Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro
Abstract
:1. Introduction
2. Results
2.1. Preparation and Physicochemical Properties of Resveratrol-Stearate-Solid Lipid Nanoparticles (RV-SLN)
2.2. Antioxidant Ability of RV-SLN
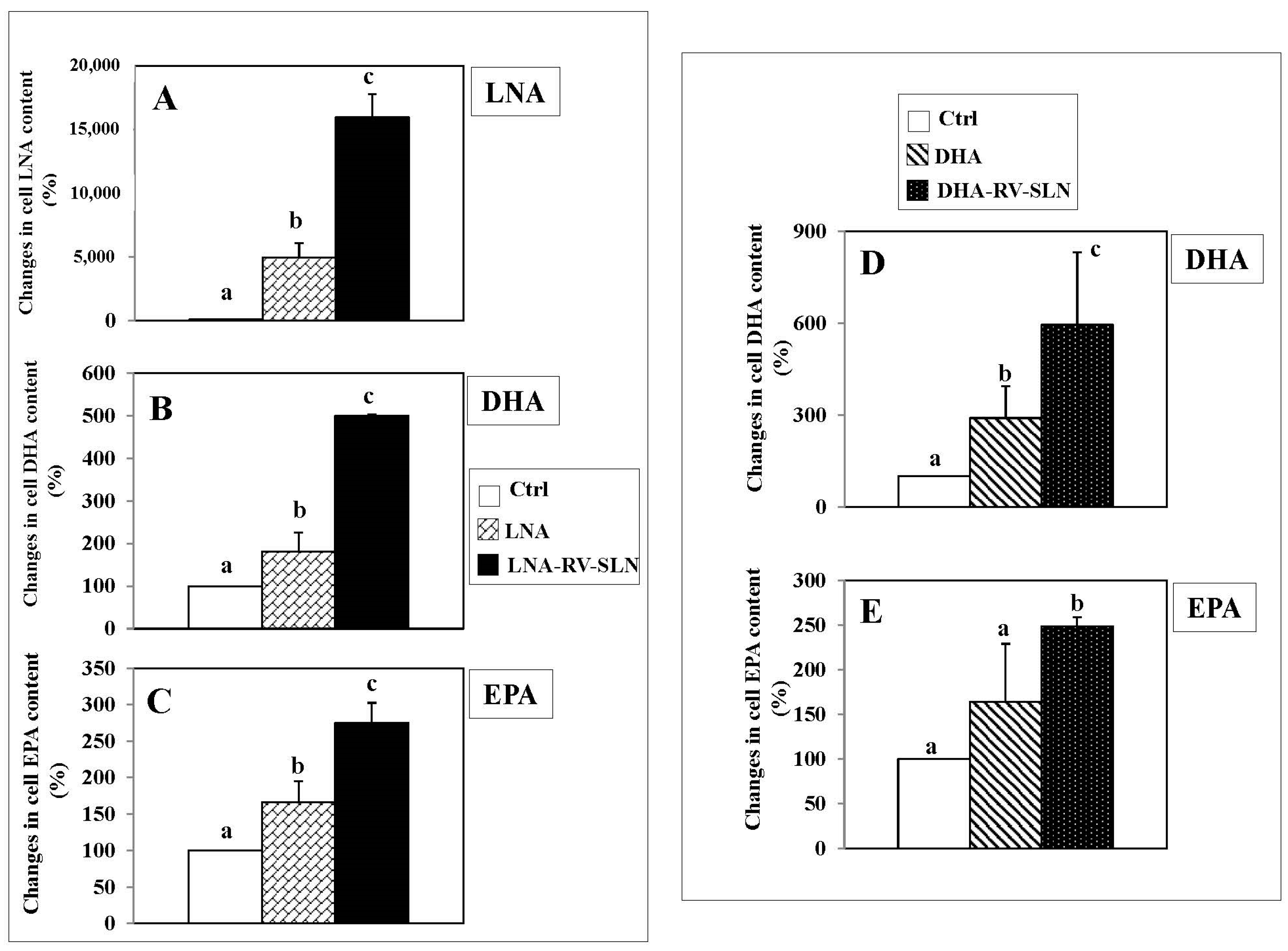
2.3. Omega-3 Incorporation in HT-29 Colorectal Cancer Cells (CRC)
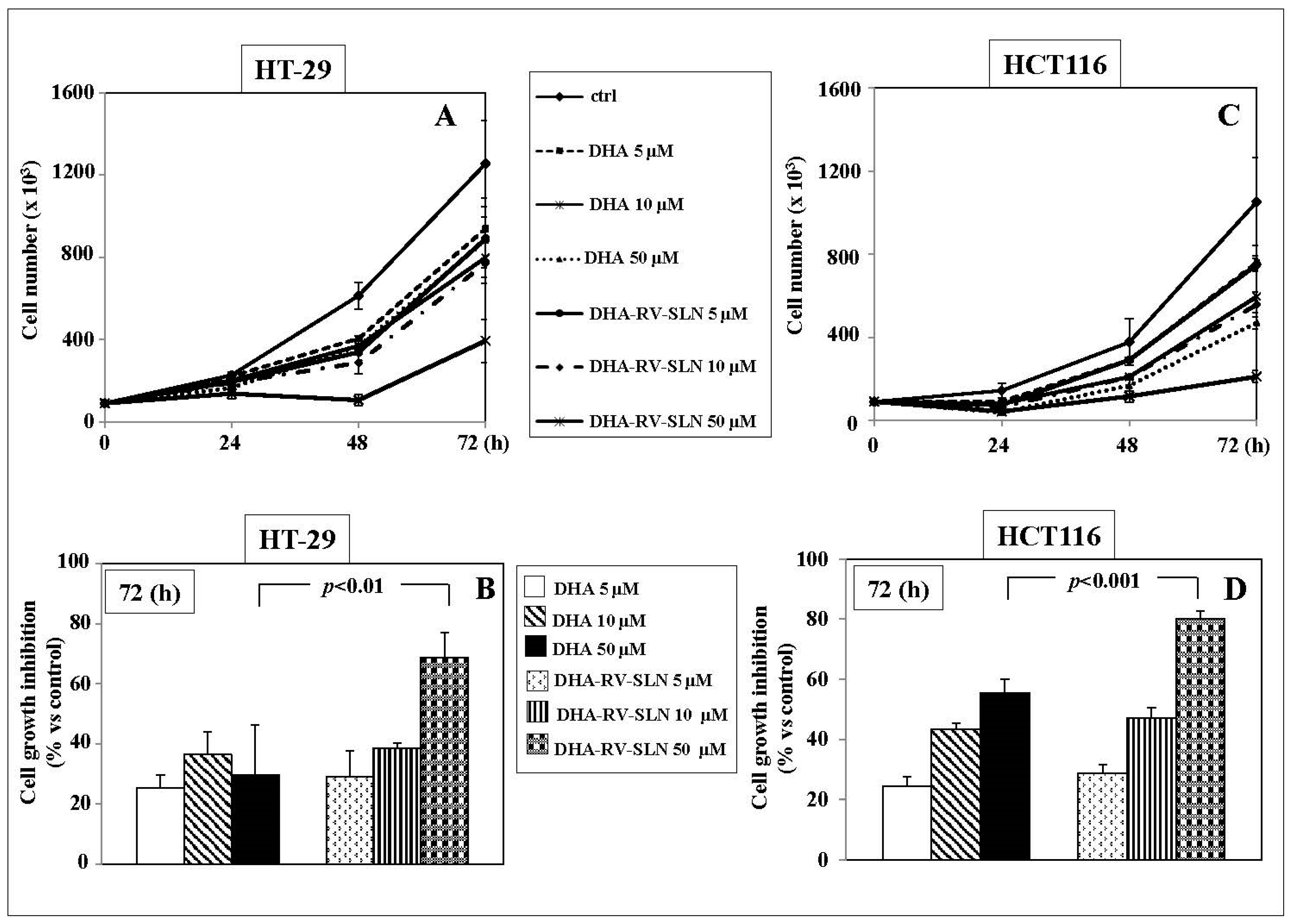
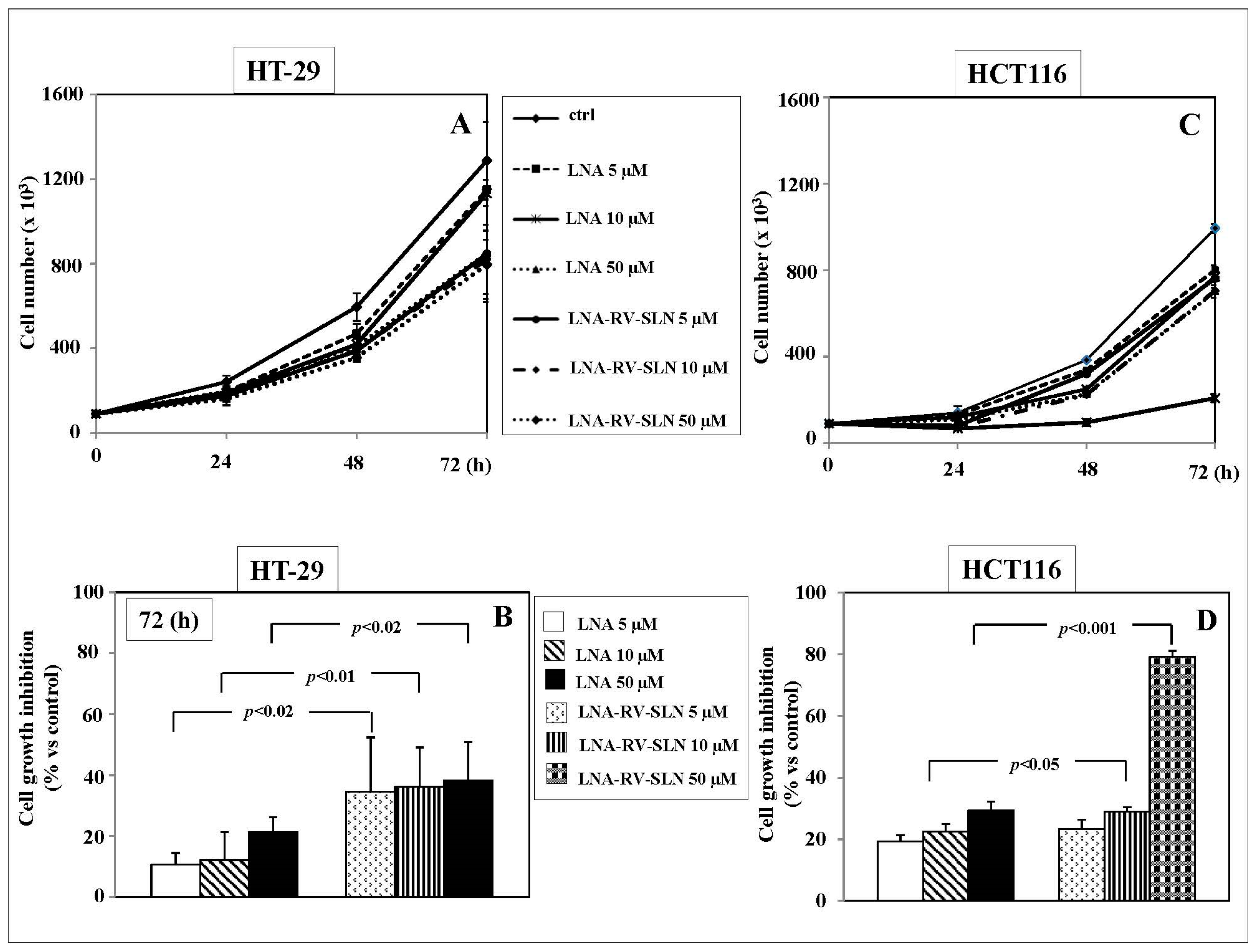
2.4. Effects of RV-SLN on Tumor Cell Growth
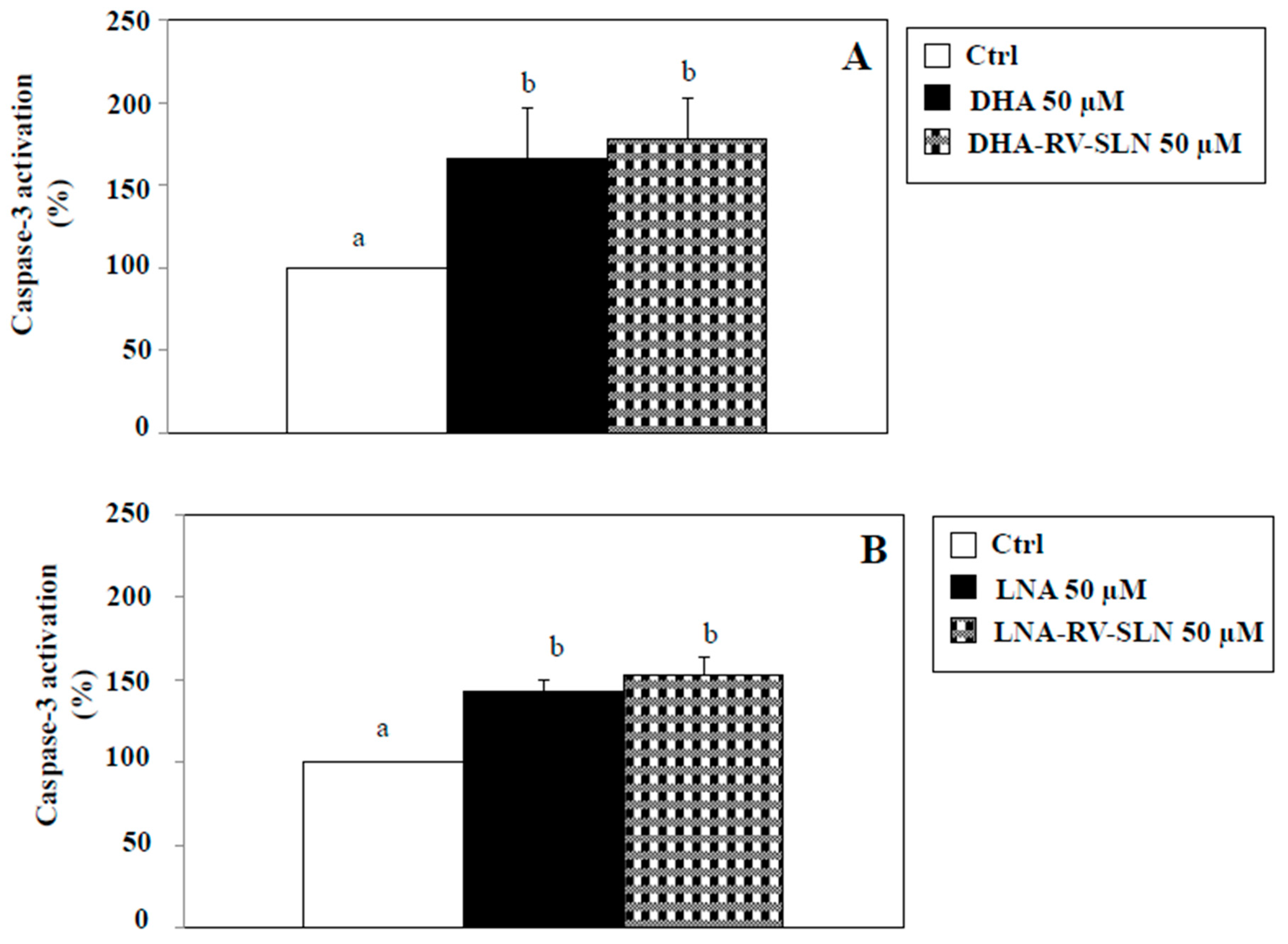
2.5. Effects of RV-SLN on Apoptosis Induction
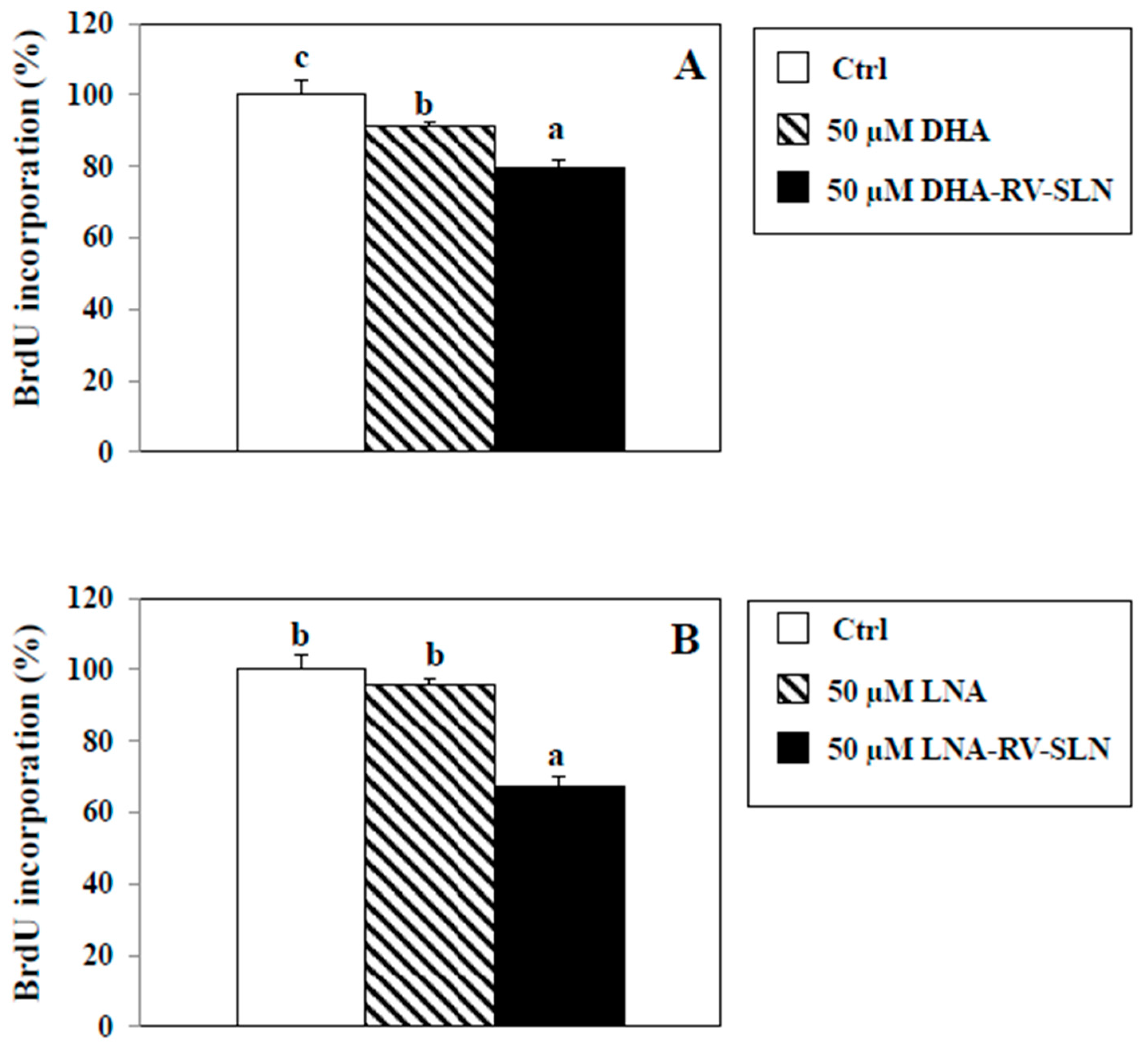
2.6. Effects of RV-SLN on Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Instruments
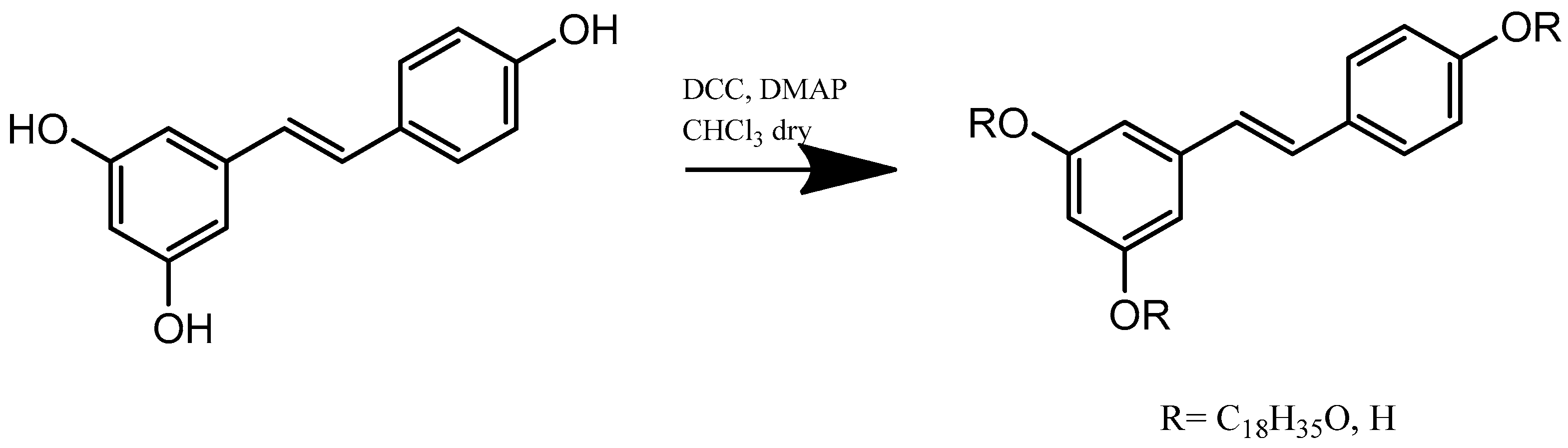
4.3. Synthesis of Resveratrol Stearate
4.4. SLN Preparation and Characterization
4.5. Percentage of Entrapped DHA or LNA into RV-SLN
4.6. Evaluation of Nanoparticle Antioxidant Activity
4.7. Lipid Extraction and Fatty Acid Analysis
4.8. Cell Lines and Treatments
4.9. Cell Growth Evaluation
4.10. Analysis of Apoptosis
4.11. Cell Proliferation Analysis
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammoud, S.S.; Cairns, B.R.; Jones, D.A. Epigenetic regulation of colon cancer and intestinal stem cells. Curr. Opin. Cell Biol. 2013, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Tuan, J.; Chen, Y.X. Dietary and lifestyle factors associated with colorectal cancer risk and interactions with microbiota: Fiber, red or processed meat and alcoholic drinks. Gastrointest. Tumors 2016, 3, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A. Chemotherapy for stage II colon cancer. Clin. Colon Rectal Surg. 2015, 28, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Willaert, W.; Ceelen, W. Extent of surgery in cancer of the colon: Is more better? World J. Gastroenterol. 2015, 21, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, M.; Gasche, C. Chemoprevention of colorectal cancer. Dig. Dis. 2015, 33, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Feng, J.; Qin, H.; Ma, Y. Molecular therapy of colorectal cancer: Progress and future directions. Int. J. Cancer 2015, 136, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Ribecco, A.S.; Pino, M.S.; Cipriani, G.; Marinozzi, C.; Fioretto, L. Molecularly targeted therapy: Toxicity and quality of life considerations in advanced colorectal cancer. Expert Rev. Anticancer Ther. 2013, 13, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Kozovska, Z.; Gabrisova, V.; Kucerova, L. Colon cancer: Cancer stem cells markers, drug resistance and treatment. Biomed. Pharmacother. 2014, 68, 91191–91196. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Calviello, G. Long-chain omega-3 fatty acids and cancer: Any cause for concern? Curr. Opin. Clin. Nutr. Metab. Care 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Jeansen, S.; Witkamp, R.F.; Garthoff, J.A.; van Helvoort, A.; Calder, P.C. Fish oil LC-PUFAs do not affect blood coagulation parameters and bleeding manifestations: Analysis of 8 clinical studies with selected patient groups on omega-3-enriched medical nutrition. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opin. Ther. Targets 2016, 20, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Cittadini, A.; Calviello, G. Long-chain n-3 PUFA against breast and prostate cancer: Which are the appropriate doses for intervention studies in animals and humans? Crit. Rev. Food Sci. Nutr. 2017, 57, 2245–2262. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Calviello, G. Modulation of Ras/ERK and phosphoinositide signaling by long-chain n-3 PUFA in breast cancer and their potential complementary role in combination with targeted drugs. Nutrients 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Piccioni, E.; Merendino, N.; Calviello, G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis 2009, 14, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Resci, F.; Serini, S.; Piccioni, E.; Toesca, A.; Boninsegna, A.; Monego, G.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic acid induces proteasome-dependent degradation of beta-catenin, down-regulation of survivin and apoptosis in human colorectal cancer cells not expressing COX-2. Carcinogenesis 2007, 28, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim. Biophys. Acta 2012, 1822, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Di Nicuolo, F.; Serini, S.; Piccioni, E.; Boninsegna, A.; Maggiano, N.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother. Pharmacol. 2005, 55, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Calviello, G.; Di Nicuolo, F.; Gragnoli, S.; Piccioni, E.; Serini, S.; Maggiano, N.; Tringali, G.; Navarra, P.; Ranelletti, F.O.; Palozza, P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis 2004, 25, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Trombino, S.; Oliva, F.; Piccioni, E.; Monego, G.; Resci, F.; Boninsegna, A.; Picci, N.; Ranelletti, F.O.; Calviello, G. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis 2008, 13, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Fasano, E.; Piccioni, E.; Monego, G.; Cittadini, A.R.; Celleno, L.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis and differentiation in human melanoma cells in vitro: Involvement of HuR-mediated COX-2 mRNA stabilization and β-catenin nuclear translocation. Carcinogenesis 2012, 33, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Messa, C.; Refolo, M.G.; Tutino, V.; Miccolis, A.; Caruso, M.G. Polyunsaturated fatty acids reduce fatty acid synthase and hydroxy-methyl-glutaryl CoA-reductase gene expression and promote apoptosis in HepG2 cell line. Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Tutino, V.; de Nunzio, V.; Dituri, F.; Caruso, M.G.; Giannelli, G. Dietary ω-3 polyunsaturated fatty acids inhibit tumor growth in transgenic ApcMin/+ mice, correlating with CB1 receptor up-regulation. Int. J. Mol. Sci. 2017, 18, 485. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; Darke, A.K.; Song, X.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L., Jr.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2013, 105, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.; Calviello, G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem. Res. Toxicol. 2011, 24, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Hadian, Z. A Review of nanoliposomal delivery system for stabilization of bioactive Omega-3 fatty acids. Electron Phys. 2016, 8, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [PubMed]
- Cassano, R.; Mellace, S.; Marrelli, M.; Conforti, F.; Trombino, S. α-Tocopheryl linolenate solid lipid nanoparticles for the encapsulation, protection, and release of the omega-3 polyunsaturated fatty acid: In vitro anti-melanoma activity evaluation. Colloids Surf. B Biointerfaces 2017, 151, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Ferrarelli, T.; Mauro, M.V.; Cavalcanti, P.; Picci, N.; Trombino, S. Preparation, characterization and in vitro activities evaluation of solid lipid nanoparticles based on PEG-40 stearate for antifungal drugs vaginal delivery. Drug Deliv. 2016, 23, 1047–1056. [Google Scholar] [PubMed]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Muzzalupo, R.; Pingitore, A.; Cione, E.; Picci, N. Stearyl ferulate-based solid lipid nanoparticles for the encapsulation and stabilization of β-carotene and α-tocopherol. Colloids Surf. B Biointerfaces 2009, 72, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Song, E.A.; Kim, H. Docosahexaenoic acid induces oxidative DNA damage and apoptosis, and enhances the chemosensitivity of cancer cells. Int. J. Mol. Sci. 2016, 17, 1257. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Omega-3 fatty acids and cancer cell cytotoxicity: Implications for multi-targeted cancer therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P. Effects of stearic acid on plasma lipid and lipoproteins in humans. Lipids 2005, 40, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Demirbilek, M.; Laçin Türkoglu, N.; Aktürk, S.; Akça, C. VitD3-loaded solid lipid nanoparticles: Stability, cytotoxicity and cytokine levels. J. Microencapsul. 2017, 34, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, G.; Kumar, R.; Malik, R.; Singh, B.; Katare, O.P.; Raza, K. Stearic acid based, systematically designed oral lipid nanoparticles for enhanced brain delivery of dimethyl fumarate. Nanomedicine 2017, 12, 2607–2621. [Google Scholar] [CrossRef] [PubMed]
- Shilpi, D.; Kushwah, V.; Agrawal, A.K.; Jain, S. Improved stability and enhanced oral bioavailability of atorvastatin loaded stearic acid modified gelatin nanoparticles. Pharm. Res. 2017, 34, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Mamalakis, G.; Kafatos, A.; Kalogeropoulos, N.; Andrikopoulos, N.; Daskalopulos, G.; Kranidis, A. Prostate cancer vs. hyperplasia: Relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 467–477. [Google Scholar] [CrossRef]
- Crowe, F.L.; Allen, N.E.; Appleby, P.N.; Overvad, K.; Aardestrup, I.V.; Johnsen, N.F.; Tjønneland, A.; Linseisen, J.; Kaaks, R.; Boeing, H.; et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2008, 88, 1353–1363. [Google Scholar] [PubMed]
- Liu, Y.Z.; Wu, K.; Huang, J.; Liu, Y.; Wang, X.; Meng, Z.J.; Yuan, S.X.; Wang, D.X.; Luo, J.Y.; Zuo, G.W.; et al. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int. J. Oncol. 2014, 45, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, L.; Goupille, C.; Blanc, C.; Pinault, M.; Domingo, I.; Guimaraes, C.; Bougnoux, P.; Chevalier, S.; Mahéo, K. Long chain n-3 polyunsaturated fatty acids increase the efficacy of docetaxel in mammary cancer cells by downregulating Akt and PKCε/δ-induced ERK pathways. Biochim. Biophys. Acta 2016, 1861, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Narayanan, N.K.; Reddy, B.S. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int. J. Oncol. 2001, 19, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, A.M.; Toit-Kohn, J.L.; Ellis, B.; Thomas, M.; Nell, T.; Smith, R. Differential induction of apoptosis and inhibition of the PI3-kinase pathway by saturated, monounsaturated and polyunsaturated fatty acids in a colon cancer cell model. Apoptosis 2008, 13, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Reddivari, L.; Radhakrishnan, S.; Tarver, C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer 2010, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.A.; Agha, A.M.; Merzabani, M.M.; Shouman, S.A. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: Calorie restriction is the force to the cytotoxicity. Hum. Exp. Toxicol. 2013, 32, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. How plausible is the use of dietary n-3 PUFA in the adjuvant therapy of cancer? Nutr. Res. Rev. 2016, 29, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell. Physiol. Biochem. 2011, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.; Preet, R.; Choudhuri, M.; Choudhuri, T.; Kundu, C.N. 5-fluorouracil increases the chemopreventive potentials of resveratrol through DNA damage and MAPK signaling pathway in human colorectal cancer cells. Oncol. Res. 2011, 19, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Hu, X.J.; Zhao, Y.M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. Br. Med. J. 2013, 346. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014, 6, 5184–5223. [Google Scholar] [CrossRef] [PubMed]
- West, N.J.; Clark, S.K.; Phillips, R.K.; Hutchinson, J.M.; Leicester, R.J.; Belluzzi, A.; Hull, M.A. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut 2010, 59, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Fini, L.; Piazzi, G.; Ceccarelli, C.; Daoud, Y.; Belluzzi, A.; Munarini, A.; Graziani, G.; Fogliano, V.; Selgrad, M.; Garcia, M.; et al. Highly purified eicosapentaenoic acid as free fatty acids strongly suppresses polyps in ApcMin/+ mice. Clin. Cancer Res. 2010, 16, 5703–5711. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, G.; D’Argenio, G.; Prossomariti, A.; Lembo, V.; Mazzone, G.; Candela, M.; Biagi, E.; Brigidi, P.; Vitaglione, P.; Fogliano, V.; et al. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int. J. Cancer 2014, 135, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Hanley, M.P.; Zha, R.; Igarashi, Y.; Hull, M.A.; Mathias, G.; Sciavolino, F.; Grady, J.J.; Rosenberg, D.W. A novel bioactive derivative of eicosapentaenoic acid (EPA) suppresses intestinal tumor development in ApcΔ14/+ mice. Carcinogenesis 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Istfan, N.W. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, H.; Shen, Y.; Ni, X.; Shen, S.; Das, U.N. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch. Med. Sci. 2015, 11, 1081–1094. [Google Scholar] [PubMed]
- Bommareddy, A.; Zhang, X.Y.; Kaushik, R.S.; Dwivedi, C. Effects of components present in flaxseed on human colon adenocarcinoma Caco-2 cells: Possible mechanisms of flaxseed on colon cancer development in animals. Drug Discov. Ther. 2010, 4, 184–189. [Google Scholar] [PubMed]
- Kim, J.Y.; Park, H.D.; Park, E.; Chon, J.W.; Park, Y.K. Growth-inhibitory and proapoptotic effects of alpha-linolenic acid on estrogen-positive breast cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Shen, J.; Pan, W.; Shen, S.; Das, U.N. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, A.K.; Kharotia, S.; Mason, J.K.; Thompson, L.U. α-Linolenic acid reduces growth of both triple negative and luminal breast cancer cells in high and low estrogen environments. Nutr. Cancer 2015, 67, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rawat, A.K.; Sammi, S.R.; Devi, U.; Singh, M.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Singh, L.; Ansari, M.N.; et al. Alpha-linolenic acid stabilizes HIF-1 α and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget 2017, 8, 70049–70071. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, J.P.; Moon, H.S. Down-regulation of malignant potential by alpha linolenic acid in human and mouse colon cancer cells. Fam. Cancer 2015, 14, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Oliveira, L.T.; Oger, C.; Galano, J.M.; Bultel-Poncé, V.; Richard, S.; Guimaraes, A.G.; Vilela, J.M.; Andrade, M.S.; Durand, T.; et al. Polymeric nanocapsules prevent oxidation of core-loaded molecules: Evidence based on the effects of docosahexaenoic acid and neuroprostane on breast cancer cells proliferation. J. Exp. Clin. Cancer Res. 2015, 34, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasco, M.R. Solid lipid nanospheres from warm microemulsions. Pharm. Techn. Eur. 1997, 9, 52–58. [Google Scholar]
- Koppel, D.E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: Method of cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comp. Phys. Comm. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Provencher, S.W. Contin: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comp. Phys. Comm. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R. Solid lipid nanoparticles for topical drug delivery. In Drug Delivery Approaches and Nanosystems; Keservani, R.K., Sharma, A.K., Kesharwani, R.K., Eds.; Apple Academic Press, Inc.: Waretown, NJ, USA, 2017; Volume 2, pp. 317–332. ISBN 978-1-77188-584-3. [Google Scholar]
- Aoun, M.; Corsetto, P.A.; Nugue, G.; Montorfano, G.; Ciusani, E.; Crouzier, D.; Hogarth, P.; Gregory, A.; Hayflick, S.; Zorzi, G.; et al. Changes in Red Blood Cell membrane lipid composition: A new perspective into the pathogenesis of PKAN. Mol. Genet. Metab. 2017, 121, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Tacconi, C.; Massimino, L.; Corsetto, P.A.; Correale, C.; Fonteyne, P.; Piontini, A.; Garzarelli, V.; Calcaterra, F.; Della Bella, S.; et al. MFSD2A promotes endothelial generation of inflammation-resolving lipid mediators and reduces colitis in mice. Gastroenterology 2017, 153, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Formulation | Mean Particle Size (nm) | Polydispersity Index (PI) |
|---|---|---|
| RV-SLN | 571 ± 6 | 0.198 ± 0.023 |
| LNA-RV-SLN | 842.2 ± 1.3 | 0.126 ± 0.017 |
| DHA-RV-SLN | 1000 ± 1.8 | 0.220 ± 0.020 |
| Resveratrol Stearate (g) | Tween 80 (mL) | 1-Butanol (mL) | Biliary Salt (g) | Water (mL) | DHA (g) | LNA (g) |
|---|---|---|---|---|---|---|
| 0.1 | 0.62 | 0.026 | 0.036 | 0.29 | 6.1 × 10−3 | 5.2 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 586. https://doi.org/10.3390/ijms19020586
Serini S, Cassano R, Corsetto PA, Rizzo AM, Calviello G, Trombino S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. International Journal of Molecular Sciences. 2018; 19(2):586. https://doi.org/10.3390/ijms19020586
Chicago/Turabian StyleSerini, Simona, Roberta Cassano, Paola Antonia Corsetto, Angela Maria Rizzo, Gabriella Calviello, and Sonia Trombino. 2018. "Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro" International Journal of Molecular Sciences 19, no. 2: 586. https://doi.org/10.3390/ijms19020586









