Alternative Respiratory Pathway Component Genes (AOX and ND) in Rice and Barley and Their Response to Stress
Abstract
:1. Introduction
2. Results
2.1. Identification of the Genes Encoding Alternative Respiratory Pathway (AP) Components in Rice and Barley
2.1.1. Alternative Oxidase
2.1.2. Type-II NADH Dehydrogenase Family
2.2. Subcellular Localisation and Structural Similarities
2.3. Expression of AP Components under Chemical-Induced Stress
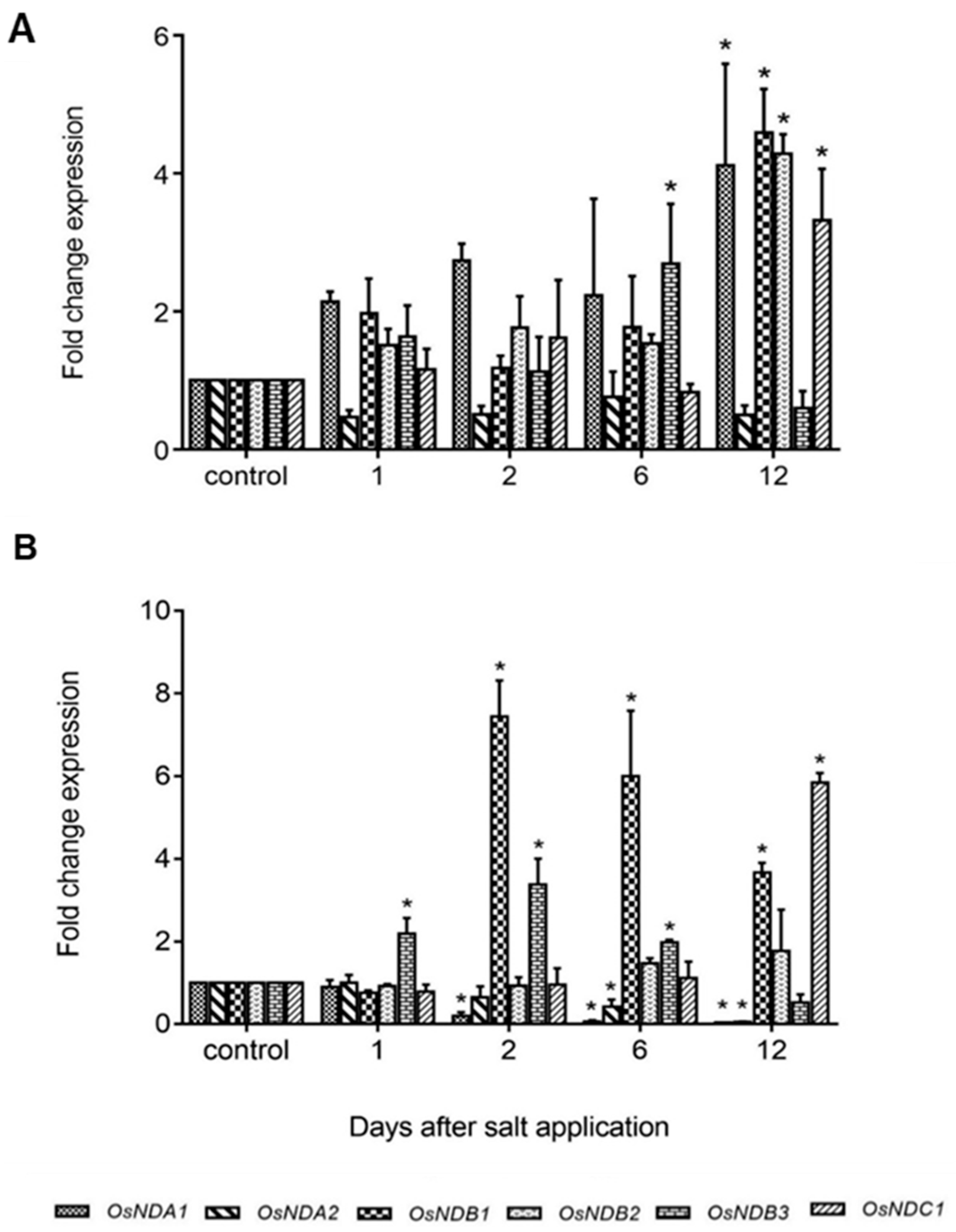
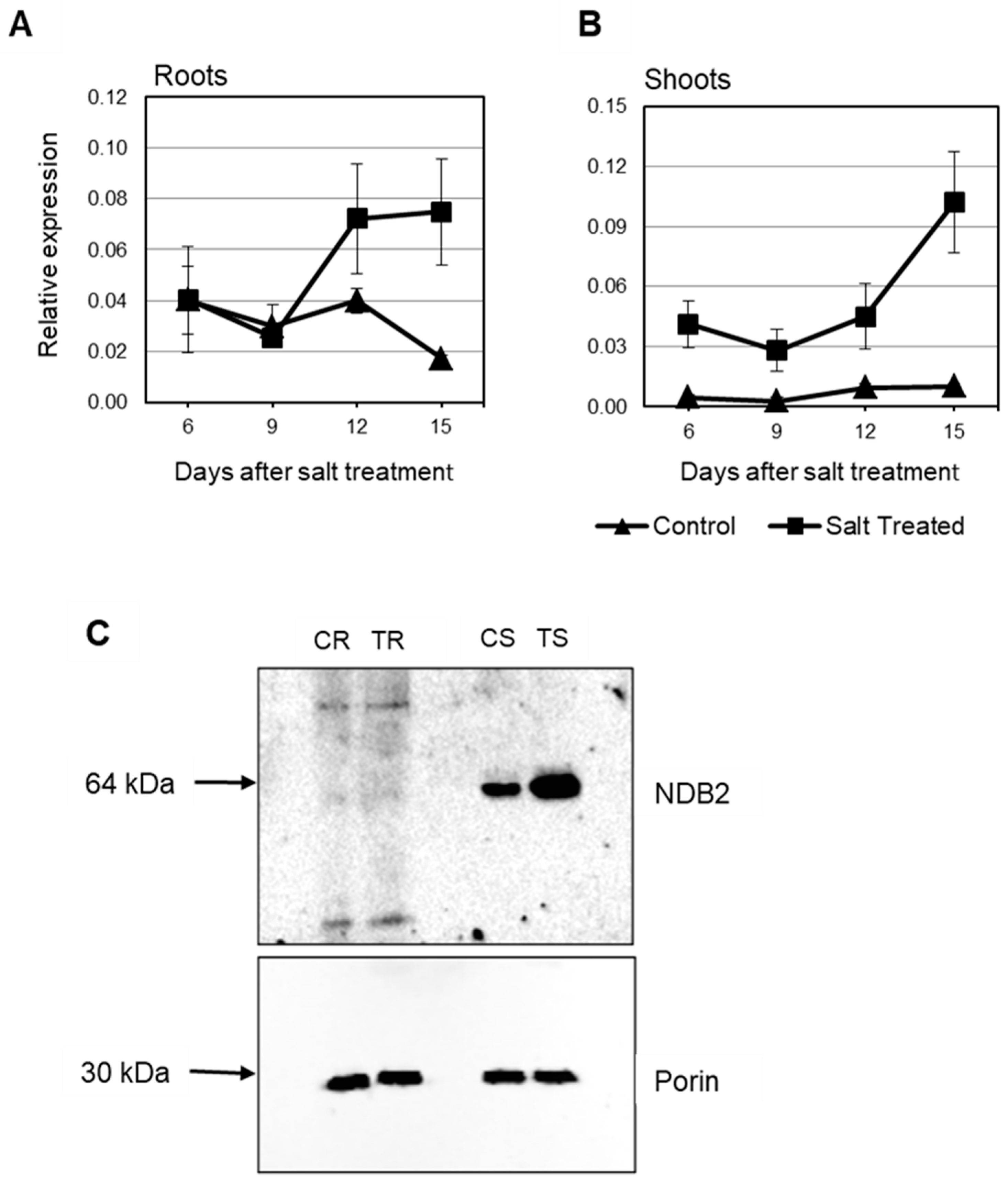
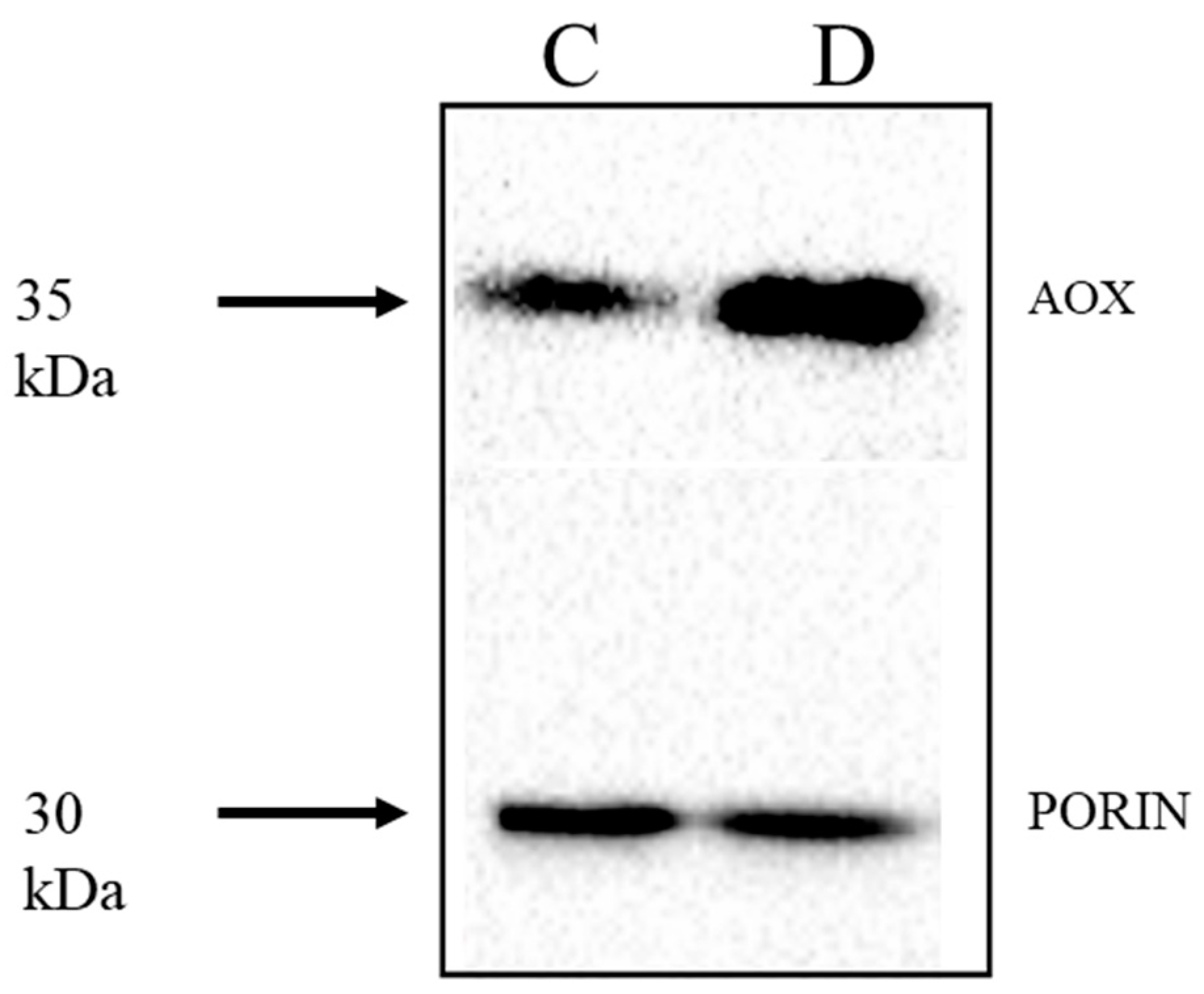
2.4. Expression of Rice and Barley AP Components under Abiotic Stress
3. General Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Gene Identification
4.3. Protein Relationship Analyses
4.4. Subcellular Localization
4.5. Genevestigator Expression Analyses
4.6. Screening for Responsive AP Genes under Oxidative Stress
4.7. Gene Expression Analysis Using qRT-PCR
4.8. Protein and Enzyme Activity Assays in Isolated Rice Tissue Mitochondria
4.9. Oxygen Electrode Assays
4.10. Immunoblot Analysis
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Millar, A.H.; Whelan, J.; Soole, K.L.; Day, D.A. Organization and Regulation of Mitochondrial Respiration in Plants. In Annual Review of Plant Biology; Merchant, S.S., Briggs, W.R., Ort, D., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 62, pp. 79–104. [Google Scholar]
- Finnegan, P.M.; Soole, K.L.; Umbach, A.L. Alternative Mitochondrial Electron Transport Proteins in Higher Plants. In Plant Mitochondria: From Genome to Function; Day, D.A., Millar, H., Whelan, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 163–230. [Google Scholar]
- Rasmusson, A.G.; Soole, K.L.; Elthon, T.E. Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu. Rev. Plant Biol. 2004, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Wiskich, J.T.; Whelan, J.; Day, D.A. Organic-acid activation of the alternative oxidase of plant-mitochondria. FEBS Lett. 1993, 329, 259–262. [Google Scholar] [CrossRef]
- Hoefnagel, M.H.N.; Millar, A.H.; Wiskich, J.T.; Day, D.A. Cytochrome and alternative respiratory pathways compete for electrons in the presence of pyruvate in soybean mitochondria. Arch. Biochem. Biophys. 1995, 318, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Purvis, A.C. Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiol. Plant. 1997, 100, 165–170. [Google Scholar] [CrossRef]
- Popov, V.N.; Purvis, A.C.; Skulachev, V.P.; Wagner, A.M. Stress-induced changes in ubiquinone concentration and alternative oxidase in plant mitochondria. Biosci. Rep. 2001, 21, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.D.; Carr, T.G.; Harris, S.A.; Hughes, C.E. Characterization of angiosperm nrDNA polymorphism, paralogy, and pseudogenes. Mol. Phylgenet. Evol. 2003, 29, 435–455. [Google Scholar] [CrossRef]
- Moore, C.S.; Cook-Johnson, R.J.; Rudhe, C.; Whelan, J.; Day, D.A.; Wiskich, J.T.; Soole, K.L. Identification of AtNDI1, an internal non-phosphorylating NAD(P)H dehydrogenase in Arabidopsis mitochondria. Plant Physiol. 2003, 133, 1968–7198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhafez, D.; Murcha, M.W.; Clifton, R.; Soole, K.L.; Day, D.A.; Whelan, J. Characterization of mitochondrial alternative NAD(P)H dehydrogenases in arabidopsis: Intraorganelle location and expression. Plant Cell Physiol. 2006, 47, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Saish, D.; Nakazono, M.; Lee, K.H.; Tsutsumi, N.; Akita, S.; Hirai, A. The gene for alternative oxidase-2 (AOX2) from Arabidopsis thaliana consists of five exons unlike other AOX genes and is transcribed at an early stage during germination. Genes Genet. Syst. 2001, 76, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Borecky, J.; Nogueira, F.T.; de Oliveira, K.A.; Maia, I.G.; Vercesi, A.E.; Arruda, P. The plant energy-dissipating mitochondrial systems: Depicting the genomic structure and the expression profiles of the gene families of uncoupling protein and alternative oxidase in monocots and dicots. J. Exp. Bot. 2006, 57, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Clifton, R.; Lister, R.; Parker, K.L.; Sappl, P.G.; Elhafez, D.; Millar, A.H.; Day, D.A.; Whelan, J. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Mol. Biol. 2005, 58, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rajakulendran, N.; Amirsadeghi, S.; Vanlerberghe, G.C. Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol. Plant. 2011, 142, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Saisho, D.; Nakazono, M.; Tsutsumi, N.; Hirai, A. Transcript levels of tandem-arranged alternative oxidase genes in rice are increased by low temperature. Gene 1997, 203, 121–129. [Google Scholar] [CrossRef]
- Considine, M.J.; Holtzapffel, R.C.; Day, D.A.; Whelan, J.; Millar, A.H. Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol. 2002, 129, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Saika, H.; Ohtsu, K.; Hamanaka, S.; Nakazono, M.; Tsutsumi, N.; Hirai, A. AOX1c, a novel rice gene for alternative oxidase; comparison with rice AOX1a and AOX1b. Genes Genet. Syst. 2002, 77, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.H.; McDonald, A.E.; Arnholdt-Schmitt, B.; de Melo, D.F. A classification scheme for alternative oxidases reveals the taxonomic distribution and evolutionary history of the enzyme in angiosperms. Mitochondrion 2014, 19, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, K.; Ito, Y.; Saika, H.; Nakazono, M.; Tsutsumi, N.; Hirai, A. ABA-independent expression of rice alternative oxidase genes under envinronmental stresses. Plant Biotechnol. 2002, 19, 187–190. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wang, Y.F.; Li, H.Y.; Wang, R.F.; Sun, K.; Jia, L.Y. Salt stress-induced expression of rice AOX1a is mediated through an accumulation of hydrogen peroxide. Biologia 2010, 65, 868–873. [Google Scholar] [CrossRef]
- Li, C.R.; Liang, D.D.; Xu, R.F.; Li, H.; Zhang, Y.P.; Qin, R.Y.; Li, L.; Wei, P.C.; Yang, J.B. Overexpression of an alternative oxidase gene, OsAOX1a, improves cold tolerance in Oryza sativa L. Genet. Mol. Res. 2013, 12, 5424–5432. [Google Scholar] [CrossRef] [PubMed]
- Oono, Y.; Yazawa, T.; Kawahara, Y.; Kanamori, H.; Kobayashi, F.; Sasaki, H.; Mori, S.; Wu, J.Z.; Handa, H.; Itoh, T.; et al. Genome-wide transcriptome analysis reveals that cadmium stress signaling controls the expression of genes in drought stress signal pathways in rice. PLoS ONE 2014, 9, e96946. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Law, S.R.; Murcha, M.W.; Whelan, J.; Carrie, C. The dual targeting ability of type II NAD(P)H dehydrogenases arose early in land plant evolution. BMC Plant Biol. 2013, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Z.; Wang, X.M.; Wang, X.Y.; Wu, K.L.; Li, P.; Chang, N.; Wang, J.F.; Wang, F.; Li, J.L.; Bi, Y.R. Glucose-6-phosphate dehydrogenase and alternative oxidase are involved in the cross tolerance of highland barley to salt stress and UV-B radiation. J. Plant Physiol. 2015, 181, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.M.; Zhao, C.Z.; Wang, J.F.; Li, P.; Dou, Y.Q.; Bi, Y.R. Alternative pathway is involved in the tolerance of highland barley to the low-nitrogen stress by maintaining the cellular redox homeostasis. Plant Cell Rep. 2016, 35, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Arnholdt-Schmitt, B.; Costa, J.H.; de Melo, D.F. AOX—A functional marker for efficient cell reprogramming under stress? Trends Plant Sci. 2006, 11, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Mangelsen, E.; Kilian, J.; Harter, K.; Jansson, C.; Wanke, D.; Sundberg, E. Transcriptome analysis of high-temperature stress in developing barley caryopses: Early stress responses and effects on storage compound biosynthesis. Mol. Plant 2011, 4, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Michalecka, A.M.; Svensson, A.S.; Johansson, F.I.; Agius, S.C.; Johanson, U.; Brennicke, A.; Binder, S.; Rasmusson, A.G. Arabidopsis genes encoding mitochondrial type IINAD(P)H dehydrogenases have different evolutionary orgin and show distinct responses to light. Plant Physiol. 2003, 133, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Carrie, C.; Murcha, M.W.; Kuehn, K.; Duncan, O.; Barthet, M.; Smith, P.M.; Eubel, H.; Meyer, E.; Day, D.A.; Millar, A.H.; et al. Type II NAD(P)H dehydrogenases are targeted to mitochondria and chloroplasts or peroxisomes in Arabidopsis thaliana. FEBS Lett. 2008, 582, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Umbach, A.L.; Ng, V.S.; Siedow, J.N. Regulation of plant alternative oxidase activity: A tale of two cysteines. Biochimica Et Biophysica Acta-Bioenergetics 2006, 1757, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.M.; Umbach, A.L.; Sweet, C.R.; Lennon, A.M.; Rauch, G.S.; Siedow, J.N. Regulation of the cyanide-resistant alternative oxidase of plant mitochondria—Identification of the cysteine residue involved in α-keto acid stimulation and intersubunit disulfide bond formation. J. Biol. Chem. 1998, 273, 30750–30756. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C.; Yio, J.Y.H.; Parsons, H.L. In organello and in vivo evidence of the importance of the regulatory sulfhydryl/disulfide system and pyruvate for alternative oxidase activity in tobacco. Plant Physiol. 1999, 121, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Djajanegara, I.; Holtzapffel, R.; Finnegan, P.M.; Hoefnagel, M.H.N.; Berthold, D.A.; Wiskich, J.T.; Day, D.A. A single amino acid change in the plant alternative oxidase alters the specificity of organic acid activation. FEBS Lett. 1999, 454, 220–224. [Google Scholar] [PubMed]
- Selinski, J.; Hartmann, A.; Kordes, A.; Deckers-Hebestreit, G.; Whelan, J.; Scheibe, R. Analysis of posttranslational activation of alternative oxidase isoforms. Plant Physiol. 2017, 174, 2113–2127. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Hoefnagel, M.H.N.; Day, D.A.; Wiskich, J.T. Specificity of the organic acid activation of alternative oxidase in plant mitochondria. Plant Physiol. 1996, 111, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Holtzapffel, R.C.; Castelli, J.; Finnegan, P.M.; Millar, A.H.; Whelan, J.; Day, D.A. A tomato alternative oxidase protein with altered regulatory properties. Biochim. Biophys. Acta 2003, 1606, 153–162. [Google Scholar] [CrossRef]
- Michalecka, A.M.; Agius, S.C.; Møller, I.M.; Rasmusson, A.G. Identification of a mitochondrial external NADPH dehydrogenase by overexpression in transgenic Nicotiana sylvestris. Plant J. 2004, 37, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Geisler, D.A.; Broselid, C.; Hederstedt, L.; Rasmusson, A.G. Ca2+-binding and Ca2+-independent respiratory NADH and NADPH dehydrogenases of Arabidopsis thaliana. J. Biol. Chem. 2007, 282, 28455–28464. [Google Scholar] [CrossRef] [PubMed]
- Fatihi, A.; Latimer, S.; Schmollinger, S.; Block, A.; Dussault, P.H.; Vermaas, W.F.J.; Merchant, S.S.; Basset, G.J. A dedicated type II NADPH dehydrogenase performs the penultimate step in the biosynthesis of Vitamin K-1 in Synechocystis and Arabidopsis. Plant Cell 2015, 27, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.P.; Roberts, T.H.; Moller, I.M. Evidence for the presence of two rotenone-insensitive NAD(P)H dehydrogenases on the inner surface of the inner membrane of potato tuber mitochondria. Biochim. Biophys. Acta 1996, 1276, 133–139. [Google Scholar] [CrossRef]
- Sweetman, C.; Waterman, C.D.; Rainbird, B.M.; Smith, P.M.C.; Jenkins, C.L.D.; Day, D.A.; Soole, K.L. AtNDB2 is the main external NADH dehydrogenase in Arabidopsis mitochondria and is important for tolerance to environmental stress. Unpublished work. 2018. [Google Scholar]
- Rasmusson, A.G.; Svensson, A.S.; Knoop, V.; Grohmann, L.; Brennicke, A. Homologues of yeast and bacterial rotenone-insensitive NADH dehydrogenases in higher eukaryotes: Two enzymes are present in potato mitochondria. Plant J. 1999, 20, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, D.P.; Wang, Y.; McIntosh, L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. USA 1999, 96, 8271–8276. [Google Scholar] [CrossRef] [PubMed]
- Tanudji, M.; Djajanegara, I.N.; Daley, D.O.; McCabe, T.C.; Finnegan, P.M.; Day, D.A.; Whelan, J. The multiple alternative oxidase proteins of soybean. Aust. J. Plant Physiol. 1999, 26, 337–344. [Google Scholar] [CrossRef]
- Smith, C.A.; Melino, V.J.; Sweetman, C.; Soole, K.L. Manipulation of alternative oxidase can influence salt tolerance in Arabidopsis thaliana. Physiol. Plant. 2009, 137, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Florez-Sarasa, I.; Ribas-Carbo, M.; Del-Saz, N.F.; Schwahn, K.; Nikoloski, Z.; Fernie, A.R.; Flexas, J. Unravelling the in vivo regulation and metabolic role of the alternative oxidase pathway in C-3 species under photoinhibitory conditions. New Phytol. 2016, 212, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Vanlerberghe, G.C. Alternative oxidase respiration maintains both mitochondrial and chloroplast function during drought. New Phytol. 2017, 213, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovska, M.; Dahal, K.; Alber, N.A.; Jin, C.; Cheung, M.; Vanlerberghe, G.C. Knockdown of mitochondrial alternative oxidase induces the ‘stress state’ of signaling molecule pools in Nicotiana tabacum, with implications for stomatal function. New Phytol. 2014, 203, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.H.; Santos, C.P.; de Sousa E Lima, B.; Moreira Netto, A.N.; Saraiva, K.D.; Arnholdt-Schmitt, B. In silico identification of alternative oxidase 2 (AOX2) in monocots: A new evolutionary scenario. J. Plant Physiol. 2017, 210, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Q.; Hou, X.L.; Li, X.; Sun, K.; Wang, R.F.; Zhang, T.G.; Ding, Y.P. Cell death of rice roots under salt stress may be mediated by cyanide-resistant respiration. Z. Naturforsch. C 2013, 68, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Taylor, N.L.; Millar, A.H.; Lambers, H.; Day, D.A. Response of mitochondria to light intensity in the leaves of sun and shade species. Plant Cell Environ. 2005, 28, 760–771. [Google Scholar] [CrossRef]
- Ribas-Carbo, M.; Taylor, N.L.; Giles, L.; Busquets, S.; Finnegan, P.M.; Day, D.A.; Lambers, H.; Medrano, H.; Berry, J.A.; Flexas, J. Effects of water stress on respiration in soybean leaves. Plant Physiol. 2005, 139, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Abu-Romman, S.; Shatnawi, M.; Hasan, M.; Qrunfleh, I.; Omar, S.; Salem, N. cDNA cloning and expression analysis of a putative alternative oxidase HsAOX1 from wild barley (Hordeum spontaneum). Genes Genom. 2012, 34, 59–66. [Google Scholar] [CrossRef]
- The Arabidopsis Information Resource. Available online: https://www.arabidopsis.org (accessed on 19 March 2018).
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Gramene. Available online: www.gramene (accessed on 19 March 2018).
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov (accessed on 19 March 2018).
- IPK Barley Blast Server (International Barley Sequencing Consortium). Available online: http://webblast.ipk-gatersleben.de/barley_ibsc (accessed on 19 March 2018).
- ExPASy Bioinformatics Resource Portal. Available online: https://web.expasy.org (accessed on 19 March 2018).
- Saitou, N.; Nei, M. The neighbor-joining method—A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Clustal Omega Tool. Available online: https://www.ebi.ac.uk/Tools/msa/clustalo (accessed on 19 March 2018).
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Predotar Tool, Plant and Fungi Data Integration Web Site. Available online: https://urgi.versailles.inra.fr/Tools/Predotar (accessed on 19 March 2018).
- Emanuelsson, O.; Nielsen, H.; von Heijne, G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 1999, 8, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.; Shen, H. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef] [PubMed]
- Reumann, S.; Buchwald, D.; Lingner, T. PredPlantPTS1: A web server for the prediction of plant peroxisomal proteins. Front. Plant. Sci. 2012, 3, 194. [Google Scholar] [CrossRef] [PubMed]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator V3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. 2008, 420747. [Google Scholar] [CrossRef] [PubMed]
- Cotsaftis, O.; Plett, D.; Johnson, A.A.T.; Walia, H.; Wilson, C.; Ismail, A.M.; Close, T.J.; Tester, M.; Baumann, U. Root-Specific Transcript Profiling of Contrasting Rice Genotypes in Response to Salinity Stress. Mol. Plant 2011, 4, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Bovill, J.; Afzal, I.; Hayes, J.E.; Roy, S.J.; Tester, M.; Collins, N.C. HVP10 encoding V-PPase is a prime candidate for the barley HvNax3 sodium exclusion gene: Evidence from fine mapping and expression analysis. Planta 2013, 237, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Kim, B.-R.; Nam, H.-Y.; Kim, S.-U.; Kim, S.-I.; Chang, Y.-J. Normalization of reverse transcription quantitative-PCR with housekeeping genes in rice. Biotechnol. Lett. 2003, 25, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, B.K.; Askerlund, P.; Bykova, N.V.; Egsgaard, H.; Moller, I.M. Identification of oxidised proteins in the matrix of rice leaf mitochondria by immunoprecipitation and two-dimensional liquid chromatography-tandem mass spectrometry. Phytochemistry 2004, 65, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Umbach, A.L.; Fiorani, F.; Siedow, J.N. Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol. 2005, 139, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, P.M.; Wooding, A.R.; Day, D.A. An alternative oxidase monoclonal antibody recognises a highly conserved sequence among alternative oxidase subunits. FEBS Lett. 1999, 447, 21–24. [Google Scholar] [CrossRef]
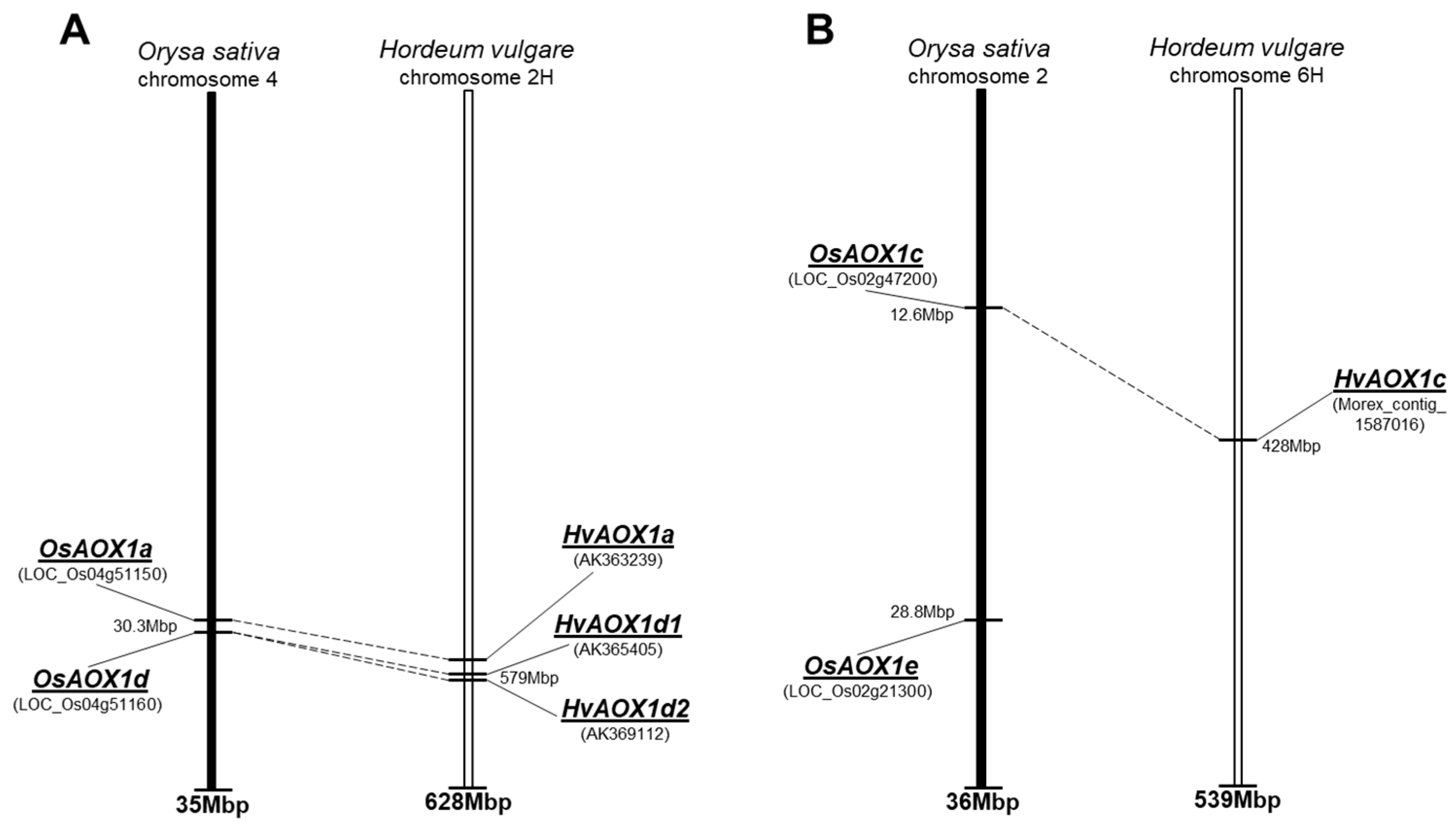

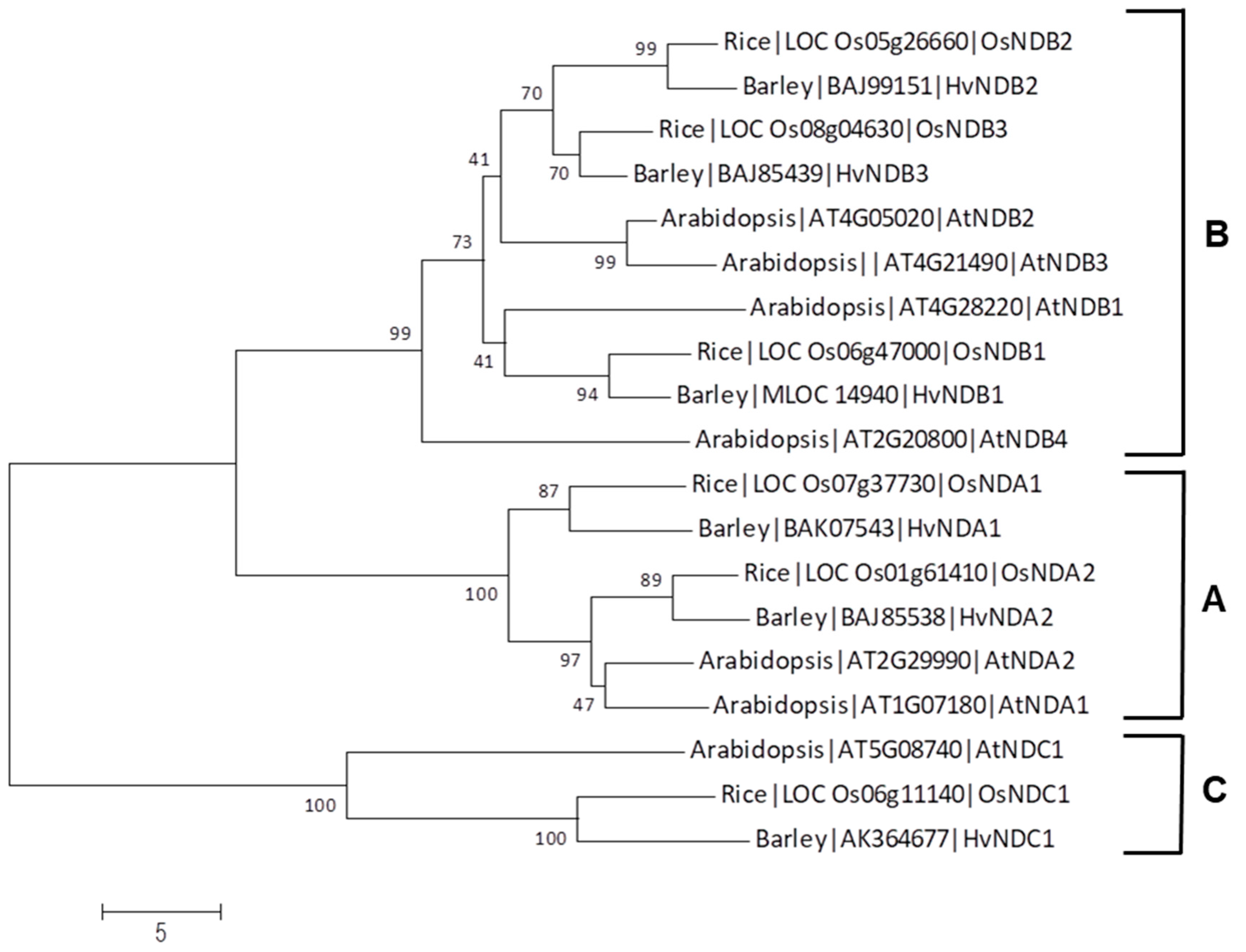


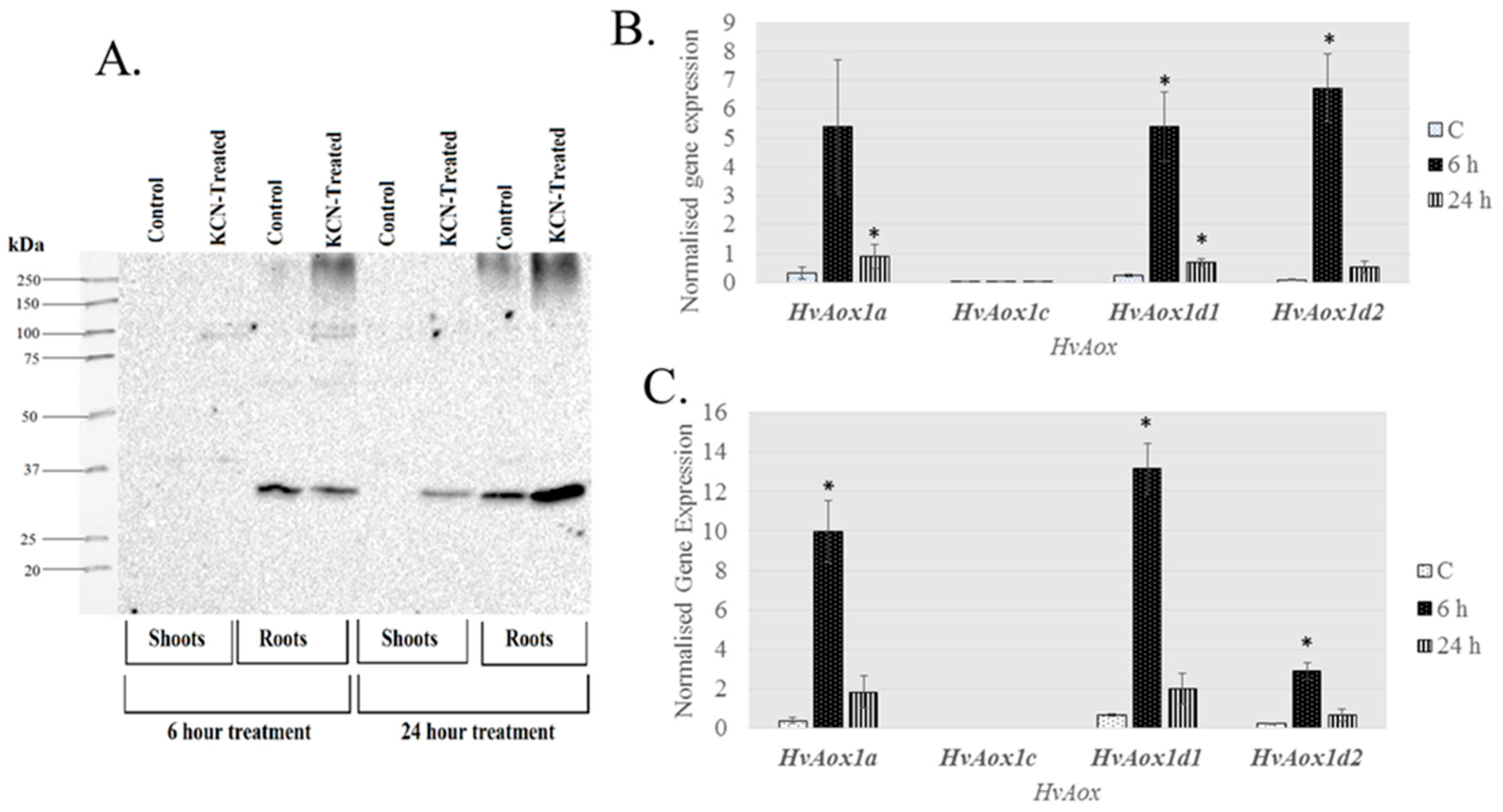
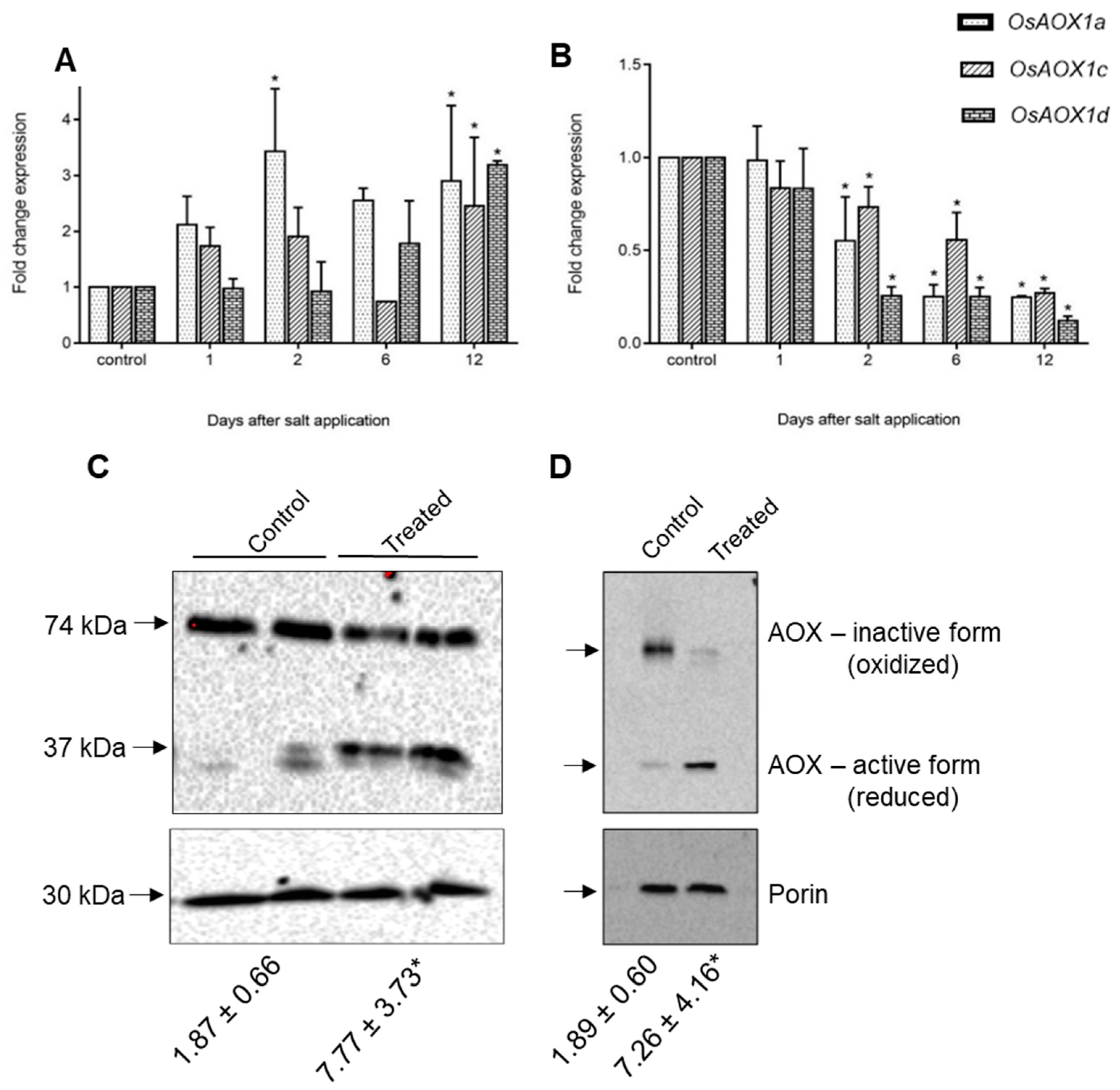
| Gene | Chromosome Location chr (Chromosome Number) cM (centi-Morgan) | Contig ID (Genomic Sequence/s | Relevant cDNA Accession Number/s | Relevant Protein Accession Number/s (Predicted from cDNA Sequence) | Predicted Protein Size (kDa) |
|---|---|---|---|---|---|
| HvAOX1A | chr = 2H cM = 105.87 chr2H:695364063-695501552 | morex_contig_38523 | AK363239 | AK363239 (on IBSC) HORVU2Hr1G101980.1 | 36.36 |
| HvAOX1C | chr = 6H cM = 63.67 chr = 6H cM = 63.46 chr6H:472176203-472177532 | morex_contig_1576377 morex_contig_1587016 | HORVU6Hr1G068150.2 * GH226429.1 * AK251266.1 * | HORVU6Hr1G068150.2 * | 36.13 |
| HvAOX1D1 | chr = 2H cM = 106.86 | morex_contig_99492 | AK365405.1 | BAJ96608.1 HORVU0Hr1G005420.4 | 36.38 |
| HvAOX1D2 | chr = 2H cM = 106.86 chr2H:695390581-695392039 | morex_contig_242686 | AK369112.1 | BAK00314.1 HORVU2Hr1G101990.1 | 37.08 |
| HvNDA1 | chr = 2H cM = 59.5 chr7H:445843858-445845235 | morex_contig_2565566 morex_contig_363823 | AK376348.1 | BAK07543.1 HORVU7Hr1G076330.1 | 60.06 |
| HvNDA2 | chr = 3H cM = 99.58 | morex_contig_7977 morex_contig_348032 morex_contig_189545 morex_contig_2081103 | AK354319.1 | BAJ85538.1 HORVU3Hr1G087850.1 HORVU4Hr1G058410.1 HORVU5Hr1G020920.2 HORVU7Hr1G040660.3 HORVU0Hr1G000930.1 HORVU2Hr1G101830.2 HORVU2Hr1G040130.1 | 54.21 |
| HvNDB1 | chr = 7H | morex_contig_1569455 | HORVU7Hr1G101500.3 | HORVU7Hr1G101500.3 | 65.08 |
| HvNDB2 | chr3H: 570908294-570913236 | morex_contig_36877 | AK367948.1 | BAJ99151.1 HORVU3Hr1G076920.2 | 64.23 |
| HvNDB3 | chr = 7H cM = 70.61 | morex_contig_47987 | AK354220.1 AK362739.1 AK371807.1 AK252091.1 AK371114.1 | BAJ85439.1 BAJ93943.1 BAK03005.1 BAK02312.1 | 64.59 |
| HvNDC1 | chr = 7H cM = 61.76 | morex_contig_49824 morex_contig_60206 | AK364677.1 | AK364677 (on IBSC) HORVU7Hr1G035810.4 | 59.33 |
| Tissue | OsAOX1a | OsAOX1c | OsAOX1d | OsNDA1 | OsNDA2 | OsNDB1 | OsNDB2 | OsNDB3 | OsNDC1 |
|---|---|---|---|---|---|---|---|---|---|
| 6 h shoots | 58.2 ± 15.51 * | 0.3 ± 0.08 | 248.6 ± 63.97 * | 17.4 ± 3.13 * | 0.2 ± 0.04 | 0.2 ± 0.11 | 5.2 ± 0.91 * | 18.3 ± 0.75 * | 0.6 ± 0.07 |
| 24 h shoots | 1.0 ± 0.29 | 0.1 ± 0.02 | 348.6 ± 78.37 * | 0.7 ± 0.05 | 1.1 ± 0.20 | 0.9 ± 0.66 | 1.9 ± 0.42 | 0.7 ± 0.05 | 0.2 ± 0.11 |
| 6 h roots | 44.0 ± 4.52 * | 1.7 ± 0.25 | 355.4 ± 46.55 * | 56.9 ± 13.04 * | 3.0 ± 0.81 | 1.6 ± 1.13 | 2.0 ± 0.37 | 29.7 ± 5.64 * | 1.0 ± 0.19 |
| 24 h roots | 5.1 ± 1.01 | 0.1 ± 0.02 | 11.3 ± 1.36 | 2.0 ± 0.15 | 6.0 ± 1.33* | 0.9 ± 0.23 | 7.1 ± 1.63 * | 1.0 ± 0.38 | 0.4 ± 0.14 |
| Tissue | HvAOX1a | HvAOX1c | HvAOX1d1 | HvAOX1d2 | HvNDA1 | HvNDA2 | HvNDB1 | HvNDB2 | HvNDB3 | HvNDC1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 h shoots | 16.5 ± 7.2 | 0.6 ± 0.3 | 23 ± 5 * | 80 ± 14 * | N.D. | 26 ± 19 | 1.4 ± 0.25 | 2.5 ± 1.0 | 17 ± 1.8 * | 1.2 ± 0.6 |
| 24 h shoots | 10.1 ± 4.7 | 0.3 ± 0.1 * | 3.5 ± 1.7 * | 3.5 ± 1.5 | N.D. | 1.2 ± 0.1 | 3.3 ± 1.0 | 3.2 ± 1.2 | 2.2 ± 0.5 * | 0.1 ± 0.02 |
| 6 h roots | 27 ± 1.6 * | N.D. | 17 ± 3.4 * | 14 ± 3 * | N.D. | 4.8 ± 1.0 | 0.8 ± 0.2 | 1.6 ± 0.3 * | 4.5 ± 1.3 * | 3.4 ± 1.8 |
| 24 h roots | 6.7 ± 1.8 | N.D. | 4.1 ± 1.7 | 2.2 ± 1.2 | N.D. | 1.8 ± 0.2 * | 0.7 ± 0.1 | 1.5 ± 0.3 | 1.7 ± 0.3 | 0.2 ± 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanniarachchi, V.R.; Dametto, L.; Sweetman, C.; Shavrukov, Y.; Day, D.A.; Jenkins, C.L.D.; Soole, K.L. Alternative Respiratory Pathway Component Genes (AOX and ND) in Rice and Barley and Their Response to Stress. Int. J. Mol. Sci. 2018, 19, 915. https://doi.org/10.3390/ijms19030915
Wanniarachchi VR, Dametto L, Sweetman C, Shavrukov Y, Day DA, Jenkins CLD, Soole KL. Alternative Respiratory Pathway Component Genes (AOX and ND) in Rice and Barley and Their Response to Stress. International Journal of Molecular Sciences. 2018; 19(3):915. https://doi.org/10.3390/ijms19030915
Chicago/Turabian StyleWanniarachchi, Vajira R., Lettee Dametto, Crystal Sweetman, Yuri Shavrukov, David A. Day, Colin L. D. Jenkins, and Kathleen L. Soole. 2018. "Alternative Respiratory Pathway Component Genes (AOX and ND) in Rice and Barley and Their Response to Stress" International Journal of Molecular Sciences 19, no. 3: 915. https://doi.org/10.3390/ijms19030915





