Mathematical Modelling of Nitric Oxide/Cyclic GMP/Cyclic AMP Signalling in Platelets
Abstract
:1. Introduction
2. Results
2.1. The Modelling Approach and Aims
2.2. A Steady State Model of the NO-cGMP-PKG Pathway
2.3. Effects of the Moderate PDE5-Inhibitor Dipyridamole on cGMP Levels
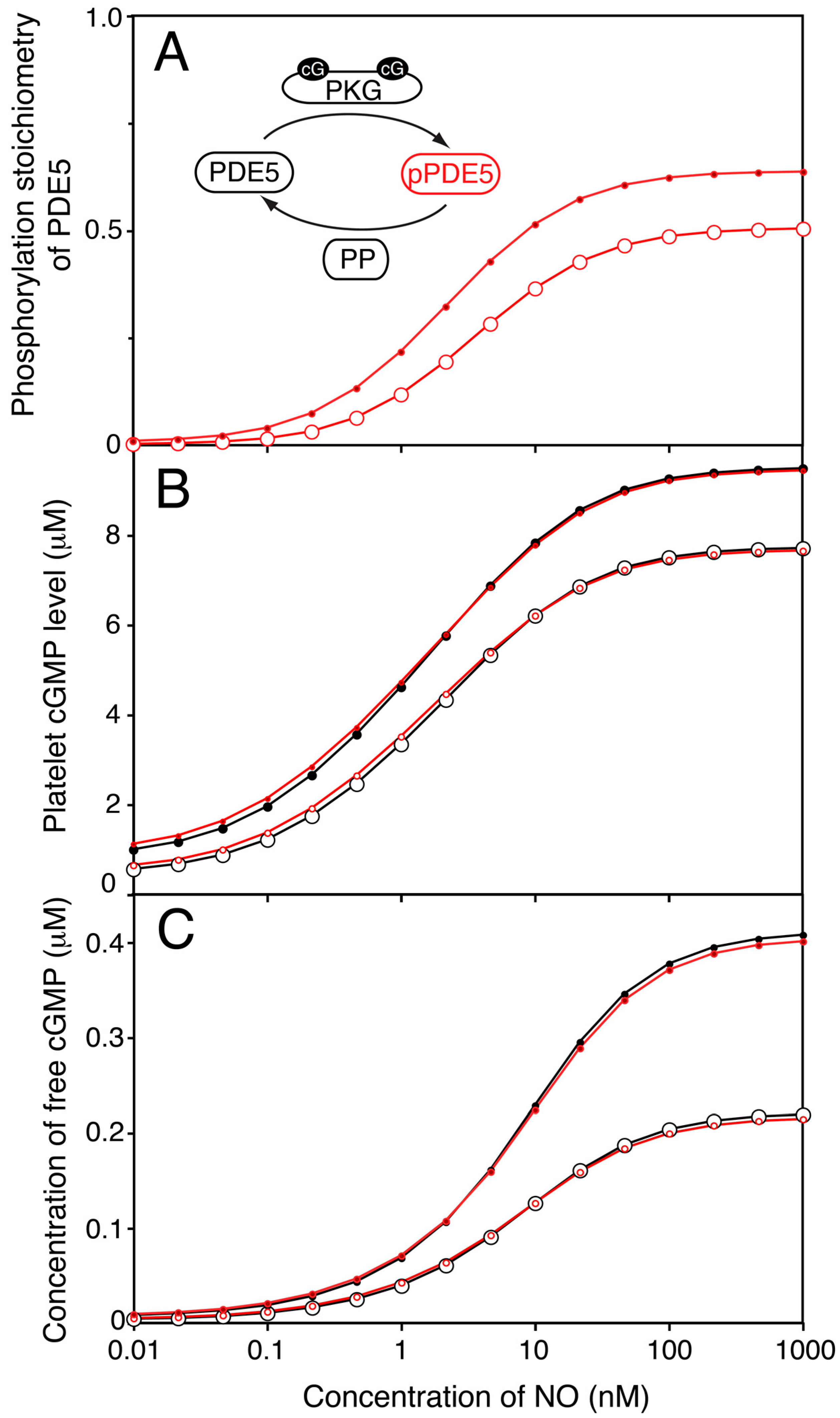
2.4. PKG-Mediated PDE5 Phosphorylation Has Little Influence on the Steady State NO-cGMP Response Curve
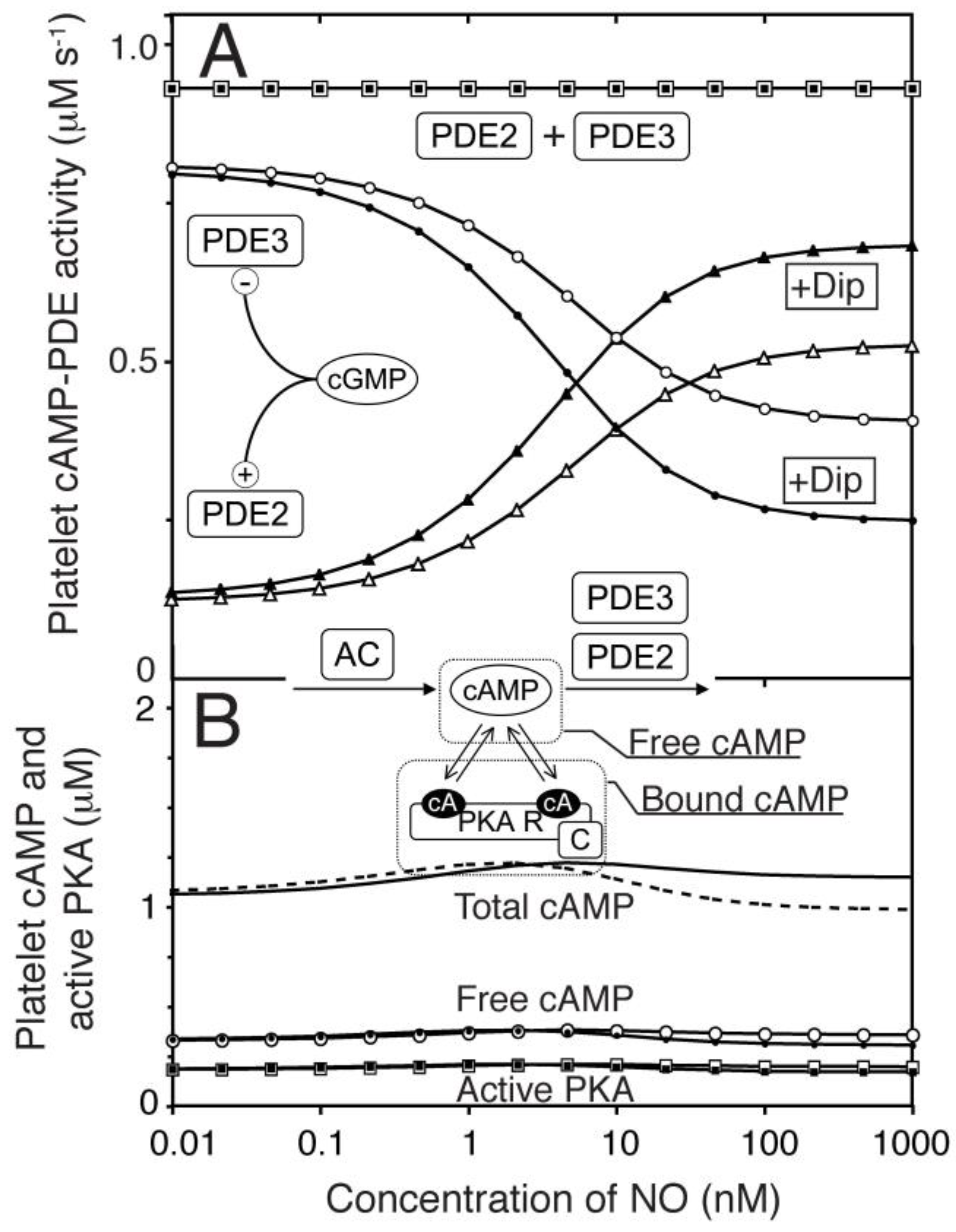
2.5. Modelling the Influence of cGMP on Platelet cAMP Levels through PDE3
2.6. Modelling Global Platelet cGMP to cAMP Cross-Talk
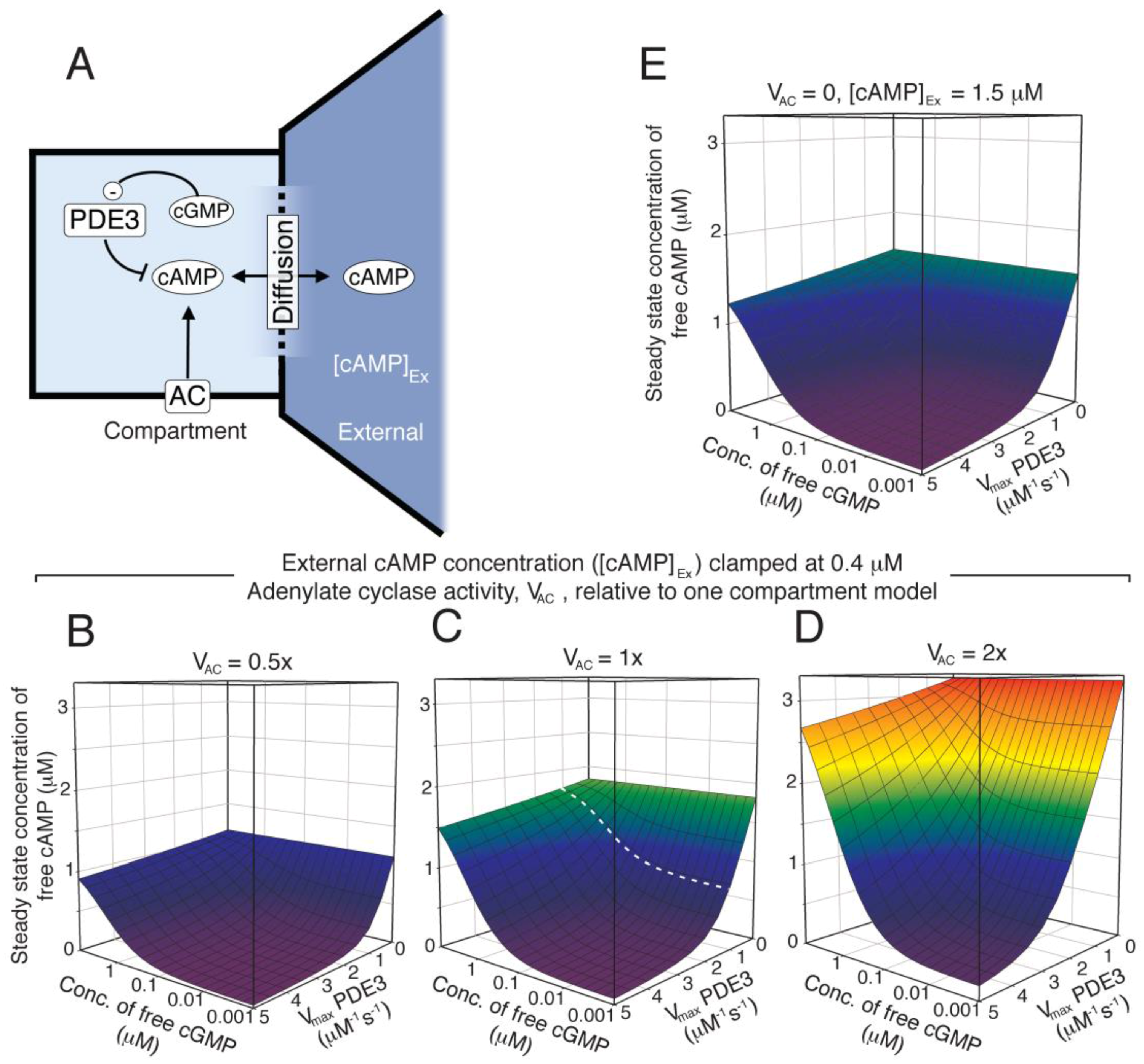
2.7. Two-Compartment Modelling of cGMP/cAMP Crosstalk through PDE3
2.8. A Modest Redistribution of Platelet PDEs Suffice to Generate NO Mediated PKA Activation
3. Discussion
4. Materials and Methods
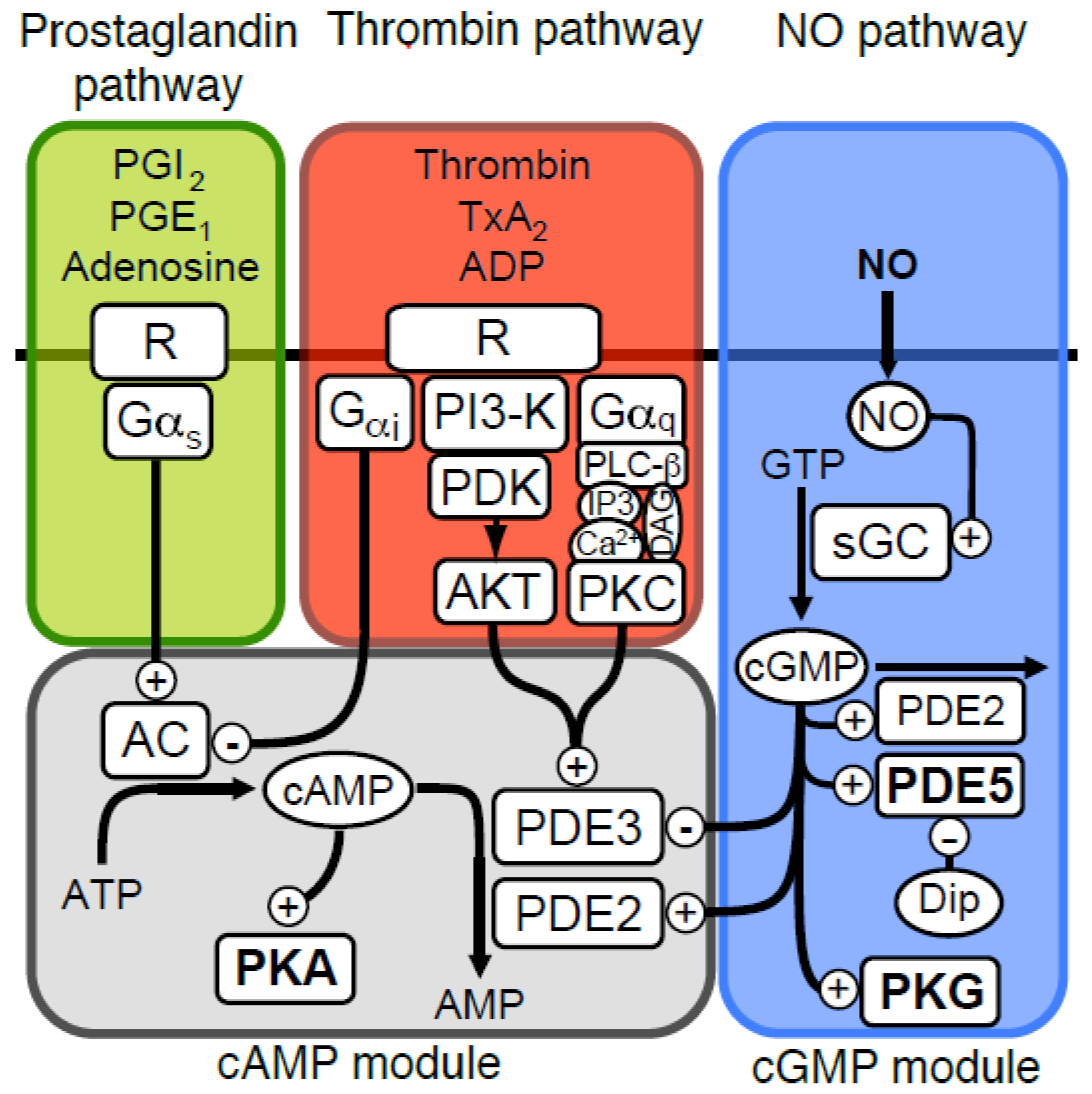
4.1. Signalling Pathway and Literature Quantitative Information
4.2. Modelling Approach
4.3. Parameter Estimation
4.4. Simulations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwarz, U.R.; Walter, U.; Eigenthaler, M. Taming platelets with cyclic nucleotides. Biochem. Pharmacol. 2001, 62, 1153–1161. [Google Scholar] [CrossRef]
- Pepine, C.J. The impact of nitric oxide in cardiovascular medicine: Untapped potential utility. Am. J. Med. 2009, 122, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Smolenski, A. Novel roles of cAMP/cGMP-dependent signalling in platelets. J. Thromb. Haemost. 2012, 10, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Mullershausen, F.; Russwurm, M.; Thompson, W.J.; Liu, L.; Koesling, D.; Friebe, A. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J. Cell Biol. 2001, 155, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Gopal, V.K.; Francis, S.H.; Corbin, J.D. Allosteric sites of phosphodiesterase-5 (PDE5). A potential role in negative feedback regulation of cGMP signalling in corpus cavernosum. Eur. J. Biochem. 2001, 268, 3304–3312. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Francis, S.H.; Corbin, J.D. Characterization of a purified bovine lung cGMP-binding cGMP phosphodiesterase. J. Biol. Chem. 1990, 265, 14964–14970. [Google Scholar] [PubMed]
- Corbin, J.D.; Turko, I.V.; Beasley, A.; Francis, S.H. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur. J. Biochem. 2000, 267, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.O.; Selheim, F.; Doskeland, S.O.; Gear, A.R.; Holmsen, H. Protein kinase A mediates inhibition of the thrombin-induced platelet shape change by nitric oxide. Blood 2004, 104, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Movsesian, M.A. cAMP and cGMP Signalling Cross-Talk: Role of Phosphodiesterases and Implications for Cardiac Pathophysiology. Circ. Res. 2007, 100, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Worner, R.; Lukowski, R.; Hofmann, F.; Wegener, J.W. cGMP signals mainly through cAMP kinase in permeabilized murine aorta. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H237–H244. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zeng, M.; Curry, F.E. Dominant role of cAMP in regulation of microvessel permeability. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1124–H1133. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Ren, J.; Zhang, J.E.; Zhong, W.; Luo, M. Natriuretic peptides block synaptic transmission by activating phosphodiesterase 2A and reducing presynaptic PKA activity. Proc. Natl. Acad. Sci. USA 2012, 109, 17681–17686. [Google Scholar] [CrossRef] [PubMed]
- Dunkern, T.R.; Hatzelmann, A. The effect of Sildenafil on human platelet secretory function is controlled by a complex interplay between phosphodiesterases 2, 3 and 5. Cell Signal. 2005, 17, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wangorsch, G.; Butt, E.; Mark, R.; Hubertus, K.; Geiger, J.; Dandekar, T.; Dittrich, M. Time-resolved in silico modelling of fine-tuned cAMP signalling in platelets: Feedback loops, titrated phosphorylations and pharmacological modulation. BMC Syst. Biol. 2011, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.O.; Kleppe, R.; Kopperud, R.; Nygaard, G.; Doskeland, S.O.; Holmsen, H.; Selheim, F. Dipyridamole synergizes with nitric oxide to prolong inhibition of thrombin-induced platelet shape change. Platelets 2011, 22, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Halbrugge, M.; Friedrich, C.; Eigenthaler, M.; Schanzenbacher, P.; Walter, U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP- elevating vasodilators. J. Biol. Chem. 1990, 265, 3088–3093. [Google Scholar] [PubMed]
- Group, E.S.; Halkes, P.; van Gijn, J.; Kappelle, L.; Koudstaal, P.; Algra, A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischemia of arterial origin (ESPRIT): Randomised controlled trial. Lancet 2006, 20, 1665–1673. [Google Scholar]
- Mischnik, M.; Hubertus, K.; Geiger, J.; Dandekar, T.; Timmer, J. Dynamical modelling of prostaglandin signalling in platelets reveals individual receptor contributions and feedback properties. Mol. Biosyst. 2013, 9, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Purvis, J.E.; Chatterjee, M.S.; Brass, L.F.; Diamond, S.L. A molecular signalling model of platelet phosphoinositide and calcium regulation during homeostasis and P2Y1 activation. Blood 2008, 112, 4069–4079. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.S.; Elbatarny, H.S.; Crawley, S.W.; Bennett, B.M.; Maurice, D.H. Compartmentation and compartment-specific regulation of PDE5 by protein kinase G allows selective cGMP-mediated regulation of platelet functions. Proc. Natl. Acad. Sci. USA 2008, 105, 13650–13655. [Google Scholar] [CrossRef] [PubMed]
- Mo, E.; Amin, H.; Bianco, I.H.; Garthwaite, J. Kinetics of a cellular nitric oxide/cGMP/phosphodiesterase-5 pathway. J. Biol. Chem. 2004, 279, 26149–26158. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Halvey, E.J.; Garthwaite, J. An enzyme-linked receptor mechanism for nitric oxide-activated guanylyl cyclase. J. Biol. Chem. 2008, 283, 18841–18851. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Garthwaite, J. Nitric oxide activation of guanylyl cyclase in cells revisited. Proc. Natl. Acad. Sci. USA 2006, 103, 12185–12190. [Google Scholar] [CrossRef] [PubMed]
- Emmons, T.L.; Mathis, K.J.; Shuck, M.E.; Reitz, B.A.; Curran, D.F.; Walker, M.C.; Leone, J.W.; Day, J.E.; Bienkowski, M.J.; Fischer, H.D.; et al. Purification and characterization of recombinant human soluble guanylate cyclase produced from baculovirus-infected insect cells. Protein Expr. Purif. 2009, 65, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.G.; Beavo, J.A. Inhibition and stimulation of photoreceptor phosphodiesterases by dipyridamole and M&B 22,948. Mol. Pharmacol. 1989, 36, 773–781. [Google Scholar] [PubMed]
- Wada, H.; Osborne, J.C., Jr.; Manganiello, V.C. Effects of temperature on allosteric and catalytic properties of the cGMP-stimulated cyclic nucleotide phosphodiesterase from calf liver. J. Biol. Chem. 1987, 262, 5139–5144. [Google Scholar] [PubMed]
- Grant, P.G.; Mannarino, A.F.; Colman, R.W. Purification and characterization of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from the cytosol of human platelets. Thromb. Res. 1990, 59, 105–119. [Google Scholar] [CrossRef]
- Moss, J.; Manganiello, V.C.; Vaughan, M. Substrate and effector specificity of a guanosine 3′:5′-monophosphate phosphodiesterase from rat liver. J. Biol. Chem. 1977, 252, 5211–5215. [Google Scholar] [PubMed]
- Harrison, S.A.; Reifsnyder, D.H.; Gallis, B.; Cadd, G.G.; Beavo, J.A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: A receptor for new cardiotonic drugs. Mol. Pharmacol. 1986, 29, 506–514. [Google Scholar] [PubMed]
- Kotera, J.; Grimes, K.A.; Corbin, J.D.; Francis, S.H. cGMP-dependent protein kinase protects cGMP from hydrolysis by phosphodiesterase-5. Biochem. J. 2003, 372, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Dao, K.K.; Teigen, K.; Kopperud, R.; Hodneland, E.; Schwede, F.; Christensen, A.E.; Martinez, A.; Doskeland, S.O. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J. Biol. Chem. 2006, 281, 21500–21511. [Google Scholar] [CrossRef] [PubMed]
- Viste, K.; Kopperud, R.K.; Christensen, A.E.; Doskeland, S.O. Substrate enhances the sensitivity of type I protein kinase a to cAMP. J. Biol. Chem. 2005, 280, 13279–13284. [Google Scholar] [CrossRef] [PubMed]
- Eigenthaler, M.; Nolte, C.; Halbrugge, M.; Walter, U. Concentration and regulation of cyclic nucleotides, cyclic-nucleotide-dependent protein kinases and one of their major substrates in human platelets. Estimating the rate of cAMP-regulated and cGMP-regulated protein phosphorylation in intact cells. Eur. J. Biochem. 1992, 205, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Halvey, E.J.; Vernon, J.; Roy, B.; Garthwaite, J. Mechanisms of activity-dependent plasticity in cellular nitric oxide-cGMP signalling. J. Biol. Chem. 2009, 284, 25630–25641. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Mullershausen, F.; Friebe, A.; Feil, R.; Thompson, W.J.; Hofmann, F.; Koesling, D. Direct activation of PDE5 by cGMP: Long-term effects within NO/cGMP signalling. J. Cell Biol. 2003, 160, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Colman, R. Platelet cAMP and cGMP phosphodiesterases. Platelets 1995, 6, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ashby, B. Model of prostaglandin-regulated cyclic AMP metabolism in intact platelets: Examination of time-dependent effects on adenylate cyclase and phosphodiesterase activities. Mol. Pharmacol. 1989, 36, 866–873. [Google Scholar] [PubMed]
- De Oliveira, S.K.; Hoffmeister, M.; Gambaryan, S.; Muller-Esterl, W.; Guimaraes, J.A.; Smolenski, A.P. Phosphodiesterase 2A forms a complex with the co-chaperone XAP2 and regulates nuclear translocation of the aryl hydrocarbon receptor. J. Biol. Chem. 2007, 282, 13656–13663. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, R.; Krall, J.; Tikishvili, E.; Honeggar, M.; Ahmad, F.; Manganiello, V.C.; Movsesian, M.A. Isoforms of cyclic nucleotide phosphodiesterase PDE3 and their contribution to cAMP hydrolytic activity in subcellular fractions of human myocardium. J. Biol. Chem. 2005, 280, 39168–39174. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, E.; Uhart, M.; Li, C.C.; Ahmad, F.; Pacheco-Rodriguez, G.; Manganiello, V.C.; Moss, J.; Vaughan, M. Interaction of phosphodiesterase 3A with brefeldin A-inhibited guanine nucleotide-exchange proteins BIG1 and BIG2 and effect on ARF1 activity. Proc. Natl. Acad. Sci. USA 2009, 106, 6158–6163. [Google Scholar] [CrossRef] [PubMed]
- Russwurm, C.; Zoidl, G.; Koesling, D.; Russwurm, M. Dual acylation of PDE2A splice variant 3: Targeting to synaptic membranes. J. Biol. Chem. 2009, 284, 25782–25790. [Google Scholar] [CrossRef] [PubMed]
- Shakur, Y.; Takeda, K.; Kenan, Y.; Yu, Z.X.; Rena, G.; Brandt, D.; Houslay, M.D.; Degerman, E.; Ferrans, V.J.; Manganiello, V.C. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). Two N-terminal domains are required for the efficient targeting to and association of, PDE3 with endoplasmic reticulum. J. Biol. Chem. 2000, 275, 38749–38761. [Google Scholar] [CrossRef] [PubMed]
- Cutler, L.S.; Christian, C.P.; Feinstein, M.B. Cytochemical localization of adenylate cyclase in the dense tubule system of human blood platelets stimulated by forskolin, prostacyclin and prostaglandin D2. Biochim. Biophys. Acta 1985, 845, 403–410. [Google Scholar] [CrossRef]
- Lambrechts, A.; Kwiatkowski, A.V.; Lanier, L.M.; Bear, J.E.; Vandekerckhove, J.; Ampe, C.; Gertler, F.B. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J. Biol. Chem. 2000, 275, 36143–36151. [Google Scholar] [CrossRef] [PubMed]
- Mongillo, M.; Tocchetti, C.G.; Terrin, A.; Lissandron, V.; Cheung, Y.F.; Dostmann, W.R.; Pozzan, T.; Kass, D.A.; Paolocci, N.; Houslay, M.D.; et al. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ. Res. 2006, 98, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Qvigstad, E.; Moltzau, L.R.; Aronsen, J.M.; Nguyen, C.H.; Hougen, K.; Sjaastad, I.; Levy, F.O.; Skomedal, T.; Osnes, J.B. Natriuretic peptides increase beta-1-adrenoceptor signalling in failing hearts through phosphodiesterase 3 inhibition. Cardiovasc. Res. 2010, 85, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Gruner, S.; Konrad, I.; Garcia Arguinzonis, M.I.; Eigenthaler, M.; Hemler, K.; Kersting, J.; Schulz, C.; Muller, I.; Besta, F.; et al. Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood 2004, 103, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Aszodi, A.; Pfeifer, A.; Ahmad, M.; Glauner, M.; Zhou, X.H.; Ny, L.; Andersson, K.E.; Kehrel, B.; Offermanns, S.; Fassler, R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation but is dispensable for smooth muscle function. EMBO J. 1999, 18, 37–48. [Google Scholar] [CrossRef] [PubMed]
- White, J.G. Platelets; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 45–73. [Google Scholar]
- Havrylenko, S.; Noguera, P.; Abou-Ghali, M.; Manzi, J.; Faqir, F.; Lamora, A.; Guerin, C.; Blanchoin, L.; Plastino, J. WAVE binds Ena/VASP for enhanced Arp2/3 complex-based actin assembly. Mol. Biol. Cell 2015, 26, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bluthgen, N.; Bruggeman, F.J.; Legewie, S.; Herzel, H.; Westerhoff, H.V.; Kholodenko, B.N. Effects of sequestration on signal transduction cascades. FEBS J. 2006, 273, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Tasken, K.; Aandahl, E.M. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 2004, 84, 137–167. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.R.; Verde, I.; Cooper, D.M.; Fischmeister, R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 2006, 113, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Haslam, R.J.; Dickinson, N.T.; Jang, E.K. Cyclic nucleotides and phosphodiesterases in platelets. Thromb. Haemost. 1999, 82, 412–423. [Google Scholar] [PubMed]
- Elbatarny, H.S.; Maurice, D.H. Leptin-mediated activation of human platelets: Involvement of a leptin receptor and phosphodiesterase 3A-containing cellular signalling complex. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E695–E702. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.; Laufs, U.; Huang, Z.; Nakamura, T.; Huang, P.; Moskowitz, M.A.; Liao, J.K. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1998, 95, 8880–8885. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Sawada, N.; Soydan, G.; Lee, H.S.; Zhou, Z.; Hwang, S.K.; Waeber, C.; Moskowitz, M.A.; Liao, J.K. Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J. Cereb. Blood Flow Metab. 2008, 28, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Greenstein, J.L.; Winslow, R.L. Interaction between phosphodiesterases in the regulation of the cardiac beta-adrenergic pathway. J. Mol. Cell. Cardiol. 2015, 88, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Doskeland, S.O.; Vintermyr, O.K.; Corbin, J.D.; Ogreid, D. Studies on the interactions between the cyclic nucleotide-binding sites of cGMP-dependent protein kinase. J. Biol. Chem. 1987, 262, 3534–3540. [Google Scholar] [PubMed]
- Rannels, S.R.; Cobb, C.E.; Landiss, L.R.; Corbin, J.D. The regulatory subunit monomer of cAMP-dependent protein kinase retains the salient kinetic properties of the native dimeric subunit. J. Biol. Chem. 1985, 260, 3423–3430. [Google Scholar] [PubMed]
- Iancu, R.V.; Jones, S.W.; Harvey, R.D. Compartmentation of cAMP signalling in cardiac myocytes: A computational study. Biophys. J. 2007, 92, 3317–3331. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.C.; Fagan, K.A.; Nakata, H.; Schaack, J.; Cooper, D.M.; Karpen, J.W. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J. Gen. Physiol. 2000, 116, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, U.S. Signalling in small subcellular volumes. I. Stochastic and diffusion effects on individual pathways. Biophys. J. 2004, 87, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, U.S. Signalling in small subcellular volumes. II. Stochastic and diffusion effects on synaptic network properties. Biophys. J. 2004, 87, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Nishikawa, M.; Fujioka, M.; Miyahara, M.; Isaka, N.; Shiku, H.; Nakano, T. Characterization of the isoenzymes of cyclic nucleotide phosphodiesterase in human platelets and the effects of E4021. Cell Signal. 1996, 8, 575–581. [Google Scholar] [CrossRef]
- Hoops, S.; Sahle, S.; Gauges, R.; Lee, C.; Pahle, J.; Simus, N.; Singhal, M.; Xu, L.; Mendes, P.; Kummer, U. COPASI—A COmplex PAthway SImulator. Bioinformatics 2006, 22, 3067–3074. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.; Hoops, S.; Sahle, S.; Gauges, R.; Dada, J.; Kummer, U. Computational modelling of biochemical networks using COPASI. Methods Mol. Biol. 2009, 500, 17–59. [Google Scholar] [PubMed]


| Enzymes | Reactions | Parameters | Ref. |
|---|---|---|---|
| sGC | sGC + NO ⟺ sGC:NO | kf = 300 µM−1 s−1, kb = 14 s−1 K1 = kb/kf = 46.7 nM | [23] |
| sGC:NO ⟺ sGC* 1 | kf = 1000 s−1, kb = 280 s−1 K2 = kb/kf = 0.28 | [23] | |
| GTP ⟶ cG 3 | 2 = 0.05, = 100 µM | [4,24] | |
| sGC* | GTP ⟶ cG | = 20, = 50 µM | [4] |
| PDE5/pPDE5 4 | PDE5* ⟺ PDE5 + cG | = 130 nM | [7] |
| pPDE5* ⟺ pPDE5 + cG | = 30 nM | [7] | |
| cG ⟶ GMP | = 39.0, = 4.60 µM, = 0.70 µM | [5,6,25] | |
| PDE5*/pPDE5* | cG ⟶ GMP | = 117, = 1.00 µM, = 0.70 µM | |
| PDE2 | PDE2* ⟺ PDE2 + cG | = 2.00 µM | [26] |
| PDE2* ⟺ PDE2 + cA | = 25.0 µM | [26] | |
| cG ⟶ GMP | = 24.0, = 120 µM | [27,28] | |
| PDE2* | cG ⟶ GMP | = 240, = 15.0 µM | [27,28] |
| PDE2 | cA 5 ⟶ AMP | = 24.0, = 120 µM, = 120 µM | [27,28] |
| PDE2* | cA ⟶ AMP | = 240, = 20.0 µM, = 22.0 µM | [27,28] |
| PDE3/pPDE3 6 | cA ⟶ AMP | = 1.2, = 150 nM, = 60.0 nM | [29] |
| AC | ⟶ cA | 0.93 µM−1s−1 | |
| PKG | PKG(cG) ⟺ PKG + cG | KD = 55.0 nM | [30] |
| PKG(cG2) ⟺ PKG(cG) + cG | KD = 750 nM | [30] | |
| PKA | R(cA)C ⟺ RC + cA | KD = 2.90 µM | [31] |
| R(cA2)C ⟺ R(cA)C + cA | KD = 2.90 µM | [31] | |
| R(cA2)C ⟺ R(cA2) + C | KD = 1.00 µM | [32] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleppe, R.; Jonassen, I.; Døskeland, S.O.; Selheim, F. Mathematical Modelling of Nitric Oxide/Cyclic GMP/Cyclic AMP Signalling in Platelets. Int. J. Mol. Sci. 2018, 19, 612. https://doi.org/10.3390/ijms19020612
Kleppe R, Jonassen I, Døskeland SO, Selheim F. Mathematical Modelling of Nitric Oxide/Cyclic GMP/Cyclic AMP Signalling in Platelets. International Journal of Molecular Sciences. 2018; 19(2):612. https://doi.org/10.3390/ijms19020612
Chicago/Turabian StyleKleppe, Rune, Inge Jonassen, Stein Ove Døskeland, and Frode Selheim. 2018. "Mathematical Modelling of Nitric Oxide/Cyclic GMP/Cyclic AMP Signalling in Platelets" International Journal of Molecular Sciences 19, no. 2: 612. https://doi.org/10.3390/ijms19020612





