ON the Nature of Ionic Liquid Gating of La2−xSrxCuO4
Abstract
:1. Introduction
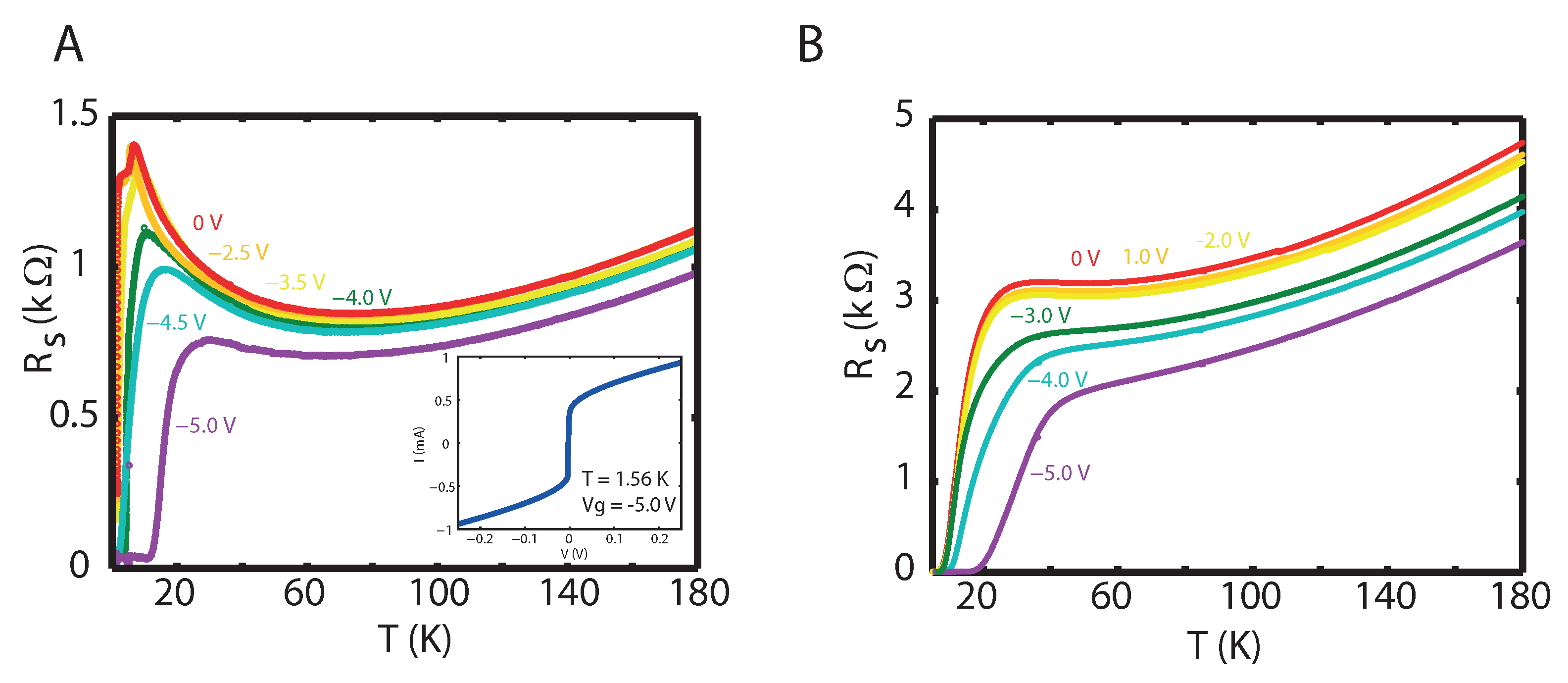
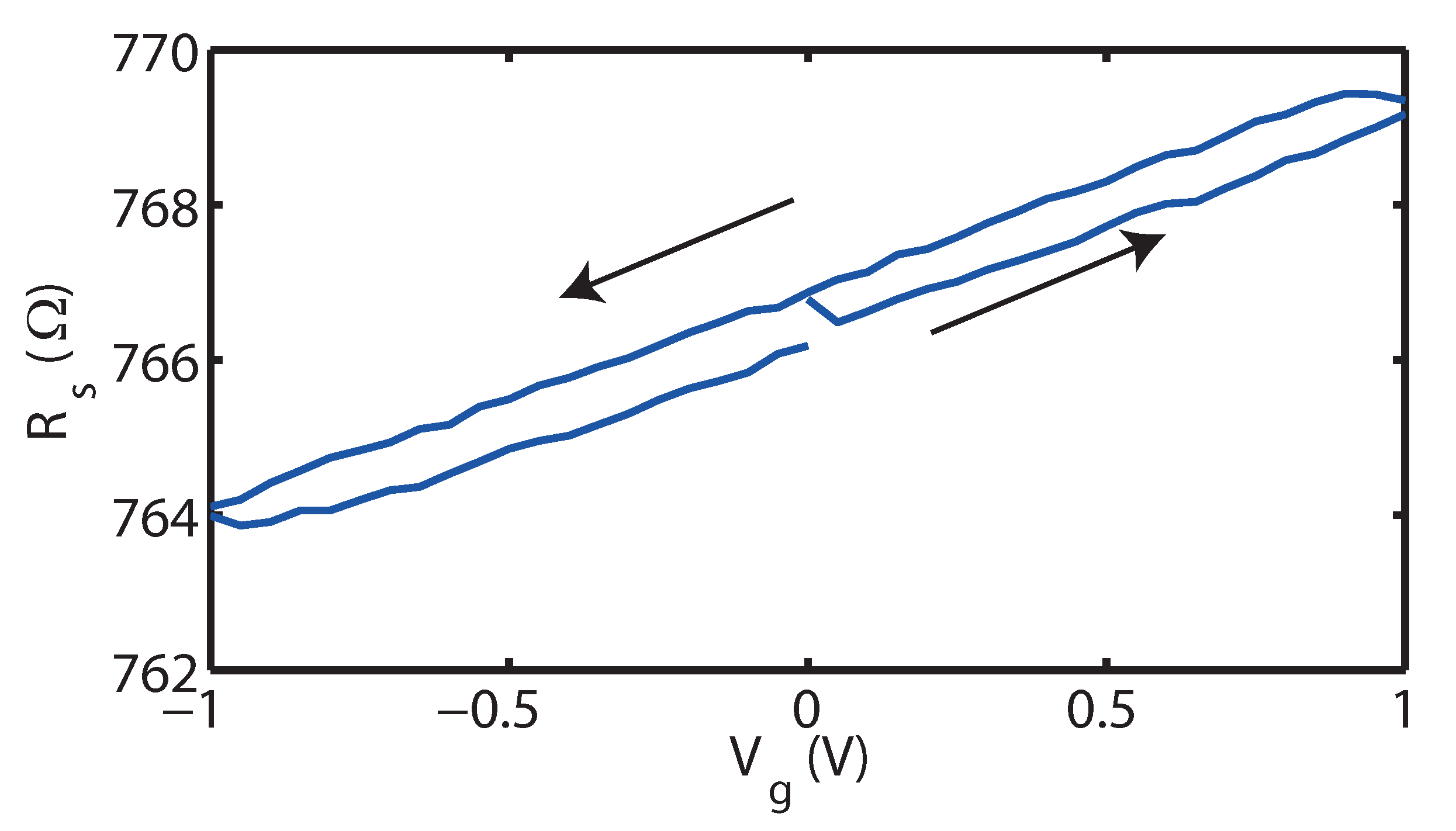
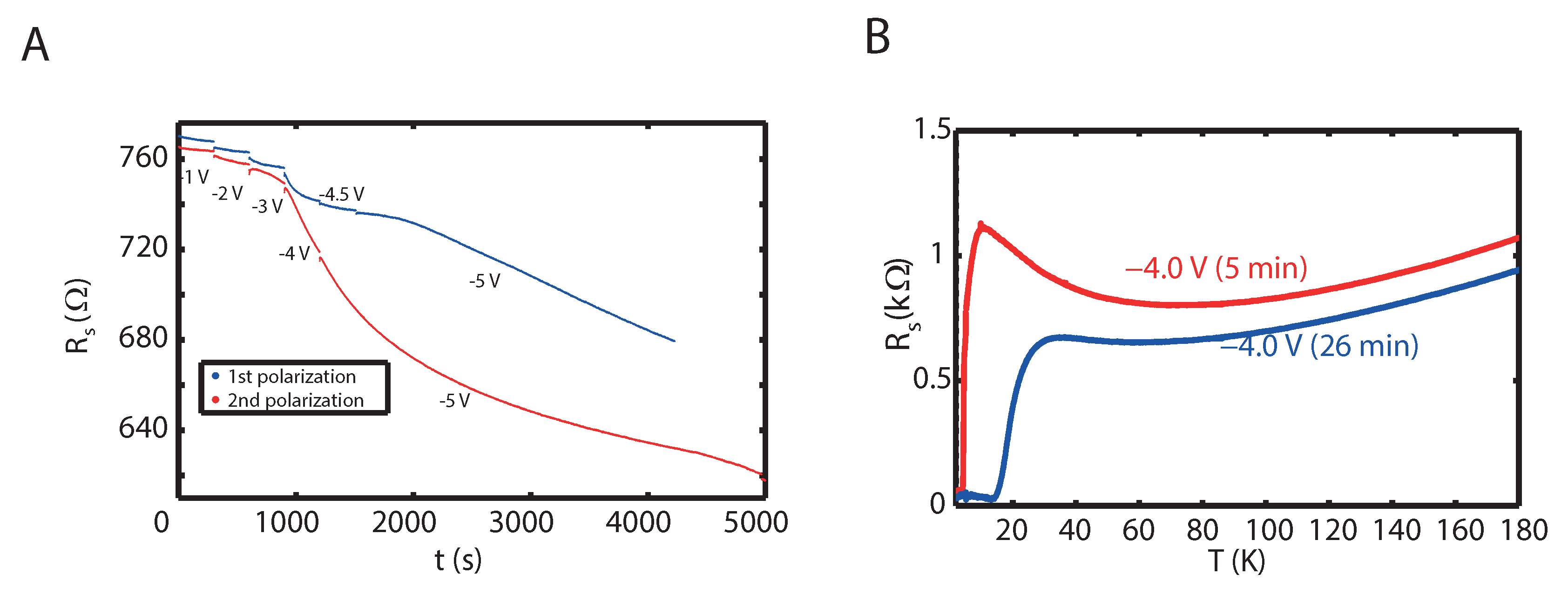
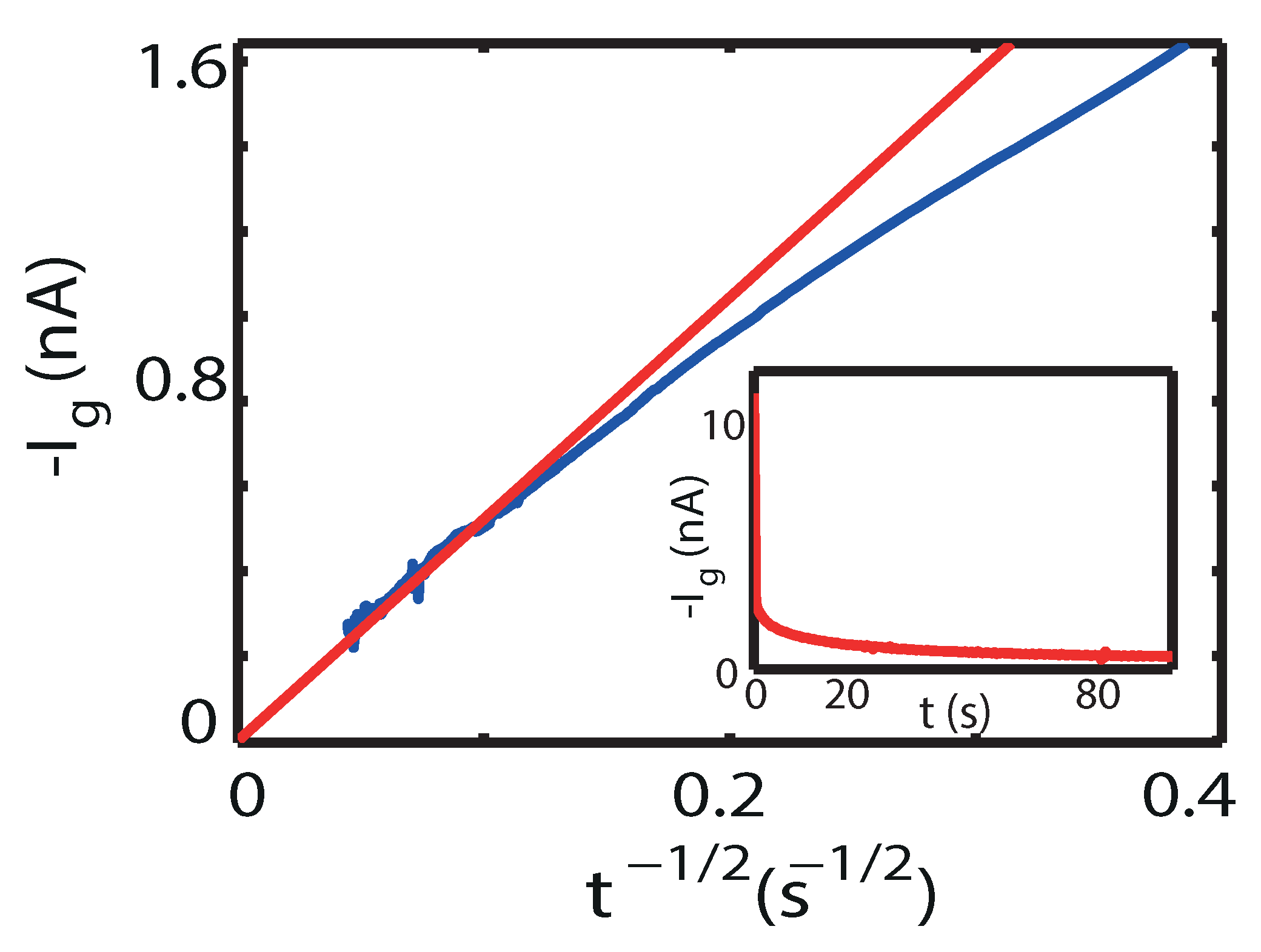
2. Results
3. Discussion
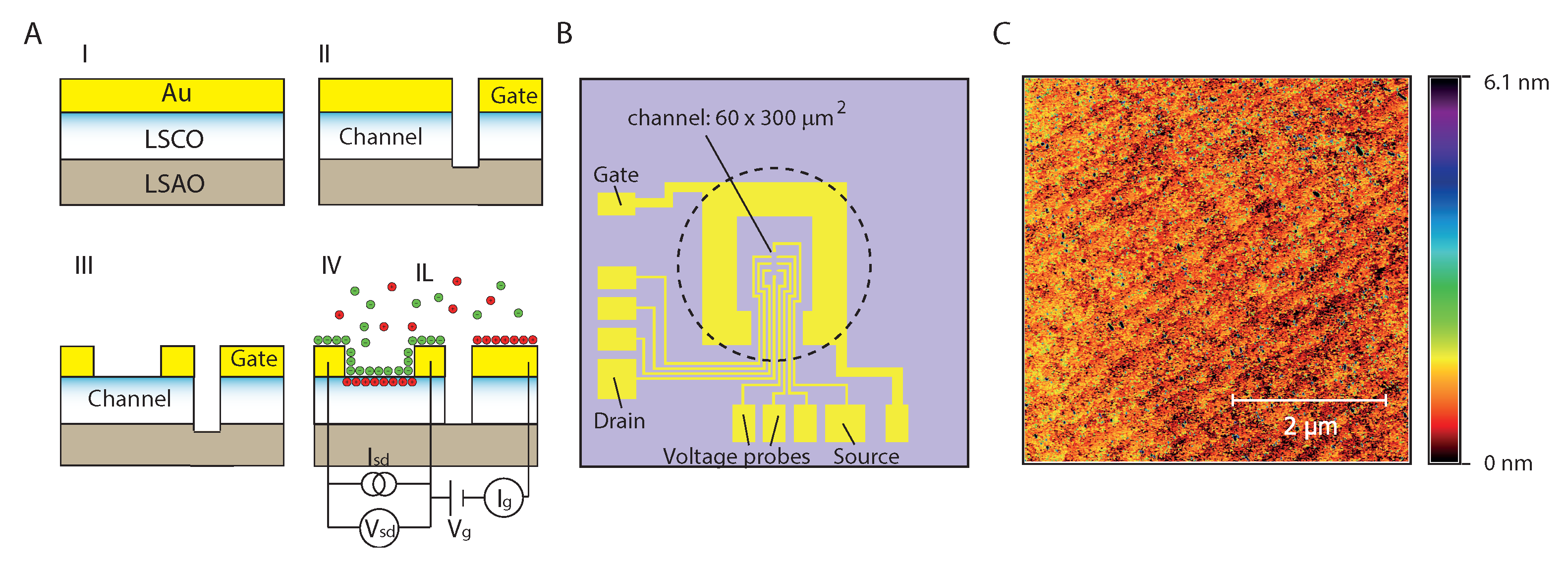
4. Materials and Method
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yuan, H.; Shimotani, H.; Tsukazaki, A.; Ohtomo, A.; Kawasaki, M.; Iwasa, Y. High-Density Carrier Accumulation in ZnO Field-Effect Transistors Gated by Electric Double Layers of Ionic Liquids. Adv. Funct. Mater. 2009, 19, 1046–1053. [Google Scholar] [CrossRef]
- Leng, X.; Garcia-Barriocanal, J.; Bose, S.; Lee, Y.; Goldman, A.M. Electrostatic Control of the Evolution from a Superconducting Phase to an Insulating Phase in Ultrathin YBa2Cu3O7−x Films. Phys. Rev. Lett. 2011, 107, 027001. [Google Scholar] [CrossRef] [PubMed]
- Dubuis, G.; Bollinger, A.T.; Pavuna, D.; Bozovič, I. Electric field effect on superconductivity in La2−xSrxCuO4. J. Appl. Phys. 2012, 111, 112632. [Google Scholar] [CrossRef]
- Ueno, K.; Nakamura, S.; Shimotani, H.; Yuan, H.T.; Kimura, N.; Nojima, T.; Aoki, H.; Iwasa, Y.; Kawasaki, M. Discovery of superconductivity in KTaO3 by electrostatic carrier doping. Nat. Nanotechnol. 2011, 6, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Aetukuri, N.; Graf, T.; Schladt, T.D.; Samant, M.G.; Parkin, S.S.P. Suppression of Metal-Insulator Transition in VO2 by Electric Field-Induced Oxygen Vacancy Formation. Science 2013, 339, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Shibuya, K.; Okuyama, D.; Hatano, T.; Ono, S.; Kawasaki, M.; Iwasa, Y.; Tokura, Y. Collective bulk carrier delocalization driven by electrostatic surface charge accumulation. Nature 2012, 487, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Iwasa, Y. Ambipolar Insulator-to-Metal Transition in Black Phosphorus by Ionic-Liquid Gating. ACS Nano 2015, 9, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Nakamura, S.; Shimotani, H.; Ohtomo, A.; Kimura, N.; Nojima, T.; Aoki, H.; Iwasa, Y.; Kawasaki, M. Electric-field-induced superconductivity in an insulator. Nat. Mater. 2008, 7, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, P.; Lee, M.; Petach, T.A.; Stanwyck, S.W.; Williams, J.R.; Watanabe, K.; Taniguchi, T.; Goldhaber-Gordon, D. A high-mobility electronic system at an electrolyte-gated oxide surface. Nat. Commun. 2015, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, W.; Jeong, J.; Samant, M.G.; Parkin, S.S.P. Suppression of Ionic Liquid Gate-Induced Metallization of SrTiO3(001) by Oxygen. Nano Lett. 2013, 13, 4675–4678. [Google Scholar] [CrossRef] [PubMed]
- Dubuis, G.; Yacoby, Y.; Zhou, H.; He, X.; Bollinger, A.T.; Pavuna, D.; Pindak, R.; Bozovič, I. Oxygen Displacement in Cuprates under Ionic Liquid Field-Effect Gating. Sci. Rep. 2016, 6, 32378. [Google Scholar] [CrossRef] [PubMed]
- Fete, A.; Rossi, L.; Augieri, A.; Senatore, C. Ionic liquid gating of ultra-thin YBa2Cu3O7−x films. Appl. Phys. Lett. 2016, 109, 192601. [Google Scholar] [CrossRef]
- Perez-Muñoz, A.M.; Schio, P.; Poloni, R.; Fernandez-Martinez, A.; Rivera-Calzada, A.; Cezar, J.C.; Salas-Colera, E.; Castro, G.R.; Kinney, J.; Leon, C.; et al. In operando evidence of deoxygenation in ionic liquid gating of YBa2Cu3O7−x. Proc. Natl. Acad. Sci. USA 2017, 114, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barriocanal, J.; Kobrinskii, A.; Leng, X.; Kinney, J.; Yang, B.; Snyder, S.; Goldman, A.M. Electronically driven superconductor-insulator transition in electrostatically doped La2CuO4+δ thin films. Phys. Rev. B 2013, 87, 024509. [Google Scholar] [CrossRef]
- Chen, C.Y.; Birgeneau, R.J.; Kastner, M.A.; Preyer, N.W.; Thio, T. Frequency and magnetic-field dependence of the dielectric constant and conductivity of La2CuO4+y. Phys. Rev. B 1991, 43, 392. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ochi, M.; Higuchi, T.; Terabe, K.; Aono, M. Effect of Ionic Conductivity on Response Speed of SrTiO3-Based All-Solid-State Electric-Double-Layer Transistor. ACS Appl. Mater. Interfaces 2015, 7, 12254–12260. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, K.; Sato, H.; Hiramatsu, H.; Kamiya, T.; Hosono, H. Electric field-induced superconducting transition of insulating FeSe thin film at 35 K. Proc. Natl. Acad. Sci. USA 2016, 113, 3986–3990. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, A.T.; Dubuis, G.; Yoon, J.; Pavuna, D.; Misewich, J.; Bozovič, I. Superconductor-insulator transition in La2−xSrxCuO4 at the pair quantum resistance. Nature 2011, 472, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Shimotani, H.; Ye, J.; Yoon, S.; Aliah, H.; Tsukazaki, A.; Kawasaki, M.; Iwasa, Y. Electrostatic and Electrochemical Nature of Liquid-Gated Electric-Double-Layer Transistors Based on Oxide Semiconductors. J. Am. Chem. Soc. 2010, 132, 18402–18407. [Google Scholar] [CrossRef] [PubMed]
- Jänsch, T.; Wallauer, J.; Roling, B. Influence of Electrode Roughness on Double Layer Formation in Ionic Liquids. J. Phys. Chem. C 2015, 119, 4620–4626. [Google Scholar] [CrossRef]
- Atkin, R.; Borisenko, N.; Drüschler, M.; El Abedin, S.Z.; Endres, F.; Hayes, R.; Huber, B.; Roling, B. An in situ STM/AFM and impedance spectroscopy study of the extremely pure 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate/Au(111) interface: Potential dependent solvation layers and the herringbone reconstruction. Phys. Chem. Chem. Phys. 2011, 13, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Bozovič, I. Controlling Superconductivity in La2−xSrxCuO4+δ by Ozone and Vacuum Annealing. J. Supercond. Nov. Magn. 2015, 28, 71–74. [Google Scholar] [CrossRef]
- Bozovič, I.; Logvenov, G.; Verhoeven, M.A.J.; Caputo, P.; Goldobin, E.; Beasley, M.R. Giant Proximity Effect in Cuprate Superconductors. Phys. Rev. Lett. 2004, 93, 157002. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, N.; Valls, O.T.; Goldman, A.M. Mechanism for electric field effects observed in YBa2Cu3O7−x films. Phys. Rev. Lett. 1993, 71, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Arrouy, F.; Locquet, J.-P.; Williams, E.J.; Mächler, E.; Berger, R.; Gerber, C.; Monroux, C.; Grenier, J.-C.; Wattiaux, A. Growth, microstructure, and electrochemical oxidation of MBE-grown c-axis La2CuO4 thin films. Phys. Rev. B 1996, 54, 7512–7520. [Google Scholar] [CrossRef]
- Locquet, J.-P.; Arrouy, F.; Mächler, E.; Despont, M.; Bauer, P.; Williams, E.J. Local electrochemical oxidation/reduction: First step towards a new lithography? Appl. Phys. Lett. 1996, 68, 1999–2001. [Google Scholar] [CrossRef]
- Ekino, T.; Matsukuma, K.; Takabatake, T.; Fujii, H. Hydrogen absorption in La2−xSrxCuO4 with mono-layer CuO6 octahedra. Phys. B 1990, 165–166, 1529–1530. [Google Scholar] [CrossRef]
- Ji, H.; Wei, J.; Natelson, D. Modulation of the Electrical Properties of VO2 Nanobeams Using an Ionic Liquid as a Gating Medium. Nano Lett. 2012, 12, 2988–2992. [Google Scholar] [CrossRef] [PubMed]
- Katase, T.; Endo, K.; Tohei, T.; Ikuhara, Y.; Ohta, H. Room-Temperature-Protonation-Driven On-Demand Metal-Insulator Conversion of a Transition Metal Oxide. Adv. Electron. Mater. 2015, 1, 1500063. [Google Scholar] [CrossRef]
- Meng, X.; Quenneville, F.; Venne, F.; Di Mauro, E.; Işık, D.; Barbosa, M.; Drolet, Y.; Natile, M.M.; Rochefort, D.; Soavi, F.; et al. Electrolyte-Gated WO3 Transistors: Electrochemistry, Structure and Device Performance. J. Phys. Chem. C 2015, 119, 21732–21738. [Google Scholar] [CrossRef]
- Terashima, T.; Bando, Y.; Iijima, K.; Yamamoto, K.; Hirata, K.; Hayashi, K.; Kamigaki, K.; Terauchi, H. Reflection high-energy electron diffraction oscillations during epitaxial growth of high-temperature superconducting oxides. Phys. Rev. Lett. 1990, 65, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-S.; Sawa, A.; Okamoto, H. Fermi level shift in La2−xSrxCuO4 probed by heteroepitaxial junctions with Nb-doped SrTiO3. Appl. Phys. Lett. 2013, 102, 111606. [Google Scholar] [CrossRef]
- Atesci, H.; Coneri, F.; Leeuwenhoek, M.; Bommer, J.; Seddon, J.R.T.; Hilgenkamp, H.; van Ruitenbeek, J.M. On the formation of a conducting surface channel by ionic liquid gating of an insulator. arXiv, 2017; arXiv:1709.01178. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atesci, H.; Gelling, W.; Coneri, F.; Hilgenkamp, H.; Van Ruitenbeek, J.M. ON the Nature of Ionic Liquid Gating of La2−xSrxCuO4. Int. J. Mol. Sci. 2018, 19, 566. https://doi.org/10.3390/ijms19020566
Atesci H, Gelling W, Coneri F, Hilgenkamp H, Van Ruitenbeek JM. ON the Nature of Ionic Liquid Gating of La2−xSrxCuO4. International Journal of Molecular Sciences. 2018; 19(2):566. https://doi.org/10.3390/ijms19020566
Chicago/Turabian StyleAtesci, Hasan, Wouter Gelling, Francesco Coneri, Hans Hilgenkamp, and Jan M. Van Ruitenbeek. 2018. "ON the Nature of Ionic Liquid Gating of La2−xSrxCuO4" International Journal of Molecular Sciences 19, no. 2: 566. https://doi.org/10.3390/ijms19020566




