Regulated Mesenchymal Stem Cells Mediated Colon Cancer Therapy Assessed by Reporter Gene Based Optical Imaging
Abstract
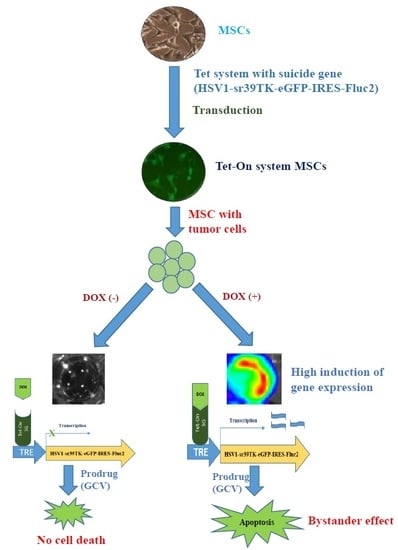
:1. Introduction
2. Results
2.1. Characterization of MSC-Tet-TK and MSC-TK
2.2. 3H-PCV Uptake Assay
2.3. Characterization of CT26/Rluc Cells
2.4. Cell Viability of MSCs after Treatment with DOX and GCV
2.5. Fluc Activity of Suicide Gene-Transduced MSCs after Treatment with GCV
2.6. Bystander Effects on Colon Cancer Cells with Suicide Gene Expressed by Engineered MSCs
2.7. Effects of Suicide Gene-Transduced MSCs on Colon Cancer in a Mouse Model
2.8. In Vivo Tumor Apoptosis Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Retroviral Transduction of MSCs
4.3. Lentiviral Transduction of Colon Cancer Cells (CT26)
4.4. Optical Imaging
4.5. 3H-Penciclovir (PCV) Uptake Assay
4.6. CCK8 Assay of Cell Viability
4.7. Bystander Effect
4.8. Tumor Model
4.9. TUNEL Staining for Ex Vivo Tumor
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer. J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; Yang, Y.; Hass, R. Interaction of MSC with tumor cells. Cell Commun. Signal. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Gjorgieva, D.; Zaidman, N.; Bosnakovski, D. Mesenchymal stem cells for anti-cancer drug delivery. Recent Pat. Anti-Cancer Drug Discov. 2013, 8, 310–318. [Google Scholar] [CrossRef]
- Sherman, L.S.; Shaker, M.; Mariotti, V.; Rameshwar, P. Mesenchymal stromal/stem cells in drug therapy: New perspective. Cytotherapy 2017, 19, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.J.; Rameshwar, P. Mesenchymal stem cells in drug/gene delivery: Implications for cell therapy. Ther. Deliv. 2012, 3, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Kalladka, D.; Muir, K.W. Brain repair: Cell therapy in stroke. Stem Cells Cloning 2014, 7, 31–44. [Google Scholar] [PubMed]
- Hsuan, Y.C.Y.; Lin, C.H.; Chang, C.P.; Lin, M.T. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016, 6, e00526. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Lin, Y.; Lennon, D.P.; Correa, D.; Schluchter, M.; Caplan, A.I. Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Transl. Med. 2012, 12, 886–897. [Google Scholar]
- Loebinger, M.R.; Eddaoudi, A.; Davies, D.; Janes, S.M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009, 69, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Greco, O.; Dachs, G.U. Gene directed enzyme/prodrug therapy of cancer: Historical appraisal and future prospectives. J. Cell. Physiol. 2001, 187, 22–36. [Google Scholar] [CrossRef]
- Dachs, G.U.; Hunt, M.A.; Syddall, S.; Singleton, D.C.; Patterson, A.V. Bystander or no bystander for gene directed enzyme prodrug therapy. Molecules 2009, 14, 4517–4545. [Google Scholar] [CrossRef] [PubMed]
- Bonini, C.; Bon, K.; Snyder, R.O.; R danza, A.; Perna, S.K.; Kaneko, S.; Traversari, C.; Ciceri, F.; Bordignon, C. The suicide gene therapy challenge: How to improve a successful gene therapy approach. Mol. Ther. 2007, 15, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Guiner, C.L.; Stieger, K.; Snyder, R.O.; Rolling, F.; Moullier, P. Immune responses to gene product of inducible promoters. Curr. Gene Ther. 2007, 7, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-C.; Deng, W.-P.; Yang, W.K.; Liu, R.-S.; Lee, C.-C.; Su, T.-C.; Lin, R.-J.; Yang, D.-M.; Chang, C.-W.; Chen, W.-H. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin. Cancer Res. 2005, 11, 7749–7756. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wang, Y.; He, N.; Wang, D.; Zhao, Q.; Feng, G.; Su, W.; Xu, Y.; Han, Z.; Kong, D. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials 2014, 35, 5162–5170. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Gangadaran, P.; Li, X.J.; Oh, J.M.; Lee, H.W.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. In Vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model. Sci. Rep. 2016, 6, 30418. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kalimuthu, S.; Ahn, B.-C. In Vivo Cell Tracking with Bioluminescence Imaging. Nucl. Med. Mol. Imaging 2015, 49, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, Y.J.; Kang, J.H. Stem cell monitoring with a direct or indirect labeling method. Nucl. Med. Mol. Imaging 2016, 50, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Miyazaki, J.-I.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ayala, M.; Thiede, B.R.; Zhang, S.C. In vitro-and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells 2008, 26, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Gossen, M.; Bender, G.; Muller, G.; Freundlieb, S. Transcriptional activation by tetracyclines in mammalian cells. Science 1995, 268, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Vink, M.; Klaver, B.; Berkhout, B.; Das, A. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 2006, 13, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- De Melo, S.M.; Bittencourt, S.; Ferrazoli, E.G.; da Silva, C.S.; da Cunha, F.F.; da Silva, F.H.; Stilhano, R.S.; Denapoli, P.M.A.; Zanetti, B.F.; Martin, P.K.M. The anti-tumor effects of adipose tissue mesenchymal stem cell transduced with HSV-Tk gene on u-87-driven brain tumor. PLoS ONE 2015, 10, e0128922. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, G.; Qiu, P.; Liu, X.; Wu, Y.; Du, B.; Li, J.; Zhou, J.; Li, J.; Tan, Y. Tanshinone IIA increases the bystander effect of herpes simplex virus thymidine kinase/ganciclovir gene therapy via enhanced gap junctional intercellular communication. PLoS ONE 2013, 8, e67662. [Google Scholar] [CrossRef] [PubMed]
- Matuskova, M.; Hlubinova, K.; Pastorakova, A.; Hunakova, L.; Altanerova, V.; Altaner, C.; Kucerova, L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010, 290, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Preuß, E.; Muik, A.; Weber, K.; Otte, J.; von Laer, D.; Fehse, B. Cancer suicide gene therapy with TK. 007: Superior killing efficiency and bystander effect. J. Mol. Med. 2011, 89, 1113–1124. [Google Scholar]
- Gangadaran, P.; Ahn, B.-C. Molecular Imaging: A Useful Tool for the Development of Natural Killer Cell-Based Immunotherapies. Front. Immunol. 2017, 8, 1090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, X.J.; Kalimuthu, S.; Gangadaran, P.; Lee, H.W.; Oh, J.M.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J. Natural Killer Cell (NK-92MI)-Based Therapy for Pulmonary Metastasis of Anaplastic Thyroid Cancer in a Nude Mouse Model. Front. Immunol. 2017, 8, 816. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, L.; Altanerova, V.; Matuskova, M.; Tyciakova, S.; Altaner, C. Adipose tissue–derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007, 67, 6304–6313. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; HAzAMA, S.; Araki, A.; Yoshimura, K.; IIzUKA, N.; Yoshino, S.; Noma, T.; Oka, M. A novel cancer vaccine strategy with combined IL-18 and HSV-TK gene therapy driven by the hTERT promoter in a murine colorectal cancer model. Int. J. Oncol. 2014, 45, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Sekar, T.V.; Foygel, K.; Ilovich, O.; Paulmurugan, R. Noninvasive theranostic imaging of HSV1-sr39TK-NTR/GCV-CB1954 dual-prodrug therapy in metastatic lung lesions of MDA-MB-231 triple negative breast cancer ind mice. Theranostics 2014, 4, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-Y.; Huang, B.; Wu, H.-B.; Wu, J.-H.; Li, L.-M.; Li, Y.-X.; Hu, Y.-L.; Han, M.; Shen, Y.-Q.; Tabata, Y. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice. J. Control. Release 2015, 209, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Luetzkendorf, J.; Mueller, L.P.; Mueller, T.; Caysa, H.; Nerger, K.; Schmoll, H.J. Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. J. Cell. Mol. Med. 2010, 14, 2292–2304. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Li, S.; Gu, C.; Gao, Y.; Koizumi, S.; Yamamoto, S.; Terakawa, S.; Namba, H. Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. Int. J. Oncol. 2009, 35, 1265–1270. [Google Scholar] [PubMed]
- Ryu, C.H.; Park, K.Y.; Kim, S.M.; Jeong, C.H.; Woo, J.S.; Hou, Y.; Jeun, S.-S. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem. Biophys. Res. Commun. 2012, 421, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, A.; Marini, F.; Amano, T.; Khan, A.; Studeny, M.; Gumin, J.; Chen, J.; Hentschel, S.; Vecil, G.; Dembinski, J. Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005, 65, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002, 62, 3603–3608. [Google Scholar] [PubMed]
- Yuan, X.; Hu, J.; Belladonna, M.L.; Black, K.L.; John, S.Y. Interleukin-23–Expressing Bone Marrow–Derived Neural Stem-Like Cells Exhibit Antitumor Activity against Intracranial Glioma. Cancer Res. 2006, 66, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yuan, X.; Belladonna, M.L.; Ong, J.M.; Wachsmann-Hogiu, S.; Farkas, D.L.; Black, K.L.; John, S.Y. Induction of potent antitumor immunity by intratumoral injection of interleukin 23–transduced dendritic cells. Cancer Res. 2006, 66, 8887–8896. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ito, Y.; Kawano, Y.; Kurozumi, K.; Kobune, M.; Tsuda, H.; Bizen, A.; Honmou, O.; Niitsu, Y.; Hamada, H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004, 11, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Matuskova, M.; Kozovska, Z.; Toro, L.; Durinikova, E.; Tyciakova, S.; Cierna, Z.; Bohovic, R.; Kucerova, L. Combined enzyme/prodrug treatment by genetically engineered AT-MSC exerts synergy and inhibits growth of MDA-MB-231 induced lung metastases. J. Exp. Clin. Cancer Res. 2015, 34. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lim, J.Y.; Park, S.I.; Jeong, C.H.; Oh, J.H.; Jeong, M.; Oh, W.; Park, S.-H.; Sung, Y.-C.; Jeun, S.-S. Gene therapy using TRAIL-secreting human umbilical cord blood–derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008, 68, 9614–9623. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalimuthu, S.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gangadaran, P.; Rajendran, R.L.; Baek, S.H.; Jeon, Y.H.; Jeong, S.Y.; Lee, S.-W.; et al. Regulated Mesenchymal Stem Cells Mediated Colon Cancer Therapy Assessed by Reporter Gene Based Optical Imaging. Int. J. Mol. Sci. 2018, 19, 1002. https://doi.org/10.3390/ijms19041002
Kalimuthu S, Zhu L, Oh JM, Lee HW, Gangadaran P, Rajendran RL, Baek SH, Jeon YH, Jeong SY, Lee S-W, et al. Regulated Mesenchymal Stem Cells Mediated Colon Cancer Therapy Assessed by Reporter Gene Based Optical Imaging. International Journal of Molecular Sciences. 2018; 19(4):1002. https://doi.org/10.3390/ijms19041002
Chicago/Turabian StyleKalimuthu, Senthilkumar, Liya Zhu, Ji Min Oh, Ho Won Lee, Prakash Gangadaran, Ramya Lakshmi Rajendran, Se Hwan Baek, Yong Hyun Jeon, Shin Young Jeong, Sang-Woo Lee, and et al. 2018. "Regulated Mesenchymal Stem Cells Mediated Colon Cancer Therapy Assessed by Reporter Gene Based Optical Imaging" International Journal of Molecular Sciences 19, no. 4: 1002. https://doi.org/10.3390/ijms19041002