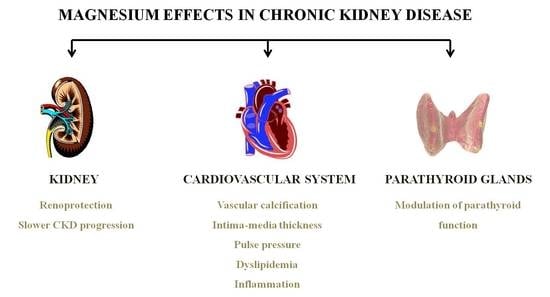
Magnesium Replacement to Protect Cardiovascular and Kidney Damage? Lack of Prospective Clinical Trials
Abstract
:1. Magnesium: Metabolism and Physiology
2. Magnesium and Enzyme Activity
3. Magnesium and Apoptosis
4. Magnesium and Oxidative Stress
5. Clinical Association between Magnesium and Cardiovascular Disease in the General Population
6. Clinical Association between Magnesium and Cardiovascular Disease in CKD
6.1. Vascular Calcification
6.2. Intima-Media Thickness
6.3. Pulse Pressure
6.4. Heart Failure
6.5. Dyslipidemia
6.6. Inflammation
7. Hypermagnesemia and Mortality
8. Magnesium and Renoprotection
9. Magnesium and CKD Progression
10. Experimental Evidence of the Beneficial Effects of Magnesium Replacement in CKD
10.1. Serum Magnesium and PTH Levels
10.2. Effect on Vascular Calcification
10.2.1. Passive effect of Magnesium Supplementation
10.2.2. Active Effect of Magnesium Supplementation
10.3. Other Effects of Magnesium
10.4. Magnesium and Bone
11. Risk of Magnesium Overdose
12. Are We Ready for Magnesium Supplements in CKD?
Acknowledgments
Conflicts of Interest
References
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Whiss, P.A. Weak relationship between ionized and total magnesium in serum of patients requiring magnesium status. Biol. Trace Elem. Res. 2007, 115, 13–21. [Google Scholar] [CrossRef]
- Speich, M.; Bousquet, B.; Nicolas, G. Reference values for ionized, complexed, and protein-bound plasma magnesium in men and women. Clin. Chem. 1981, 27, 246–248. [Google Scholar] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Kubota, K.; Oka, T.; Yamaguchi, S.; Matsumoto, A.; Hashimoto, N.; Mori, D.; Obi, Y.; Matsui, I.; et al. Anion Gap as a Determinant of Ionized Fraction of Divalent Cations in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2017, 13, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Laboratory evaluation of magnesium status. Renal function and free intracellular magnesium concentration. Clin. Lab. Med. 1993, 13, 209–223. [Google Scholar] [PubMed]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary References Intakes. Dietary References Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997.
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Regulation of magnesium balance: Lessons learned from human genetic disease. Clin. Kidney J. 2012, 5, i15–i24. [Google Scholar] [CrossRef] [PubMed]
- Kude, R.K.; Gruber, H.E. Magnesium deficiency and osteoporosis: Animal and human observations. J. Nutr. Biochem. 2004, 15, 710–716. [Google Scholar]
- Yu, A.S. Claudins and the kidney. J. Am. Soc. Nephrol. 2015, 26, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Goodenough, D.A. Claudin-16 and claudin-19 function in the thick ascending limb. Curr. Opin. Nephrol. Hypertens. 2010, 19, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Gomes, A.S.; Hou, M.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA 2009, 106, 15350–15355. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, L.; Garfinkel, D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium 1985, 4, 60–72. [Google Scholar] [PubMed]
- Lin, J.; Pan, L.P.; Chan, S.I. The subunit location of magnesium in cytochrome c oxidase. J. Biol. Chem. 1993, 268, 22210–22214. [Google Scholar] [PubMed]
- Panov, A.; Scarpa, A. Mg2+ control of respiration in isolated rat liver mitochondria. Biochemistry 1996, 35, 12849–12856. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.H. Mechanism of Ca2+ transport by Ca2+-Mg2+-ATPase pump: Analysis of major states and pathways. Am. J. Physiol. 1983, 244, G3–G12. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Chien, M.M.; Zahradka, K.E.; Newell, M.K.; Freed, J.H. Fas-induced B cell apoptosis requires an increase in free cytosolic magnesium as an early event. J. Biol. Chem. 1999, 274, 7059–7066. [Google Scholar] [CrossRef] [PubMed]
- Malpuech-Brugère, C.; Nowacki, W.; Gueux, E.; Kuryszko, J.; Rock, E.; Rayssiquier, Y.; Mazur, A. Accelerated thymus involution in magnesium-deficient rats is related to enhance apoptosis and sensitivity to oxidative stress. Br. J. Nutr. 1999, 81, 405–411. [Google Scholar] [PubMed]
- Martin, H.; Richert, L.; Berthelot, A. Magnesium deficiency induces apoptosis in primary cultures of rat hepatocytes. J. Nutr. 2003, 133, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Liu, W.; Altura, B.T.; Altura, B.M. Peroxynitrite induces apoptosis and decline in intracelular free Mg with concomitant elevation in intracellular free Mg with concomitant elevation in [Ca2+]I in rat aortic smooth muscle cells: Possible roles of extracellular and intracellular magnesium ions in peroxynitrite-induced cell death. Drug Metab. Lett. 2007, 1, 85–89. [Google Scholar] [PubMed]
- Altura, B.M.; Shah, N.C.; Jiang, X.C.; Li, Z.; Perez-Albela, J.L.; Sica, A.C.; Altura, B.T. Short-term magnesium deficiency results in decreased levels of serum sphyngomyelin, lipid peroxidation, and apoptosis in cardiovascular tissues. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H86–H92. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Guo, L.; Gao, H.; Li, X.A. Deficiency of calcium and magnesium induces apoptosis via scavenger receptor BI. Life Sci. 2011, 88, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S. Effects of magnesium deficiency on the pathogenesis of myocardial infarction. Magnesium 1986, 5, 154–164. [Google Scholar] [PubMed]
- Arsenian, M.A. Magnesium and cardiovascular disease. Prog. Cardiovasc. Dis. 1993, 35, 271–310. [Google Scholar] [CrossRef]
- Purvis, J.R.; Movahed, A. Magnesium disorders and cardiovascular diseases. Clin. Cardiol. 1992, 15, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, K.; Prakash Kumar, B. Magnesium deficiency enhances oxidative stress and collagen synthesis in vivo in the aorta of rats. Int. J. Biochem. 1997, 29, 1273–1278. [Google Scholar] [CrossRef]
- Garcia, L.A.; Dejong, S.C.; Martin, S.M.; Smith, R.S.; Buettner, G.R.; Kerber, R.E. Magnesium reduces free radicals in an in vivo coronary occlusion-reperfusion model. J. Am. Coll. Cardiol. 1998, 32, 536–539. [Google Scholar] [CrossRef]
- Dickens, B.F.; Weglicki, W.B.; Li, Y.S.; Mak, I.T. Magnesium deficiency in vitro enhances free radical-induced intracellular oxidation and cytotoxicity in endothelial cells. FEBS 1992, 311, 187–191. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferrè, S.; Maier, J.A.M. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes. Res. 2008, 21, 58–64. [Google Scholar] [PubMed]
- Touyz, R.M.; Pu, Q.; He, G.; Chen, X.; Yao, G.; Neves, M.F.; Viel, E. Effects of low dietary magnesium intake on development of hypertension in stroke-prone spontaneously hypertensive rats: Role of reactive oxygen species. J. Hypertens. 2002, 20, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.; Astier, C.; Lab, C.; Vignon, X.; Gueux, E.; Motta, C.; Rayssiquier, Y. Dietary magnesium deficiency in rats enhances free radical production in skeletal muscle. J. Nutr. 1995, 125, 1205–1210. [Google Scholar] [PubMed]
- Boparai, R.K.; Kiran, R.; Bansal, D.D. Insinuation of exacerbated oxidative stress in sucrose-fed rats with a low dietary intake of magnesium: Evidence of oxidative damage to proteins. Free Radic. Res. 2007, 41, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Araújo Sampaio, F.; Monte Feitosa, M.; Hermes Sales, C.; Costa e Silva, D.M.; Clímaco Cruz, K.J.; Oliveira, F.E.; Colli, C.; do Nascimento Marreiro, D. Influence of magnesium on biochemical parameters of iron and oxidative stress in patients with type 2 diabetes. Nutr. Hosp. 2014, 30, 570–576. [Google Scholar] [PubMed]
- Kolisek, M.; Montezano, A.C.; Sponder, G.; Anagnostopoulou, A.; Vormann, J.; Touyz, R.M.; Aschenbach, J.R. PARK7/DJ-1 dysregulation by oxidative stress leads to magnesium deficiency: Implicatioins in degenerative and chronic diseases. Clin. Sci. 2015, 129, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Vijayaraghavan, K.; Khera, S.; Sica, D.A.; Frishman, W.H. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014, 22, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Pisu, E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 2008, 19, 50–56. [Google Scholar] [CrossRef] [PubMed]
- M de Francisco, A.L.; Rodríguez, M. Magnesium—Its role in CKD. Nefrologia 2013, 33, 389–399. [Google Scholar] [PubMed]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Min, J.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Wahlqvist, M.L.; Kao, M.D.; Wang, J.L.; Lee, M.S. Optimal dietary and plasma magnesium statuses depend on dietary quality for a reduction in the risk of all-cause mortality in older adults. Nutrients 2015, 7, 5664–5683. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Jin, F.; Hao, Y.; Li, H.; Tang, T.; Wang, H.; Wan, W.; Dai, K. Magnesium and the risk of cardiovascular events: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e57720. [Google Scholar] [CrossRef] [PubMed]
- Del Globbo, L.C.; Imamura, F.; Wu, J.H.; de Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bulló, M.; Estruch, R.; Corella, D.; Martínez-González, M.A.; Ros, E.; Covas, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary magnesium intake is inversely associated with mortality in adults at high cardiovascular disease risk. J. Nutr. 2014, 144, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Sun, Q.; Curhan, G.C.; Taylor, E.N.; Spiegelman, D.; Willet, W.C.; Manson, J.E.; Rexrode, K.M.; Albert, C.M. Dietary and plasma magnesium and risk of coronary heart disease among women. J. Am. Heart Assoc. 2013, 2, e000114. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risk of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 23, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Riva, H.; Donato, G.; Gabutti, L. Ionized and total serum magnesium in hemodialysis: Predictors and variability. A longitudinal cross-sectional study. Clin. Exp. Nephrol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wyskida, K.; Witkowicz, J.; Chudek, J.; Wiecek, A. Daily magnesium intake and hypermagnesemia in hemodialysis patients with chronic kidney disease. J. Ren. Nutr. 2012, 22, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Hirayama, T.; Hibino, Y.; Fukuhara, Y.; Kanno, Y. Malnutrition as cause of hypomagnesemia. Kidney Int. 2014, 86, 856. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Chakraborti, T.; Mandal, M.; Mandal, A.; Das, S.; Ghosh, S. Protective role of magnesium in cardiovascular diseases: A review. Mol. Cell. Biochem. 2002, 238, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.; Wanner, C. Magnesium in disease. Clin. Kidney J. 2012, 5, i25–i28. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Isaka, Y. Effects of Magnesium on the Phosphate Toxicity in Chronic Kidney Disease: Time for Intervention Studies. Nutrients 2017, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.C.; Pham, P.M.; Pham, S.V.; Miller, J.M.; Pham, P.T. Hypomagnesemia in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2007, 2, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.; Joshi, S.; Shaw, J. Hypomagnesaemia is associated with diabetes: Not pre-diabetes, obesity or the metabolic síndrome. Diabetes Res. Clin. Pract. 2010, 87, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Fragoso, A.; Silva, C.; Tavares, N.; Santos, N.; Martins, H.; Gundlach, K.; Büchel, J.; Camacho, A.; Faísca, M.; et al. Magnesium and mortality in patients with diabetes and early chronic kidney disease. J. Diabetes Metab. 2014, 5, 3. [Google Scholar]
- Alexopoulos, D.; Toulgaridis, T.; Davlouros, P.; Christodoulou, J.; Sitafidis, G.; Hahalis, G.; Vagenakis, A.G. Prognostic significance of coronary artery calcium in asymptomatic subjects with usual cardiovascular risk. Am. Heart J. 2003, 145, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; O’Donnell, C.J.; Jacques, P.F.; Meigs, J.B.; Hoffmann, U.; McKeown, N.M. Magnesium intake is inversely associated with coronary artery calcification: The Framingham Heart Study. JACC Cardiovasc. Imaging 2014, 7, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Sánchez, R.; Posadas-Romero, C.; Cardoso-Saldaña, G.; Vargas-Alarcón, G.; Villarreal-Molina, M.T.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Medina-Urrutia, A.; Jorge-Galarza, E.; Juárez-Rojas, J.G.; et al. Serum magnesium is inversely associated with coronary artery calcification in the Genetics of Atherosclerotic Disease (GEA) study. Nutr. J. 2016, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Nakano, C.; Obi, Y.; Matsui, I.; Kusunoki, Y.; Mori, D.; Oka, T.; Hashimoto, N.; Takabatake, Y.; et al. Association between Density of Coronary Artery Calcification and Serum Magnesium Levels among Patients with Chronic Kidney Disease. PLoS ONE 2016, 11, e0163673. [Google Scholar] [CrossRef] [PubMed]
- Bressendorff, I.; Hansen, D.; Schou, M.; Kragelund, C.; Brandi, L. The effect of magnesium supplementation on vascular calcification in chronic kidney disease-A randomised clinical trial (MAGiCAL-CKD): Essential study design and rationale. BMJ Open 2017, 7, e016795. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.; Bruck, H.; Bahlmann, F.H.; Peter, M.; Passlick-Deetjen, J.; Kretschmer, A.; Steppan, S.; Volsek, M.; Kribben, A.; Nierhaus, M.; et al. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am. J. Nephrol. 2012, 35, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zaher, M.M.; Abdel-Salam, M.; Abdel-Salam, R.; Sabour, R.; Morsy, A.A.; Gamal, D. Serum magnesium level and vascular stiffness in children with chronic kidney disease on regular hemodialysis. Saudi J. Kidney Dis. Transpl. 2016, 27, 233–240. [Google Scholar] [PubMed]
- Turgut, F.; Kanbay, M.; Metin, M.R.; Uz, E.; Akcay, A.; Covic, A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int. Urol. Nephrol. 2008, 40, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Moeinzadeh, F.; Saadatnia, M.; Shahidi, S.; McGee, J.C.; Minagar, A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: A double-blind, randomized, placebo-controlled trial. Eur. Neurol. 2013, 69, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, A.; Silva, A.P.; Gundlach, K.; Büchel, J.; Neves, P.L. Magnesium and FGF-23 are independent predictors of pulse pressure in pre-dialysis diabetic chronic kidney disease patients. Clin. Kidney J. 2014, 7, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Alonso, A.; Michos, E.D.; Loehr, L.R.; Astor, B.C.; Coresh, J.; Folsom, A.R. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2014, 100, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Robles, N.R.; Escola, J.M.; Albarran, L.; Espada, R. Correlation of serum magnesium and serum lipid levels in hemodialysis patients. Nephron 1998, 78, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.R.; Maheshwari, N.; Shaikh, M.A.; Laghari, M.S.; Darshana; Lal, K.; Ahmed, K. Correlation of serum magnesium with dyslipidemia in patients on maintenance hemodialysis. Saudi J. Kidney Dis. Transpl. 2012, 23, 21–25. [Google Scholar] [PubMed]
- Baradaran, A.; Nasri, H. Correlation of serum magnesium with dyslipidemia in maintenance hemodialysis patients. Indian J. Nephrol. 2004, 14, 46–49. [Google Scholar]
- Dey, R.; Rajappa, M.; Parameswaran, S.; Revathy, G. Hypomagnesemia and atherogenic dyslipidemia in chronic kidney disease: Surrogate markers for increased cardiovascular risk. Clin. Exp. Nephrol. 2015, 19, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Malpuech-Brugère, C.; Nowacki, W.; Daveau, M.; Gueux, E.; Linard, C.; Rock, E.; Lebreton, J.; Mazur, A.; Rayssiguier, Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta 2000, 1501, 91–98. [Google Scholar] [CrossRef]
- Tejero-Taldo, M.I.; Kramer, J.H.; Mak, I.T.; Komarov, A.M.; Weglicki, W.B. The nerve-heart connection in the pro-oxidant response to Mg-deficiency. Heart Fail. Rev. 2006, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Fein, P.; Suda, V.; Borawsky, C.; Kapupara, H.; Butikis, A.; Matza, B.; Chattopadhyay, J.; Avra, M.M. Relationship of serum magnesium to body composition and inflammation in peritoneal dialysis patients. Adv. Perit. Dial. 2010, 26, 112–115. [Google Scholar] [PubMed]
- Liu, F.; Zhang, X.; Qi, H.; Wang, J.; Wang, M.; Zhang, Y.; Yan, H.; Zhuang, S. Correlation of serum magnesium with cardiovascular risk factors in maintenance hemodialysis patients—A cross-sectional study. Magnes. Res. 2013, 26, 100–108. [Google Scholar] [PubMed]
- Massy, Z.A.; Nistor, I.; Apetrii, M.; Brandenburg, V.M.; Bover, J.; Evenepoel, P.; Goldsmith, D.; Mazzaferro, S.; Urena-Torres, P.; Vervloet, M.G.; et al. Magnesium-based interventions for normal kidney function and chronic kidney disease. Magnes. Res. 2016, 29, 126–140. [Google Scholar] [PubMed]
- Ferrè, S.; Baldoli, E.; Leidi, M.; Maier, J.A. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFκB. Biochim. Biophys. Acta 2010, 1802, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Malpuech-Brugère, C.; Zimowska, W.; Rayssiguier, Y.; Mazur, A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim. Biophys. Acta 2004, 1689, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Qian, Q. Dysmagnesemia in Hospitalized Patients: Prevalence and Prognostic Importance. Mayo Clin Proc. 2015, 90, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.G.; Lindner, G.; Ahmad, S.S.; Sauter, T.; Wolzt, M.; Leichtle, A.B.; Fiedler, G.M.; Exadaktylos, A.K.; Fuhrmann, V. Hypermagnesemia is a strong independent risk factor for mortality in critically ill patients: Results from a cross-sectional study. Eur. J. Intern. Med. 2015, 26, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Broner, C.W.; Stidham, G.L.; Westenkirchner, D.F.; Tolley, E.A. Hypermagnesemia and hypocalcemia as predictors of high mortality in critically ill pediatric patients. Crit. Care Med. 1990, 18, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Broman, M.; Hansson, F.; Klarin, B. Analysis of hypo- and hypermagnesemia in an intensive care unit cohort. Acta Anaesthesiol. Scand. 2018. [Google Scholar] [CrossRef] [PubMed]
- Angkananard, T.; Anothaisintawee, T.; Eursiriwan, S.; Gorelik, O.; McEvoy, M.; Attia, J.; Thakkinstian, A. The association of serum magnesium and mortality outcomes in heart failure patients: A systematic review and meta-analysis. Medicine 2016, 95, e5406. [Google Scholar] [CrossRef] [PubMed]
- Naksuk, N.; Hu, T.; Krittanawong, C.; Thongprayoon, C.; Sharma, S.; Park, J.Y.; Rosenbaum, A.N.; Gaba, P.; Killu, A.M.; Sugrue, A.M.; et al. Association of Serum Magnesium on Mortality in Patients Admitted to the Intensive Cardiac Care Unit. Am. J. Med. 2017, 130, 229.e5–229.e13. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, E.; Okuno, S.; Yamakawa, T.; Inaba, M.; Nishizawa, Y. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes. Res. 2007, 20, 234–244. [Google Scholar]
- Lacson, E., Jr.; Wang, W.; Ma, L.; Passlick-Deetjen, J. Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am. J. Kidney Dis. 2015, 66, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, H.; Wang, S.X. Serum Magnesium and Mortality in Maintenance Hemodialysis Patients. Blood Purif. 2017, 43, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Pere, A.K.; Lindgren, L.; Tuomainen, P.; Krogerus, L.; Rauhala, P.; Laakso, J.; Karppanen, H.; Vapaatalo, H.; Ahonen, J.; Mervaala, E.M. Dietary potassium and magnesium supplementation in cyclosporine-induced hypertension and nephrotoxicity. Kidney Int. 2000, 58, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Lajer, H.; Daugaard, G. Cisplatin and hypomagnesemia. Cancer Treat Rev. 1999, 25, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.H.; Chatterjee, P.K.; Xue, X.; Gupta, M.; Rosales, I.; Yeboah, M.M.; Kohn, N.; Metz, C.N. Magnesium protects against cisplatin-induced acute kidney injury without compromising cisplatin-mediated killing of an ovarian tumor xenograft in mice. Am. J. Physiol. Ren. Physiol. 2015, 309, F35–F47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Solanki, M.H.; Xue, X.; Mintz, R.; Madankumar, S.; Chatterjee, P.K.; Metz, C.N. Magnesium improves cisplatin-mediated tumor killing while protecting against cisplatin-induced nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2017, 313, F339–F350. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kobayashi, M.; Yamada, T.; Kasashi, K.; Honma, R.; Takeushi, S.; Shimizu, Y.; Kinoshita, I.; Dosaka-Akita, H.; Iseki, K. Premedication with intravenous magnesium has a protective effect against cisplatin-induced nephrotoxicity. Support Cancer Care 2017, 25, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Okamoto, K.; Kobayashi, M.; Narumi, K.; Yamada, T.; Iseki, K. Magnesium attenuates cisplatin-induced nephrotoxicity by regulating the expression of renal transporters. Eur. J. Pharmacol. 2017, 811, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, M.R.; Parviz, M.; Tavangar, S.M.; Soltani, N.; Kadkhodaee, M.; Seifi, B.; Keshavarz, M. Protective effect of magnesium on renal function in STZ-induced diabetic rats. J. Diabetes Metab. Disord. 2014, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Tin, A.; Grams, M.E.; Maruthur, N.M.; Astor, B.C.; Couper, D.; Mosley, T.H.; Selvin, E.; Coresh, J.; Kao, W.H. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015, 87, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Nagler, E.V.; Verbeke, F.; Van Biesen, W.; Vanholder, R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.C.; Pham, P.M.; Pham, P.A.; Pham, S.V.; Pham, H.V.; Miller, J.M.; Yanagawa, N.; Pham, P.T. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin. Nephrol. 2005, 63, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Shoji, T.; Hayashi, T.; Suzuki, A.; Shimizu, M.; Mitsumoto, K.; Kawabata, H.; Niihata, K.; Okada, N.; Isaka, Y.; et al. Hypomagnesemia in type 2 diabetic nephropathy: A novel predictor of end-stage renal disease. Diabetes Care 2012, 35, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Iwatani, H.; Hamano, T.; Tomida, K.; Kawabata, H.; Kusunoki, Y.; Shimomura, A.; Matsui, I.; Hayashi, T.; Tsubakihara, Y.; et al. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int. 2015, 88, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Ruggenenti, P.; Perna, A.; Leonardis, D.; Tripepi, R.; Tripepi, G.; Mallamaci, F.; Remuzzi, G. REIN Study Group. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J. Am. Soc. Nephrol. 2011, 22, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Tocados, J.M.; Peralta-Ramirez, A.; Rodríguez-Ortiz, M.E.; Raya, A.I.; Lopez, I.; Pineda, C.; Herencia, C.; Montes de Oca, A.; Vergara, N.; Steppan, S.; et al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017, 92, 1084–1099. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ortiz, M.E.; Canalejo, A.; Herencia, C.; Martínez-Moreno, J.M.; Peralta-Ramírez, A.; Perez-Martinez, P.; Navarro-González, J.F.; Rodríguez, M.; Peter, M.; Gundlach, K.; et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol. Dial. Transplant. 2014, 29, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Wada, A.; Hoshino, J.; Masakane, I. Magnesium and risk of hip fracture among patients undergoing hemodialysis. J. Am. Soc. Nephrol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Fuchigami, M.; Miwa, M. Dietary magnesium supplementation suppresses bone resorption via inhibition of parathyroid hormone secretion in rats fed a high-phosphorus diet. Magnes. Res. 2010, 23, 126–130. [Google Scholar] [PubMed]
- Zhang, C.; Zhang, T.; Zou, J.; Miller, C.L.; Gorkhali, R.; Yang, J.Y.; Schilmiller, A.; Wang, S.; Huang, K.; Brown, E.M.; et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2016, 27, e1600241. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Mora, C.; Jiménez, A.; Torres, A.; Macía, M.; García, J. Relationship between serum magnesium and parathyroid hormone levels in hemodialysis patients. Am. J. Kidney Dis. 1999, 34, 43–48. [Google Scholar] [CrossRef]
- De Francisco, A.L.; Leiding, M.; Covic, A.C.; Ketteler, M.; Benedyk-Lorens, E.; Mircescu, G.M.; Scholz, C.; Ponce, P.; Passlick-Deetjen, J. Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: A controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol. Dial. Transplant. 2010, 25, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Verberckmoes, S.C.; Persy, V.; Behets, G.J.; Neven, E.; Hufkens, A.; Zebger-Gong, H.; Müller, D.; Haffner, D.; Querfeld, U.; Bohic, S.; et al. Uremia-related vascular calcification: More than apatite deposition. Kidney Int. 2007, 71, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Zelt, J.G.; McCabe, K.M.; Svajger, B.; Barron, H.; Laverty, K.; Holden, R.M.; Adams, M.A. Magnesium modifies the impact of calcitriol treatment on vascular calcification in experimental chronic kidney disease. J. Pharmacol. Exp. Ther. 2015, 355, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Magnesium stabilization of amorphous calcium phosphate: A kinetic study. Mater. Res. Bull. 1974, 9, 907–916. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Contiguglia, S.R.; Alfrey, A.C. Pathological calcifications associated with uremia: Two types of calcium phosphate deposits. Calcif. Tissue Res. 1973, 23, 175–185. [Google Scholar] [CrossRef]
- Louvet, L.; Bazin, D.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS ONE 2015, 10, e0115342. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, T.M.; Behets, G.J.; Geryl, H.; Peter, M.E.; Steppan, S.; Gundlach, K.; Passlick-Deetjen, J.; D’Haese, P.C.; Neven, E. Effect of a magnesium-based phosphate binder on medial calcification in a rat model of uremia. Kidney Int. 2013, 83, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, A.; Guerrero, F.; Martinez-Moreno, J.M.; Madueño, J.A.; Herencia, C.; Peralta, A.; Almaden, Y.; Lopez, I.; Aguilera-Tejero, E.; Gundlach, K.; et al. Magnesium inhibits Wnt/β-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS ONE 2014, 9, e89525. [Google Scholar] [CrossRef] [PubMed]
- Louvet, L.; Metzinger, L.; Büchel, J.; Steppan, S.; Massy, Z.A. Magnesium attenuates phosphate-induced deregulation of a microRNA signature and prevents modulation of Smad1 and osterix during the course of vascular calcification. BioMed Res. Int. 2016, 2016, 7419524. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bai, Y.; Jin, J.; Zhang, J.; Zhang, S.; Cui, L.; Zhang, H. Magnesium modulates the expression levels of calcification-associated factors to inhibit calcification in a time-dependent manner. Exp. Ther. Med. 2015, 9, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2012, 122, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Ravn, H.B.; Korsholm, T.L.; Falk, E. Oral magnesium supplementation induces favorable antiatherogenic changes in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.R.; D’El-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J. Hypertens. 2017, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Su, N.Y.; Peng, T.C.; Tsai, P.S.; Huang, C.J. Phosphoinositide 3-kinase/Akt pathway is involved in mediating the anti-inflammation effects of magnesium sulfate. J. Surg. Res. 2013, 185, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Akan, M.; Ozbilgin, S.; Boztas, N.; Celik, A.; Ozkardesler, S.; Ergur, B.U.; Guneli, E.; Sisman, A.R.; Akokay, P.; Meseri, R. Effect of magnesium sulfate on renal ischemia-reperfusion injury in streptozotocin-induced diabetic rats. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1642–1655. [Google Scholar] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tocados, J.M.; Herencia, C.; Martínez-Moreno, J.M.; Montes de Oca, A.; Rodríguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almadén, Y.; Rodríguez, M.; et al. Magnesium chloride promotes osteogenesis through Notch signaling activation and expansion of mesenchymal stem cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.C.; Pringa, E.; Chou, L. Effect of magnesium on the osteogenesis of normal human osteoblasts. Magnes. Res. 2017, 30, 42–52. [Google Scholar] [PubMed]
- Vissers, R.J.; Purssell, R. Iatrogenic magnesium overdose: Two case reports. J. Emerg. Med. 1996, 14, 187–191. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Bressendorff, I.; Hansen, D.; Schou, M.; Silver, B.; Pasch, A.; Bouchelouche, P.; Pedersen, L.; Rasmussen, L.M.; Brandi, L. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: Efficacy, safety, and effect on serum calcification propensity—A prospective randomized double-blinded placebo-controlled clinical trial. KI Rep. 2017, 2, 380–389. [Google Scholar] [CrossRef] [PubMed]


| Reference | Study Type | Clinical Setting | No. of Subjects | Outcome | Conclusion |
|---|---|---|---|---|---|
| Fang et al. [40] | Meta-analysis of prospective studies | General population | >1,000,000 | CVD (coronary heart disease, ischemic heart disease, stroke) and all-cause mortality | Increasing dietary Mg is associated with a reduced risk of stroke and heart failure, but not with total CVD, and all-cause mortality. |
| Huang et al. [41] | Observational | Elderly | 1400 | All-cause and cause-specific mortality | Low plasma Mg levels increase all-cause mortality. |
| Qu et al. [42] | Meta-analysis of prospective studies | General population | 532,979 | CVD | Inverse association between dietary Mg intake and CVD risk. |
| Del Globbo et al. [43] | Meta-analysis of prospective studies | General population | 313,041 | Incidence of CVD, including IHD | Plasma and dietary Mg are inversely associated with CVD risk. |
| Guasch-Ferré et al. [44] | Prospective | Individuals at high risk of CVD | 7216 | CVD and all-cause mortality | Mg intake is associated with a lower mortality risk in this population, but not with CV events. |
| Chiuve et al. [45] | Prospective | Women free of disease | 86,323 | CHD | Dietary Mg intake was inversely associated with fatal CHD. |
| Reference | Study Type | Clinical Setting | No. of Subjects | Outcome | Conclusion |
|---|---|---|---|---|---|
| Sakaguchi et al. [53] | Observational | Hemodialysis | 142,555 | Cardiovascular and non-cardiovascular mortality | Hypomagnesemia predicts cardiovascular and non-cardiovascular mortality. |
| Sakaguchi et al. [60] | Observational | Pre-dialysis | 109 | Density of CAC | CAC is inversely associated with serum Mg levels. |
| Bressendorf et al. [61] | Interventional | Pre-dialysis | 250 | Progression of CAC | Ongoing study. |
| Salem et al. [62] | Observational | Dialysis | 36 | IMT PWV | In CKD, Mg levels were inversely associated with the IMT of carotids and the PWV. |
| Zaher et al. [63] | Observational | Hemodialysis | 25 | IMT | Mg correlates inversely with IMT in pediatric CKD. |
| Turgut et al. [64] | Interventional | Hemodialysis | 47 | IMT | Carotid IMT improved following administration of Mg citrate. |
| Mortazavi et al. [65] | Interventional | Hemodialysis | 54 | IMT, FMD, CRP | Mg may be involved in the decrease in IMT in treated patients. |
| Fragoso et al. [66] | Observational | Pre-dialysis | 80 | PP | Low Mg levels are independently associated with higher PP. |
| Robles et al. [68] | Observational | Hemodialysis | 25 | Dyslipidemia | Mg is positively associated with total cholesterol, LDL-C, VLDL-C and ApoB. |
| Ansari et al. [69] | Observational | Hemodialysis | 50 | Dyslipidemia | Mg is directly associated with LP-A, HDL-C, and TG. |
| Baradaran et al. [70] | Observational | Hemodialysis | 36 | Dyslipidemia | Positive correlation between Mg and LP-A and TG. |
| Dey et al. [71] | Observational | Pre-dialysis | 90 | Dyslipidemia | Significant association between Mg, total cholesterol, HDL-C, LDL-C and non-HDL-C. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Castañeda, J.R.; Pendón-Ruiz de Mier, M.V.; Rodríguez, M.; Rodríguez-Ortiz, M.E. Magnesium Replacement to Protect Cardiovascular and Kidney Damage? Lack of Prospective Clinical Trials. Int. J. Mol. Sci. 2018, 19, 664. https://doi.org/10.3390/ijms19030664
Muñoz-Castañeda JR, Pendón-Ruiz de Mier MV, Rodríguez M, Rodríguez-Ortiz ME. Magnesium Replacement to Protect Cardiovascular and Kidney Damage? Lack of Prospective Clinical Trials. International Journal of Molecular Sciences. 2018; 19(3):664. https://doi.org/10.3390/ijms19030664
Chicago/Turabian StyleMuñoz-Castañeda, Juan R., María V. Pendón-Ruiz de Mier, Mariano Rodríguez, and María E. Rodríguez-Ortiz. 2018. "Magnesium Replacement to Protect Cardiovascular and Kidney Damage? Lack of Prospective Clinical Trials" International Journal of Molecular Sciences 19, no. 3: 664. https://doi.org/10.3390/ijms19030664