.; Tang, B.; Sun, J.; Yang, S.; Dong, H. Molecular mechanisms of calcium-sensing receptor-mediated calcium signaling in the modulation of epithelial ion transport and bicarbonate secretion. J. Biol. Chem. 2014, 289, 34642–34653. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, M.; Parks, S.K.; Salazar, E.; Levin, L.R.; Goss, G.G.; Buck, J. Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Guffey, S.; Esbaugh, A.; Grosell, M. Regulation of apical H+-ATPase activity and intestinal HCO3− secretion in marine fish osmoregulation. AJP Regul. Integr. Comp. Physiol. 2011, 301, R1682–R1691. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Taylor, J.R. Intestinal anion exchange in teleost water balance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Baker, D.W.; Schulte, P.M.; Wood, C.M. Physiological and molecular mechanisms of osmoregulatory plasticity in killifish after seawater transfer. J. Exp. Biol. 2008, 211, 2450–2459. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence (5′ to 3′) | Tm (°C) | Amplicon (bp) | NCBI Accession No. |
|---|---|---|---|---|---|
| CaSR | Fw Rv | CAACCATTGCAGTTGTAGGAG AAGCGACTAGAGGAGGCGTAG | 60 | 123 | AJ289717.1 |
| 18S | Fw Rv | AACCAGACAAATCGCTCCAC CCTGCGGCTTAATTTGACTC | 58 | 139 |
| Constituents (mmol·L−1) | Basolateral | Apical | Apical High Ca2+ | Apical Low Ca2+ |
|---|---|---|---|---|
| NaCl | 160 | 88 | 88 | 88 |
| KCl | 3 | 3 | 3 | 3 |
| MgSO4 | 1 | 126.5 | 136 | 126.5 |
| MgCl2 | 9.5 | 0 | 13.875 | |
| Na2HPO4 | 1 | 1 | 1 | |
| CaCl2 | 1.5 | 7.5 | 18.35 | 3.125 |
| NaHCO3 | 5 | |||
| Glucose | 5.5 | |||
| HEPES | 5 | |||
| NaH2PO4 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregório, S.F.; Fuentes, J. Regulation of Bicarbonate Secretion in Marine Fish Intestine by the Calcium-Sensing Receptor. Int. J. Mol. Sci. 2018, 19, 1072. https://doi.org/10.3390/ijms19041072
Gregório SF, Fuentes J. Regulation of Bicarbonate Secretion in Marine Fish Intestine by the Calcium-Sensing Receptor. International Journal of Molecular Sciences. 2018; 19(4):1072. https://doi.org/10.3390/ijms19041072
Chicago/Turabian StyleGregório, Sílvia F., and Juan Fuentes. 2018. "Regulation of Bicarbonate Secretion in Marine Fish Intestine by the Calcium-Sensing Receptor" International Journal of Molecular Sciences 19, no. 4: 1072. https://doi.org/10.3390/ijms19041072





