Determination of Proteinaceous Selenocysteine in Selenized Yeast
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of the Samples in Terms of Total Selenium, Total Selenomethionine, and Total Selenometabolite Fraction
2.2. Concerns About Selenocysteine Determination
2.2.1. Recovery
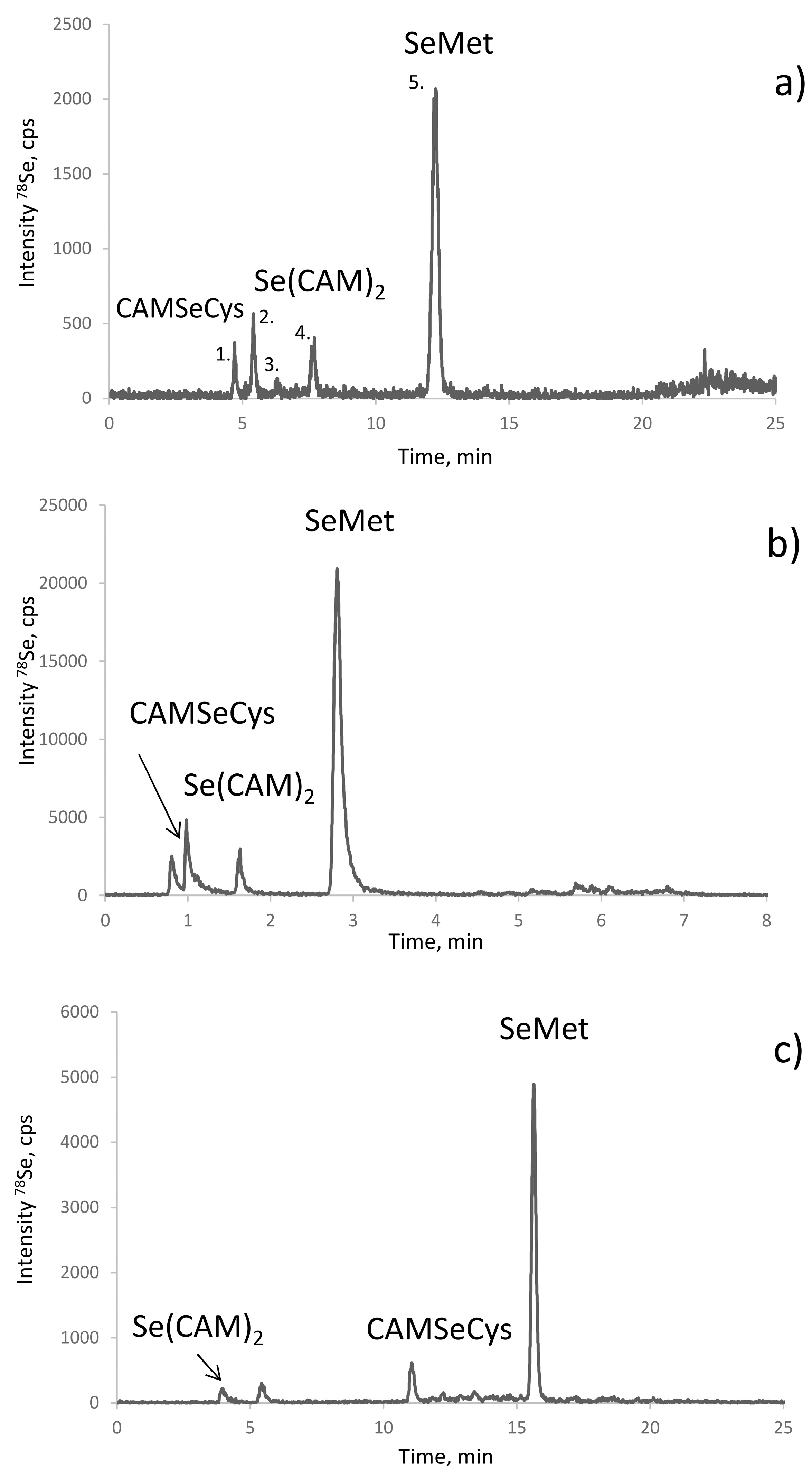
2.2.2. Chromatographic Baseline Resolution
2.2.3. Distribution of SeCys and SeMet between the Metabolite and Protein Fraction
2.3. Optimization of the Baseline Separation of CAM-SeCys in the Proteolytic Extract
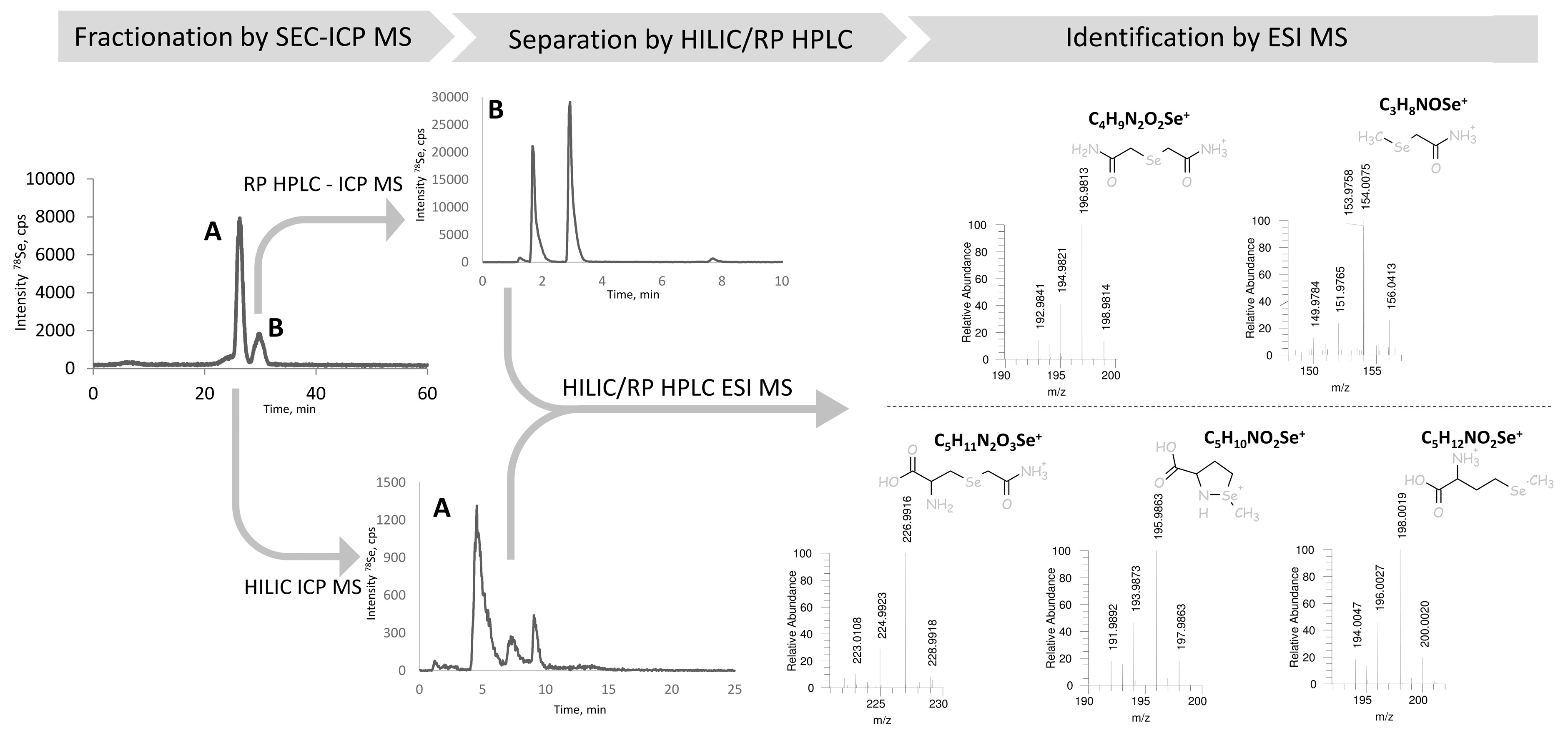
2.4. Identification of the Peaks Observed
2.5. The Origin of Dicarbamidomethylselenium (Se(CAM)2)
- (i)
- The occurrence of selenite-binding proteins: their presence in many organisms has been well documented and a binding site consisting of two neighboring Cys residues was identified [36]; such easily-accessible sites are present in many yeast proteins and are able to scavenge (bind) inorganic selenium present in the growth medium rich in selenium;
- (ii)
- (iii)
- The traces of elemental (e.g., nanoparticulate) selenium or residual post-processing Se(IV) present (trapped) in water insoluble fraction. We observed that the carbamidomethylated selenium compound (Se(CAM)2) was readily formed from any inorganic Se, including selenate, selenide, or Se0 nanoparticles; and
- (iv)
- loss of Se during derivatization of SeCys residues. This hypothesis was investigated by an increase in the iodoacetamide concentration (Figure 5). It would be expected that harsher derivatization conditions would increase the intensity of the Se(CAM)2 peak. However, hardly any effect of the SeCys signal was observed. No significant increase in the intensity of the Se(CAM)2 was observed either which would suggest that the source selenium had already been present in the sample. Note that an increase in the IAM concentration results in a decrease of selenomethionine concentration and formation of derivatization byproducts, which indicates the need for a careful optimization of the derivatization conditions should SeMet be determined together with SeCys.
2.6. Quantification of SeCys and Validation Strategies
3. Experimental
3.1. Samples
3.2. Reagents
3.3. Standards
3.4. Instrumentation
3.5. Procedures
3.5.1. Total Selenium Determination
3.5.2. Determination of Total Selenomethionine (SeMet)
3.5.3. Determination of Proteinaceous Selenocysteine (SeCys), Inorganic Se and SeMet
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hatfield, D.L.; Schweizer, U.; Tsuji, P.A.; Gladyshev, V.N.E. Selenium: Its Molecular Biology and Role in Human Health; Springer: Berlin, Germany, 2016. [Google Scholar]
- Rayman, M.P. The importance of selenium to human health. Lancet 2012, 379, 233–241. [Google Scholar] [CrossRef]
- Juniper, D.T.; Phipps, R.H.; Ramos-Morales, E.; Bertin, G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle 1. J. Anim. Sci. 2008, 86, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Garg, A.K.; Dass, R.S.; Chaturvedi, V.K.; Mudgal, V.; Varshney, V.P. Selenium supplementation influences growth performance, antioxidant status and immune response in lambs. Anim. Feed Sci. Technol. 2009, 153, 77–87. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Vignola, G.; Lambertini, L.; Mazzone, G.; Giammarco, M.; Tassinari, M.; Martelli, G.; Bertin, G. Effects of selenium source and level of supplementation on the performance and meat quality of lambs. Meat Sci. 2009, 81, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Szpunar, J.; Yiannikouris, A.; Lobinski, R. Comprehensive speciation of selenium in selenium-rich yeast. TrAC Trends Anal. Chem. 2012, 41, 122–132. [Google Scholar] [CrossRef]
- Bierla, K.; Bianga, J.; Ouerdane, L.; Szpunar, J.; Yiannikouris, A.; Lobinski, R. A comparative study of the Se/S substitution in methionine and cysteine in Se-enriched yeast using an inductively coupled plasma mass spectrometry (ICP MS)-assisted proteomics approach. J. Proteom. 2013, 87, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Mester, Z.; Willie, S.; Yang, L.; Sturgeon, R.; Caruso, J.A.; Fernández, M.L.; Fodor, P.; Goldschmidt, R.J.; Goenaga-Infante, H.; Lobinski, R.; et al. Certification of a new selenized yeast reference material (SELM-1) for methionine, selenomethinone and total selenium content and its use in an intercomparison exercise for quantifying these analytes. Anal. Bioanal. Chem. 2006, 385, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Dernovics, M.; Lobinski, R. Characterization of the selenocysteine-containing metabolome in selenium-rich yeast Part 1. Identification of new species by multi-dimensional liquid chromatography with parallel ICP-MS and electrospray Q-TOFMS/MS detection. J. Anal. At. Spectrom. 2008, 23, 72–83. [Google Scholar] [CrossRef]
- Axley, M.J.; Stadtman, T.C. Selenium metabolism and selenium-dependent enzymes in microorganisms. Ann. Rev. Nutr. 1989, 9, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Ann. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Mo, S.; Zhang, P.; Cai, Y.; Mou, S.; Jiang, G.; Wen, M. Selenium speciation by high-performance anion-exchange chromatography-post-column UV irradiation coupled with atomic fluorescence spectrometry. J. Chromatogr. A 2006, 1118, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chassaigne, H.; Chery, C.C.; Bordin, G.; Rodriguez, A.R. Development of new analytical methods for selenium speciation in selenium-enriched yeast material. J. Chromatogr. A 2002, 976, 409–422. [Google Scholar] [CrossRef]
- Uden, P.C.; Bird, S.M.; Kotrebai, M.; Nolibos, P.; Tyson, J.F.; Block, E.; Denoyer, E. Analytical selenoamino acid studies by chromatography with interfaced atomic mass spectrometry and atomic emission spectral detection. Fresenius J. Anal. Chem. 1998, 362, 447–456. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenocompounds in plants and animals and their biological significance. J. Am. Coll. Nutr. 2002, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.M.; Uden, P.C.; Tyson, J.F.; Block, E.; Denoyer, E. Speciation of selenoamino acids and organoselenium compounds in selenium-enriched yeast using high-performance liquid chromatography—Inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1997, 12, 785–788. [Google Scholar] [CrossRef]
- Müller, S.; Senn, H.; Gsell, B.; Vetter, W.; Baron, C.; Böck, A. The formation of diselenide bridges in proteins by incorporation of selenocysteine residues: biosynthesis and characterization of (Se) 2-thioredoxin. Biochemistry 1994, 33, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.; Birringer, M.; Block, E.; Kotrebai, M.; Tyson, J.F.; Uden, P.C.; Lisk, D.J. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J. Agric. Food Chem. 2000, 48, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Gilon, N.; Astruc, A.; Astruc, M.; Potin-Gautier, M. Selenoamino acid speciation using HPLC–ETAAS following an enzymic hydrolysis of selenoprotein. Appl. Organomet. Chem. 1995, 9, 623–628. [Google Scholar] [CrossRef]
- Bierla, K.; Dernovics, M.; Vacchina, V.; Szpunar, J.; Bertin, G.; Lobinski, R. etermination of selenocysteine and selenomethionine in edible animal tissues by 2D size-exclusion reversed-phase HPLC-ICP MS following carbamidomethylation and proteolytic extraction. Anal. Bioanal. Chem. 2008, 390, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Szpunar, J.; Lobinski, R. Specific determination of selenoaminoacids in whole milk by 2D size-exclusion-ion-paring reversed phase high-performance liquid chromatography–inductively coupled plasma mass spectrometry (HPLC–ICP MS). Anal. Chim. Acta 2008, 624, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Szpunar, J.; Lobinski, R. Biological selenium species and selenium speciation in biological samples. In Selenium; Springer: Berlin, Germany, 2016; pp. 413–424. [Google Scholar]
- Lipiec, E.; Siara, G.; Bierla, K.; Ouerdane, L.; Szpunar, J. Determination of selenomethionine, selenocysteine, and inorganic selenium in eggs by HPLC—inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2010, 397, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-López, B.; Dernovics, M.; Moreno-González, D.; Molina-Díaz, A.; García-Reyes, J.F. Detection of over 100 selenium metabolites in selenized yeast by liquid chromatography electrospray time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bierla, K.; Godin, S.; Lobinski, R.; Szpunar, J. Advances in electrospray mass spectrometry for the selenium speciation: focus on Se-rich yeast. TrAC Trends Anal. Chem. 2017. [Google Scholar] [CrossRef]
- Ma, S.; Caprioli, R.M.; Hill, K.E.; Burk, R.F. Loss of selenium from selenoproteins: conversion of selenocysteine to dehydroalanine in vitro. J. Am. Soc. Mass Spectrom. 2003, 14, 593–600. [Google Scholar] [CrossRef]
- Kotrebai, M.; Birringer, M.; Tyson, J.F.; Block, E.; Uden, P.C. Selenium speciation in enriched and natural samples by HPLC-ICP-MS and HPLC-ESI-MS with perfluorinated carboxylic acid ion-pairing agents. Analyst 2000, 125, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Encinar, J.R.; Ouerdane, L.; Buchmann, W.; Tortajada, J.; Lobinski, R.; Szpunar, J. Identification of water-soluble selenium-containing proteins in selenized yeast by size-exclusion-reversed-phase HPLC/ICPMS followed by MALDI-TOF and electrospray Q-TOF mass spectrometry. Anal. Chem. 2003, 75, 3765–3774. [Google Scholar] [CrossRef] [PubMed]
- Kahakachchi, C.; Boakye, H.T.; Uden, P.C.; Tyson, J.F. Chromatographic speciation of anionic and neutral selenium compounds in Se-accumulating Brassica juncea (Indian mustard) and in selenized yeast. J. Chromatogr. A 2004, 1054, 303–312. [Google Scholar] [CrossRef]
- Cankur, O.; Yathavakilla, S.K.V.; Caruso, J.A. Selenium speciation in dill (Anethum graveolens L.) by ion pairing reversed phase and cation exchange HPLC with ICP-MS detection. Talanta 2006, 70, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Lobiński, R.; Szpunar, J. Biochemical speciation analysis by hyphenated techniques. Anal. Chim. Acta 1999, 400, 321–332. [Google Scholar] [CrossRef]
- Hansen, H.R.; Raab, A.; Feldmann, J. New arsenosugar metabolite determined in urine by parallel use of HPLC-ICP-MS and HPLC-ESI-MS. J. Anal. At. Spectrom. 2003, 18, 474–479. [Google Scholar] [CrossRef]
- Sriram, S.M.; Kwon, Y.T. The molecular principles of N-end rule recognition. Nat. Struct. Mol. Biol. 2010, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Selenium-enriched yeast as source for selenium added for nutritional purposes in foods for particular nutritional uses and foods (including food supplements) for the general population. EFSA J. 2008, 766, 1–42. [Google Scholar]
- Schild, F.; Kieffer-Jaquinod, S.; Palencia, A.; Cobessi, D.; Sarret, G.; Zubieta, C.; Jourdain, A.; Dumas, R.; Forge, V.; Testemale, D.; et al. Biochemical and biophysical characterization of the selenium-binding and reducing site in Arabidopsis thaliana homologue to mammals selenium-binding protein 1. J. Biol. Chem. 2014, 289, 31765–31776. [Google Scholar] [CrossRef] [PubMed]
- Arnaudguilhem, C.; Bierla, K.; Ouerdane, L.; Preud’homme, H.; Yiannikouris, A.; Lobinski, R. Selenium metabolomics in yeast using complementary reversed-phase/hydrophilic ion interaction (HILIC) liquid chromatography–electrospray hybrid quadrupole trap/Orbitrap mass spectrometry. Anal. Chim. Acta 2012, 757, 26–38. [Google Scholar] [CrossRef] [PubMed]



| Sample | Total Se, ppm | Total SeMet *, ppm as Se | Water Soluble Se Species, ppm |
|---|---|---|---|
| SELM-1 | 1928 ± 59 ** | 1362 ± 49 ppm *** | 276 ± 8 |
| A | 1919 ± 51 | 1670 ± 132 | 185 ± 2 |
| B | 2087 ± 70 | 1283 ± 52 | 276 ± 8 |
| C | 2292 ± 30 | 1676 ± 24 | 354 ± 5 |
| Peak Area, 76Se | ||
|---|---|---|
| Added | Found, ng/mL | Error, % |
| 1 | 1.02 | 2.25 |
| 2.5 | 2.43 | −7.00 |
| 5 | 4.98 | −2.25 |
| 10 | 9.99 | −1.00 |
| Sample | Total Se, ppm | SeCys, ppm as Se | SeMet *, ppm as Se | Se(CAM)2, ppm as Se | Water Soluble Se, ppm | Sum of Species ** | Yield, % |
|---|---|---|---|---|---|---|---|
| SELM | 1928 ± 59 | 81 ± 11 | 1367 ± 93 | 139 ± 13 | 276 ± 8 | 1883 | 97.7 |
| A | 1919 ± 51 | 109 ± 14 | 1563 ± 22 | 97 ± 11 | 185 ± 2 | 1954 | 102.8 |
| B | 2087 ± 70 | 58 ± 7 | 1254 ± 70 | 352 ± 24 | 276 ± 8 | 1940 | 93.0 |
| C | 2292 ± 30 | 105 ± 15 | 1476 ± 32 | 203 ± 16 | 354 ± 5 | 2138 | 93.3 |
| Separation Mechanism | Name | Dimensions (d × l × Particle Size) | Supplier (Location) |
|---|---|---|---|
| Size-exclusion | Superdex-75 | 10 × 300 mm × 13 µm | GE Healthcare (Little Chalfont, UK) |
| Reversed-phase C8 | Alltima C8 | 4.6 × 250 mm × 5 µm | HiChrom (Theale, Reading Berkshire, UK) |
| Mixed | Hypercarb | 2.1 × 100 mm × 3 µm | Thermo Scientific (Waltham, MA, USA) |
| Reversed-phase C18 | CS Evolution AQ | 2.1 × 100 mm × 2.6 µm | Intershim (Montluçon, France) |
| Reversed-phase C18 | Zorbax Eclipse Plus | 4.6 × 100 mm × 3.5 µm | Agilent (Apple Valley, MN, USA) |
| Anion exchange | PRPX-100 | 4.1 × 250 mm × 5 µm | Hamilton Robotics (Reno, NV, USA) |
| HILIC | Phenomenex | 2.1 × 150 mm × 2.6 µm | Kinetex (Torrance, CA, USA) |
| Separation Mechanisms (Column) | Eluent | Elution Mode | Flow Rate, mL/min | Sample Volume, µL |
|---|---|---|---|---|
| Determination of selenocysteine, selenomethionine and protein-bound selenium | ||||
| RP (C8) | A: 0.1% HFBA * in water B: 0.1% HFBA in MeOH | Gradient: 0–15 min 3% B 15–18 min 3–40% B 18–21 min 40% B 21–23 min 40–3 % B 23–30 min 3% B | 0.9 | 10 |
| Mixed (Hypercarb) | A: 20 mM NFPA ** in water B: ACN | Gradient: 0–10 min 0–15 % B 10–20 min 15–26% B 20–30 min 26–50% B 30–33 min 50% B 33–34 min 50–0% B 34–40 min 0% B | 0.2 | 5 |
| RP (C18 CS) | A: 0.1% TFA in H2O B: ACN | Gradient: 0–1 min 1% B 1–5 min 1–40% B 5–6.5 min 40–80% B 6.5–8 min 80% B 8–8.5 min 80–1% B 8.5–10 min 1% B | 0.3 | 7 |
| Determination of SeMet | ||||
| Anion-exchange (PRPX-100) | A: 20 mM CH3COOH- 10 mM triethylamine (pH 4.7) B: 200 mM CH3COOH- 100 mM triethylamine (pH 4.7) | 0–5 min: 0% B 5–30 min: 0–100% B 30–40 min: 100–0% B | 0.7 | 20 |
| Identification of selenium species by ESI MS | ||||
| SEC (Superdex-75) | 100 mM ammonium acetate, pH 7.5 | isocratic | 0.9 | 100 |
| HILIC (Phenomenex) | A: 90:10 ACN: 50 mM ammonium acetate B: 40:50:10 ACN/H2O/50 mM ammonium acetate | Gradient: 0–2.5 min 0% B 2.5–10 min 0–100% B 10–12.5 min 100% B 12.5–12.6 min 100–0% B 12.6–25 min 0% B | 0.5 | 10 |
| RP (C18 Zorbax) | 0.1% FA in 5% methanol | isocratic | 1 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierla, K.; Lobinski, R.; Szpunar, J. Determination of Proteinaceous Selenocysteine in Selenized Yeast. Int. J. Mol. Sci. 2018, 19, 543. https://doi.org/10.3390/ijms19020543
Bierla K, Lobinski R, Szpunar J. Determination of Proteinaceous Selenocysteine in Selenized Yeast. International Journal of Molecular Sciences. 2018; 19(2):543. https://doi.org/10.3390/ijms19020543
Chicago/Turabian StyleBierla, Katarzyna, Ryszard Lobinski, and Joanna Szpunar. 2018. "Determination of Proteinaceous Selenocysteine in Selenized Yeast" International Journal of Molecular Sciences 19, no. 2: 543. https://doi.org/10.3390/ijms19020543