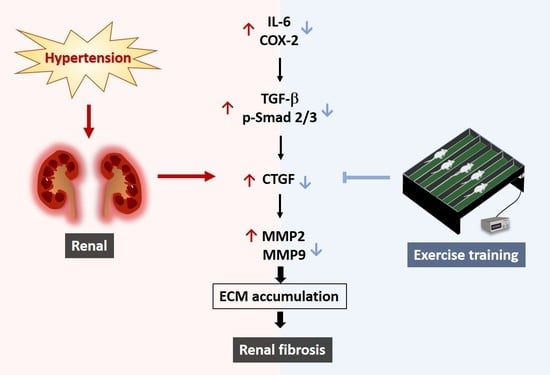
Anti-Renal Fibrotic Effect of Exercise Training in Hypertension
Abstract
:1. Introduction
2. Results
2.1. Physical and Cardiovascular Characteristics
2.2. Body Weight and Blood Pressure Changes
2.3. Histopathology of Renal Fibrosis Changes
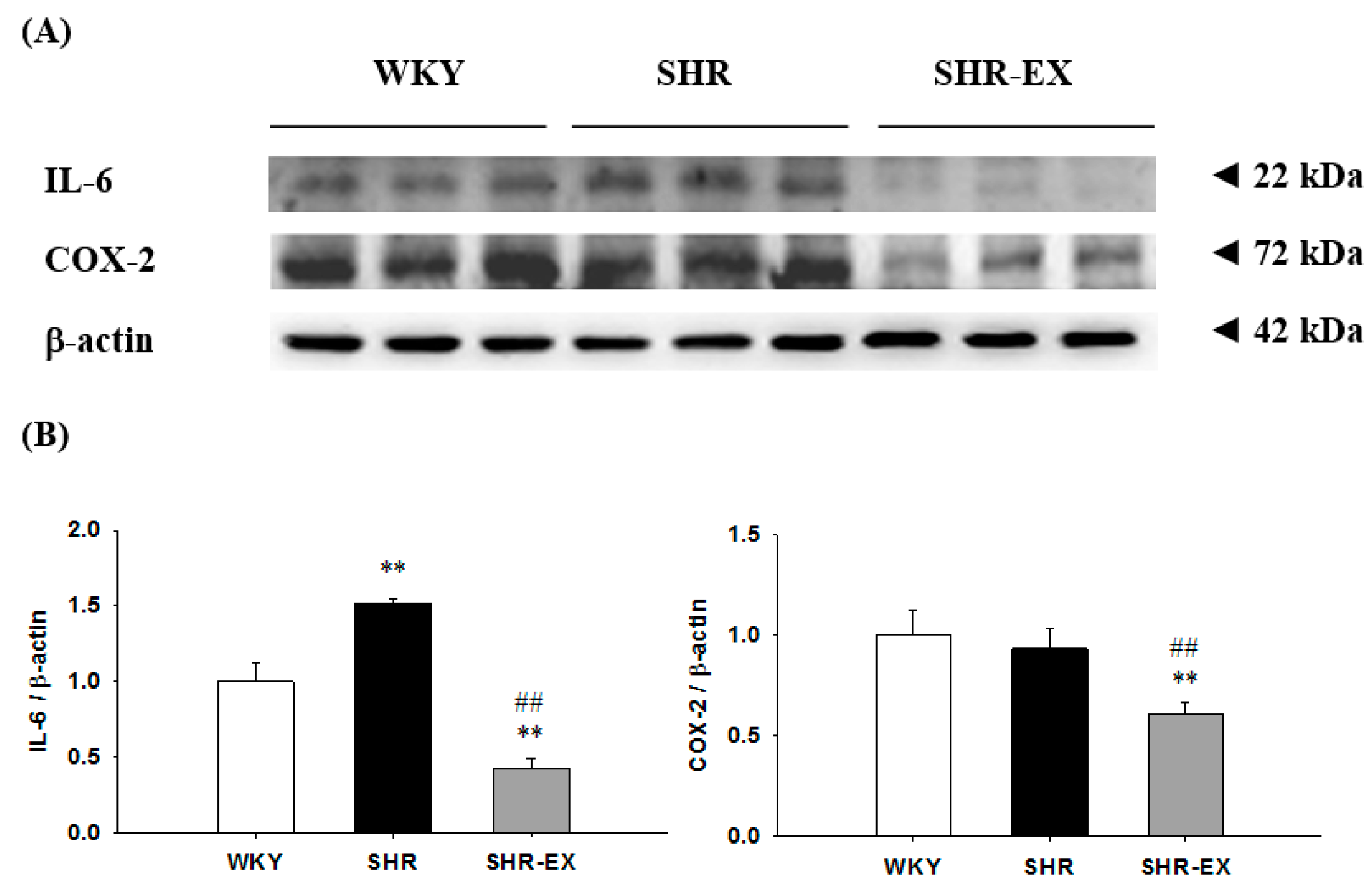
2.4. Components of Renal Inflammatory Pathway
2.5. Components of Renal Fibrotic Pathway
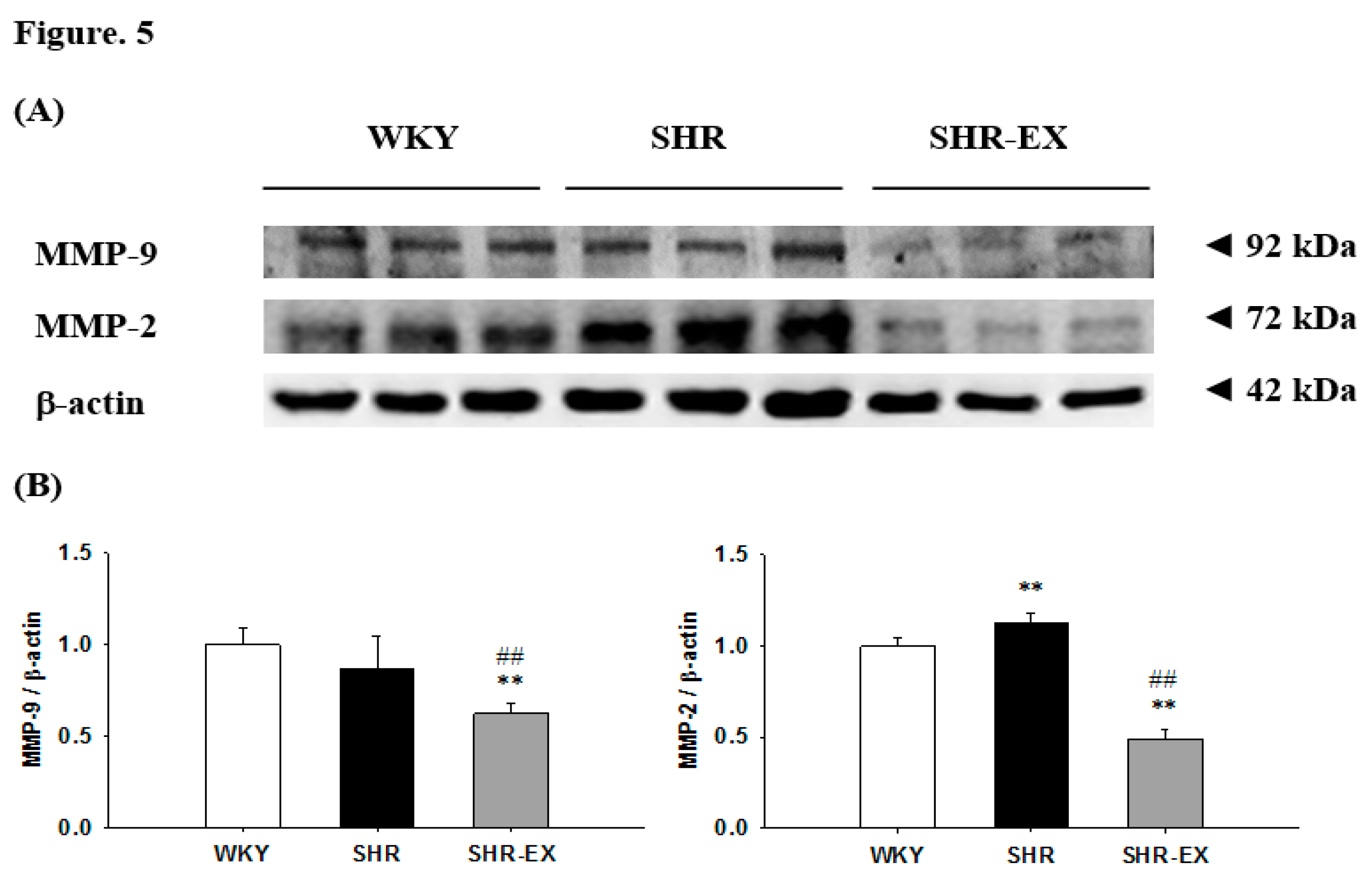
2.6. Components of Renal MMPs
3. Discussion
Hypothesized and Clinical Application
4. Experimental Section
4.1. Animals
4.2. Exercise Training
4.3. Resting Blood Pressure and Heart Rate
4.4. Masson’s Trichrome Staining
4.5. Western Immunoblotting
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Udani, S.; Lazich, I.; Bakris, G.L. Epidemiology of hypertensive kidney disease. Nat. Rev. Nephrol. 2011, 7, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Qiu, Y.; Wang, C.; Bakris, G. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999–2004. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2008, 51, S30–S37. [Google Scholar] [CrossRef] [PubMed]
- Barri, Y.M. Hypertension and kidney disease: A deadly connection. Curr. Hypertens. Rep. 2008, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Krzesinski, J.M.; Cohen, E.P. Hypertension and the kidney. Acta Clin. Belg. 2007, 62, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. Inflammation in end-stage renal disease: The hidden enemy. Nephrology 2006, 11, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Ryan, M.J. Immune and inflammatory role in renal disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [PubMed]
- Zhang, W.; Wang, W.; Yu, H.; Zhang, Y.; Dai, Y.; Ning, C.; Tao, L.; Sun, H.; Kellems, R.E.; Blackburn, M.R.; et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 2012, 59, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.D.; Yang, X.P.; Liu, Y.H.; Shesely, E.G.; Cavasin, M.A.; Kuziel, W.A.; Pagano, P.J.; Carretero, O.A. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension 2008, 52, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, E.A.; Zhou, M.S.; Pearse, D.D.; Puzis, L.; Raij, L. Upregulation of cortical COX-2 in salt-sensitive hypertension: Role of angiotensin II and reactive oxygen species. Am. J. Physiol. Ren. Physiol. 2008, 294, F385–F392. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Yuan, L.; Cao, Y.J.; Fan, Y.P.; Chen, X.L.; Huang, X.Z. Resveratrol ameliorates renal injury in spontaneously hypertensive rats by inhibiting renal micro-inflammation. Biosci. Rep. 2016, 36. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, S.A.; Ruiz-Ortega, M.; Egido, J. Angiotensin II and renal fibrosis. Hypertension 2001, 38, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumar, S.; Kundu, S.; Pryor, T.; Givvimani, S.; Lederer, E.; Tyagi, S.C.; Sen, U. Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. J. Hypertens. 2013, 31, 2270–2281, discussion 2281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, S.S.; Chen, Y.; Ahokas, R.A.; Sun, Y. Kidney fibrosis in hypertensive rats: Role of oxidative stress. Am. J. Nephrol. 2008, 28, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, A.; Kim, S.; Ohta, K.; Yagi, K.; Yukimura, T.; Miura, K.; Fukuda, T.; Iwao, H. Transforming growth factor-beta 1 expression and phenotypic modulation in the kidney of hypertensive rats. Hypertension 1995, 26, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int. J. Biol. Sci. 2011, 7, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.; Sebekova, K.; Ostendorf, T.; Floege, J. Treatment targets in renal fibrosis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial Transpl. Assoc. Eur. Ren. Assoc. 2007, 22, 3391–3407. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kim, H.; Liu, X.; Sugiura, H.; Kohyama, T.; Fang, Q.; Wen, F.Q.; Abe, S.; Wang, X.; Atkinson, J.J.; et al. Matrix metalloproteinase-9 activates TGF-beta and stimulates fibroblast contraction of collagen gels. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L1006–L1015. [Google Scholar] [CrossRef] [PubMed]
- Bolbrinker, J.; Markovic, S.; Wehland, M.; Melenhorst, W.B.; van Goor, H.; Kreutz, R. Expression and response to angiotensin-converting enzyme inhibition of matrix metalloproteinases 2 and 9 in renal glomerular damage in young transgenic rats with renin-dependent hypertension. J. Pharmacol. Exp. Ther. 2006, 316, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lelongt, B.; Legallicier, B.; Piedagnel, R.; Ronco, P.M. Do matrix metalloproteinases MMP-2 and MMP-9 (gelatinases) play a role in renal development, physiology and glomerular diseases? Curr. Opin. Nephrol. Hypertens. 2001, 10, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.; Collinson, D.J.; Manning, G. Hypertension, matrix metalloproteinases and target organ damage. J. Hypertens. 2003, 21, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Camp, T.M.; Smiley, L.M.; Hayden, M.R.; Tyagi, S.C. Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. J. Hypertens. 2003, 21, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chen, K.C.; Hsieh, C.L.; Peng, R.Y. Swimming exercise prevents fibrogenesis in chronic kidney disease by inhibiting the myofibroblast transdifferentiation. PLoS ONE 2012, 7, e37388. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.; Celec, P.; Behuliak, M.; Grancic, P.; Kebis, A.; Kukan, M.; Pronayova, N.; Liptaj, T.; Ostendorf, T.; Sebekova, K. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metab. Clin. Exp. 2009, 58, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Kurdak, H.; Sandikci, S.; Ergen, N.; Dogan, A.; Kurdak, S.S. The effects of regular aerobic exercise on renal functions in streptozotocin induced diabetic rats. J. Sport. Sci. Med. 2010, 9, 294–299. [Google Scholar]
- Garcia-Pinto, A.B.; de Matos, V.S.; Rocha, V.; Moraes-Teixeira, J.; Carvalho, J.J. Low-Intensity physical activity beneficially alters the ultrastructural renal morphology of spontaneously hypertensive rats. Clinics 2011, 66, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Entman, M.L.; Wang, Y. Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension 2013, 62, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Elks, C.M.; Reed, S.D.; Mariappan, N.; Majid, D.S.; Francis, J. Chronic exercise preserves renal structure and hemodynamics in spontaneously hypertensive rats. Antioxid. Redox Signal 2012, 16, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Liu, S.; Zhao, Z.; Liu, Y.; Li, T. The effect of connective tissue growth factor on renal fibrosis and podocyte injury in hypertensive rats. Ren. Fail. 2014, 36, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- De las Heras, N.; Ruiz-Ortega, M.; Ruperez, M.; Sanz-Rosa, D.; Miana, M.; Aragoncillo, P.; Mezzano, S.; Lahera, V.; Egido, J.; Cachofeiro, V. Role of connective tissue growth factor in vascular and renal damage associated with hypertension in rats. Interactions with angiotensin II. J. Renin Angiotensin Aldosterone Syst. 2006, 7, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, M. Inhibitors/antagonists of TGF-beta system in kidney fibrosis. Nephrol. Dial. Transplant. 2012, 27, 3686–3691. [Google Scholar] [CrossRef] [PubMed]
- Egido, J. Vasoactive hormones and renal sclerosis. Kidney Int. 1996, 49, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.P.; Schnackenberg, C.G. Renal mechanisms of angiotensin II-induced hypertension. Semin. Nephrol. 2000, 20, 417–425. [Google Scholar] [PubMed]
- Kopp, J.B.; Factor, V.M.; Mozes, M.; Nagy, P.; Sanderson, N.; Bottinger, E.P.; Klotman, P.E.; Thorgeirsson, S.S. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab. Investig. J. Tech. Methods Pathol. 1996, 74, 991–1003. [Google Scholar]
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging Dis. 2011, 2, 158–173. [Google Scholar] [PubMed]
- Frazier, K.; Williams, S.; Kothapalli, D.; Klapper, H.; Grotendorst, G.R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J. Investig. Dermatol. 1996, 107, 404–411. [Google Scholar] [CrossRef] [PubMed]
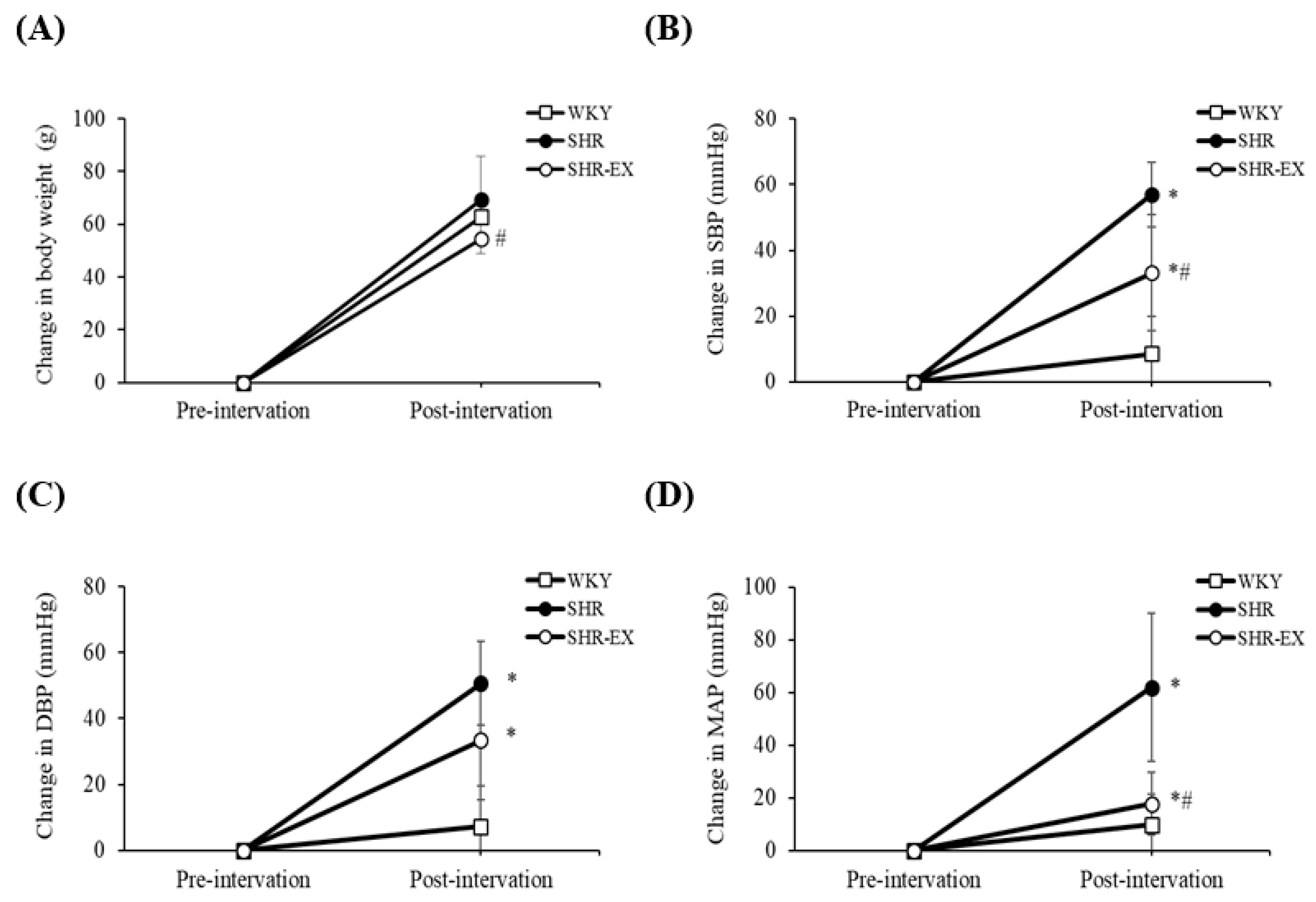



| Parameters/Groups | WKY | SHR | SHR-EX |
| Number of animals | 8 | 8 | 8 |
| Kidney Weight Index | |||
| WKW (g) | 1.28 ± 0.08 | 1.30 ± 0.12 | 1.41 ± 0.12 |
| WKW (g)/TL (mm) | 0.033 ± 0.002 | 0.036 ± 0.003 | 0.032 ± 0.014 |
| Blood pressure | |||
| initial SBP (mmHg) | 123 ± 11 | 146 ± 8 ** | 150 ± 11 ** |
| initial DBP (mmHg) | 89 ± 9 | 105 ± 15 * | 114 ± 15 ** |
| initial MAP (mmHg) | 100 ± 9 | 119 ± 12 ** | 126 ± 12 ** |
| final SBP (mmHg) | 134 ± 4 | 203 ± 8 ** | 183 ± 12 **## |
| final DBP (mmHg) | 98 ± 7 | 157 ± 8 ** | 148 ± 13 ** |
| final MAP (mmHg) | 110 ± 5 | 172 ± 7 ** | 159 ± 13 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Lin, Y.-Y.; Yang, A.-L.; Kuo, T.-W.; Kuo, C.-H.; Lee, S.-D. Anti-Renal Fibrotic Effect of Exercise Training in Hypertension. Int. J. Mol. Sci. 2018, 19, 613. https://doi.org/10.3390/ijms19020613
Huang C-C, Lin Y-Y, Yang A-L, Kuo T-W, Kuo C-H, Lee S-D. Anti-Renal Fibrotic Effect of Exercise Training in Hypertension. International Journal of Molecular Sciences. 2018; 19(2):613. https://doi.org/10.3390/ijms19020613
Chicago/Turabian StyleHuang, Chiu-Ching, Yi-Yuan Lin, Ai-Lun Yang, Tang-Wei Kuo, Chia-Hua Kuo, and Shin-Da Lee. 2018. "Anti-Renal Fibrotic Effect of Exercise Training in Hypertension" International Journal of Molecular Sciences 19, no. 2: 613. https://doi.org/10.3390/ijms19020613