AWRK6, A Synthetic Cationic Peptide Derived from Antimicrobial Peptide Dybowskin-2CDYa, Inhibits Lipopolysaccharide-Induced Inflammatory Response
Abstract
:1. Introduction
2. Results
2.1. AWRK6 Inhibited the Binding of LPS with LBP in Vitro
2.2. AWRK6 Protects Mice from Endotoxemia
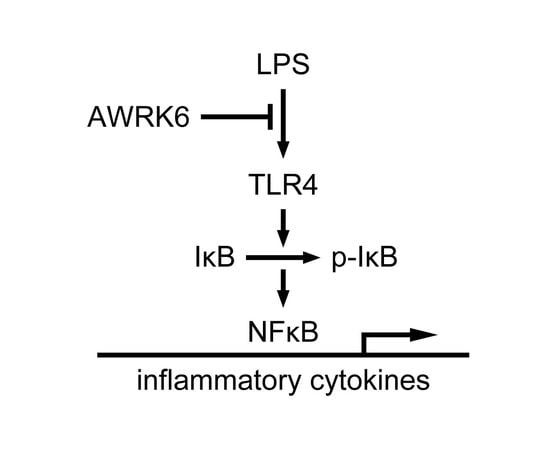
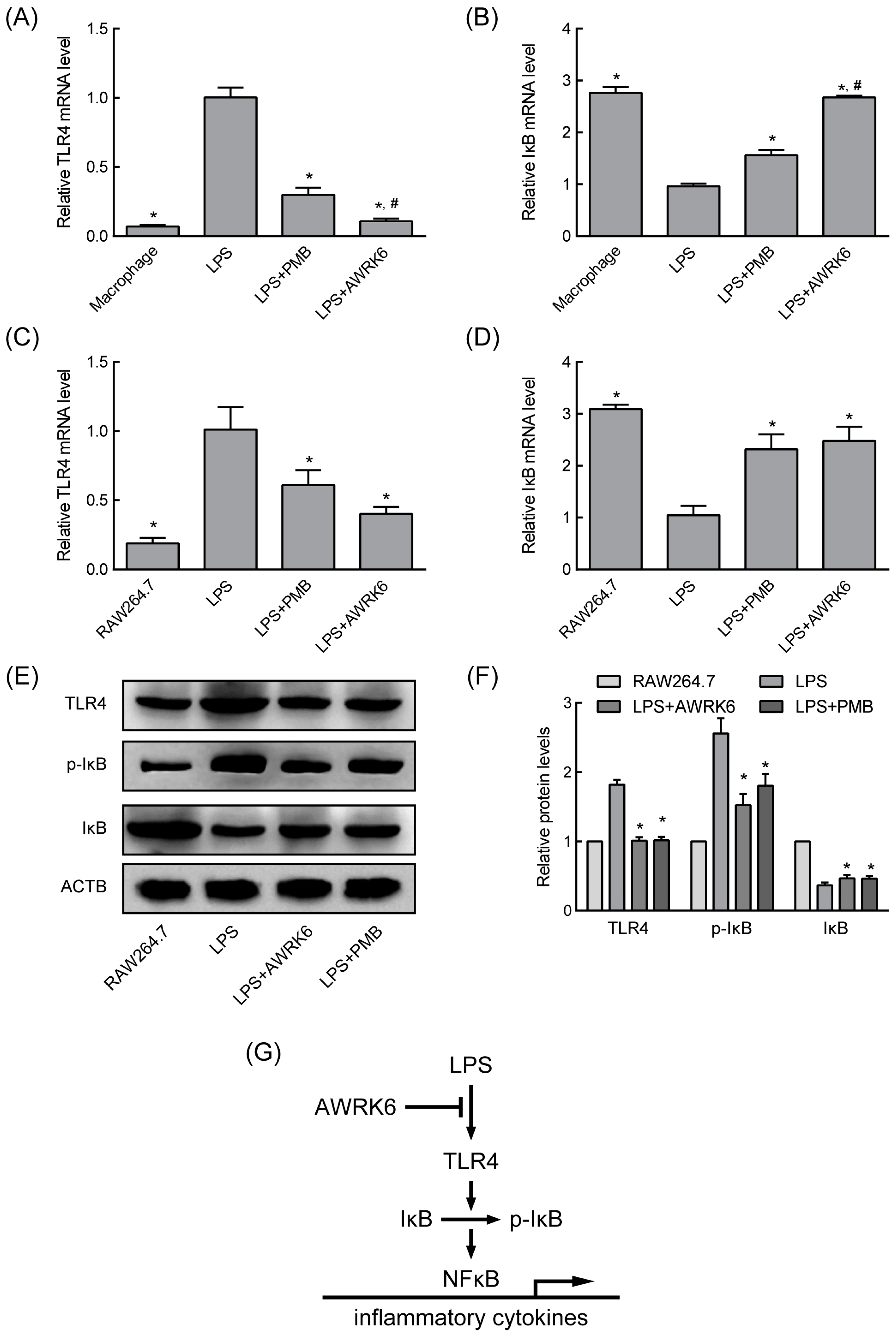
2.3. AWRK6 Modulated LPS-Activated Pro-Inflammatory Mediators
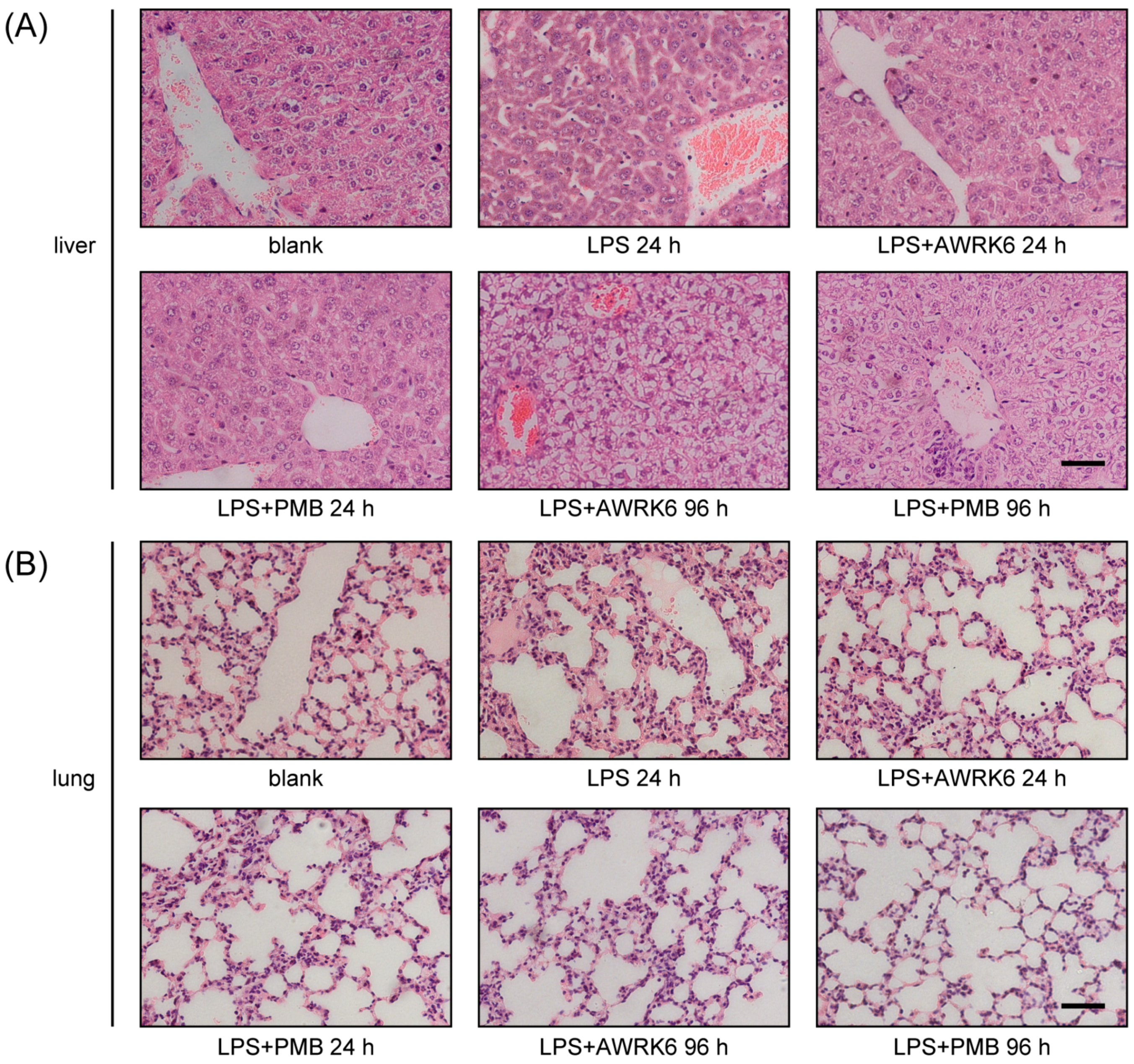
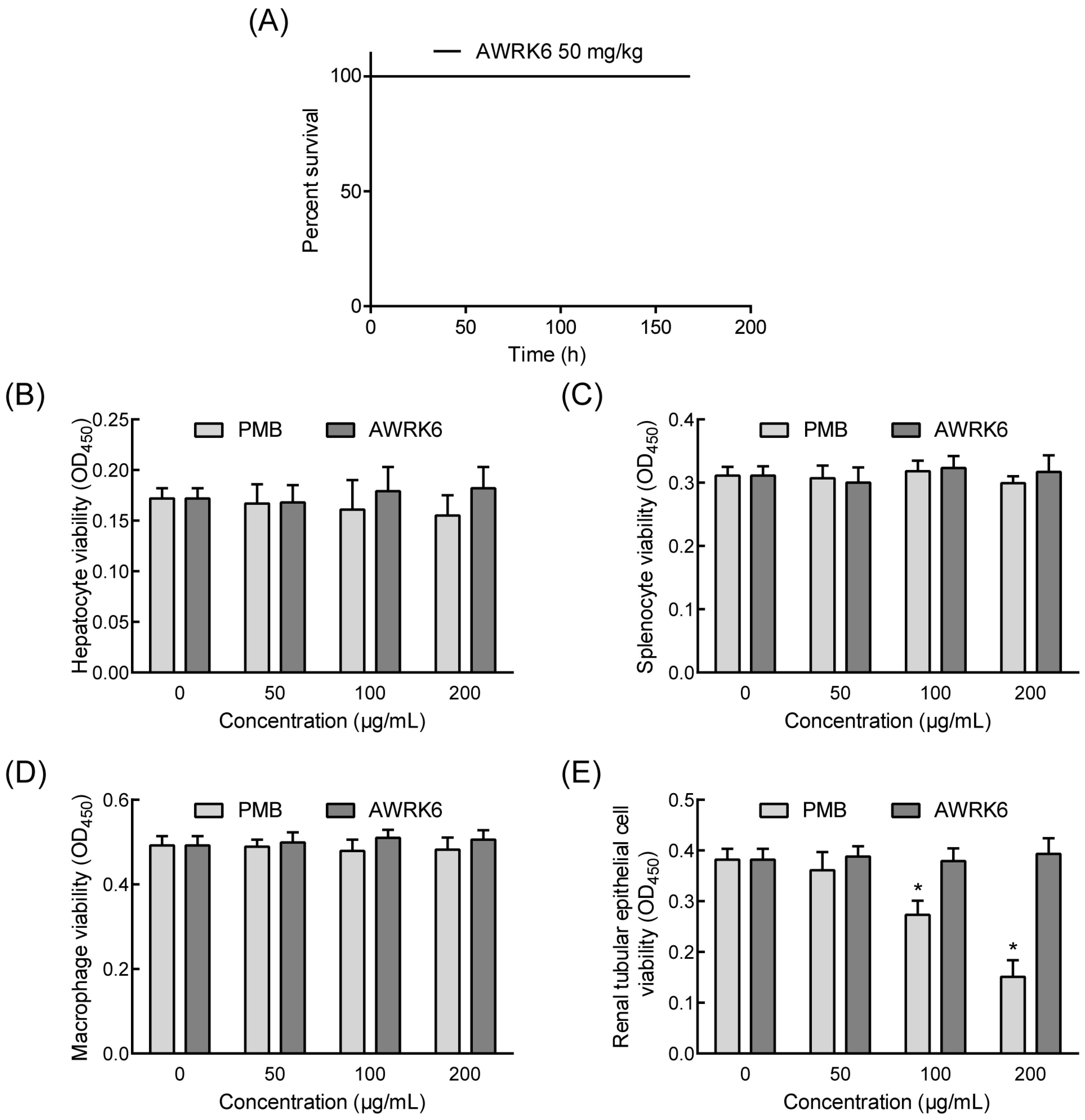
2.4. The Toxicity of AWRK6 in Vivo and in Vitro
3. Discussion
4. Materials and Methods
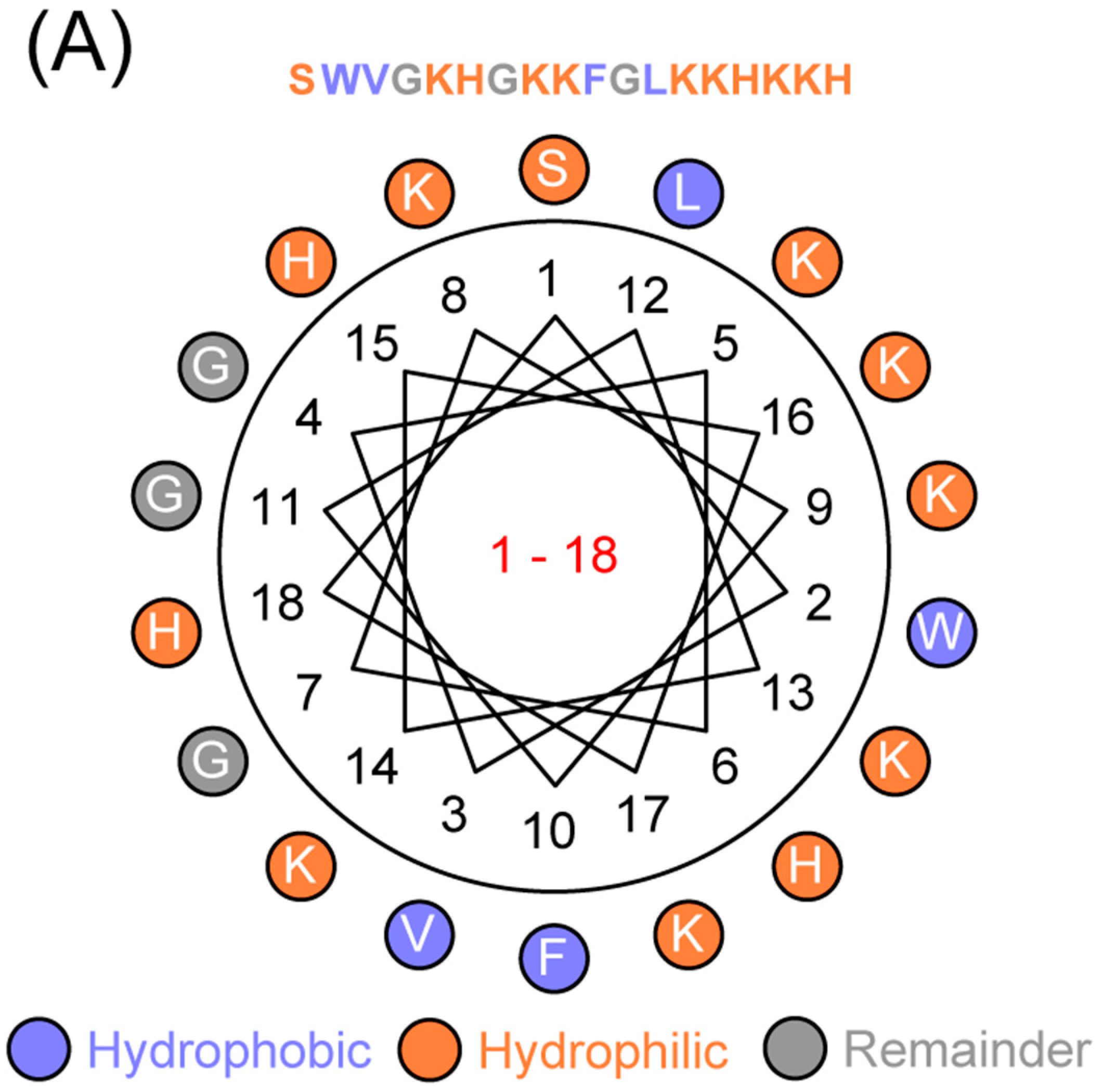
4.1. Sequence Analyses
4.2. Peptide Synthesis
4.3. ELISA
4.4. LAL Assay
4.5. Mouse Endotoxemia Models
4.6. Histopathological Examination
4.7. Cell Isolation and Culture
4.8. Quantitative Real-Time PCR
4.9. Western Blotting
4.10. Cytotoxicity Analysis
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bryant, C.E.; Spring, D.R.; Gangloff, M.; Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S. Endotoxemia-menace, marker, or mistake? J. Leukoc. Biol. 2016, 100, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Pro, C.I.; Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Falagas, M.E. Colistin versus polymyxin b for the treatment of patients with multidrug-resistant gram-negative infections: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 49, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Kramer, U.; et al. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–E1418. [Google Scholar] [CrossRef] [PubMed]
- Li, S.A.; Lee, W.H.; Zhang, Y. Efficacy of oh-cath30 and its analogs against drug-resistant bacteria in vitro and in mouse models. Antimicrob. Agents Chemother. 2012, 56, 3309–3317. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shang, D. Inhibitory effects of antimicrobial peptides on lipopolysaccharide-induced inflammation. Mediators Inflamm. 2015, 2015, 167572. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial cathelicidin peptide ll-37 inhibits the lps/atp-induced pyroptosis of macrophages by dual mechanism. PLoS ONE 2014, 9, e85765. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial cathelicidin peptide ll-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int. Immunol. 2016, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Schuerholz, T.; Brandenburg, K.; Marx, G. Antimicrobial peptides and their potential application in inflammation and sepsis. Crit. care 2012, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.L.; Li, Q.; Song, S.S.; Feng, K.; Zhang, D.B.; Wang, Q.Y.; Chen, Y.H. Characterization of antimicrobial peptides isolated from the skin of the chinese frog, rana dybowskii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Dusik, K.; Zheng, W.; Lili, J.; Hanpin, L.; Junwei, H.; John, W.H.; Qiuyu, W. Development of a novel antimicrobial peptide awrk6. Bang. J. Pharmacol. 2016, 11, 460–468. [Google Scholar] [CrossRef]
- Woodfin, A.; Beyrau, M.; Voisin, M.B.; Ma, B.; Whiteford, J.R.; Hordijk, P.L.; Hogg, N.; Nourshargh, S. Icam-1-expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia. Blood 2016, 127, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Sherwood, E.R. Immunology. Getting sepsis therapy right. Science 2015, 347, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Afacan, N.J.; Yeung, A.T.; Pena, O.M.; Hancock, R.E. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Jin, M. Potential immunotherapeutics for immunosuppression in sepsis. Biomol. Ther. 2017, 25, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Ding, R.; Chu, Z.Y.; Zhang, M.B.; Liu, X.Y.; Xie, S.H.; Zhai, Y.J.; Wang, Y.D. Role of berberine in anti-bacterial as a high-affinity lps antagonist binding to tlr4/md-2 receptor. BMC Complement. Altern. Med. 2014, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by tlr4 signal transduction pathway activation of fak and myd88. J. Immunol. 2015, 195, 4999–5010. [Google Scholar] [CrossRef] [PubMed]
- Storek, K.M.; Monack, D.M. Bacterial recognition pathways that lead to inflammasome activation. Immunol. Rev. 2015, 265, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Zhang, Q.; Dong, W.; Liang, H.; Bi, X. The effects of lps on the activity of trp-containing antimicrobial peptides against gram-negative bacteria and endotoxin neutralization. Acta Biomater. 2016, 33, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Vincent, J.L.; Adhikari, N.K.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A roadmap for future research. Lancet Infect. Dis. 2015, 15, 581–614. [Google Scholar] [CrossRef]
- Ryu, J.K.; Kim, S.J.; Rah, S.H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.O.; Park, B.S.; Yoon, T.Y.; et al. Reconstruction of lps transfer cascade reveals structural determinants within lbp, cd14, and tlr4-md2 for efficient lps recognition and transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Jiang, X.; Wu, X.; Fordjour, P.A.; Miao, L.; Zhang, H.; Zhu, Y.; Gao, X. Anti-inflammatory activity of tanshinone iia in lps-stimulated raw264.7 macrophages via mirnas and tlr4-nf-kappab pathway. Inflammation 2016, 39, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yi, M.; Guo, Q.; Wang, C.; Wang, H.; Meng, S.; Liu, C.; Fu, Y.; Ji, H.; Chen, T. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through tlr4-myd88-nf-kappab pathway. Int. Immunopharmacol. 2015, 29, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Deleage, G.; Combet, C.; Blanchet, C.; Geourjon, C. Antheprot: An integrated protein sequence analysis software with client/server capabilities. Comput. Biol. Med. 2001, 31, 259–267. [Google Scholar] [CrossRef]
- Hunter, M.M.; Wang, A.; Parhar, K.S.; Johnston, M.J.G.; Van Rooijen, N.; Beck, P.L.; Mckay, D.M. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology 2010, 138, 1395–1405. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence |
|---|---|
| TLR4 | Forward: 5′-TCTGCCTTCACTACAGAGACT-3′ |
| Reverse: 5′-AGTCTTCTCCAGAAGATGTGC-3′ | |
| JNK | Forward: 5′-TATACGCATAAGTACGGCTACA-3′ |
| Reverse: 5′-GTCCTGGTGGGAAATGAAC-3′ | |
| IκB | Forward: 5′-GCCCTTCTGGGATTTCCT-3′ |
| Reverse: 5′-GCGGCTCCGCTTCGTTCT-3′ | |
| ACTB | Forward: 5′-TTGTTACCACCTGGGACG-3′ |
| Reverse: 5′-GGCATAGAGCTCTTTACGG-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Jin, L.; Wang, H.; Tai, S.; Liu, H.; Zhang, D. AWRK6, A Synthetic Cationic Peptide Derived from Antimicrobial Peptide Dybowskin-2CDYa, Inhibits Lipopolysaccharide-Induced Inflammatory Response. Int. J. Mol. Sci. 2018, 19, 600. https://doi.org/10.3390/ijms19020600
Wang Q, Jin L, Wang H, Tai S, Liu H, Zhang D. AWRK6, A Synthetic Cationic Peptide Derived from Antimicrobial Peptide Dybowskin-2CDYa, Inhibits Lipopolysaccharide-Induced Inflammatory Response. International Journal of Molecular Sciences. 2018; 19(2):600. https://doi.org/10.3390/ijms19020600
Chicago/Turabian StyleWang, Qiuyu, Lili Jin, Huan Wang, Sijia Tai, Hongsheng Liu, and Dianbao Zhang. 2018. "AWRK6, A Synthetic Cationic Peptide Derived from Antimicrobial Peptide Dybowskin-2CDYa, Inhibits Lipopolysaccharide-Induced Inflammatory Response" International Journal of Molecular Sciences 19, no. 2: 600. https://doi.org/10.3390/ijms19020600