Metabolic Effects of FecB Gene on Follicular Fluid and Ovarian Vein Serum in Sheep (Ovis aries)
Abstract
:1. Introduction
2. Results
2.1. Measurement of Ovulation Rate, Number, and Diameter for Pre-Ovulation
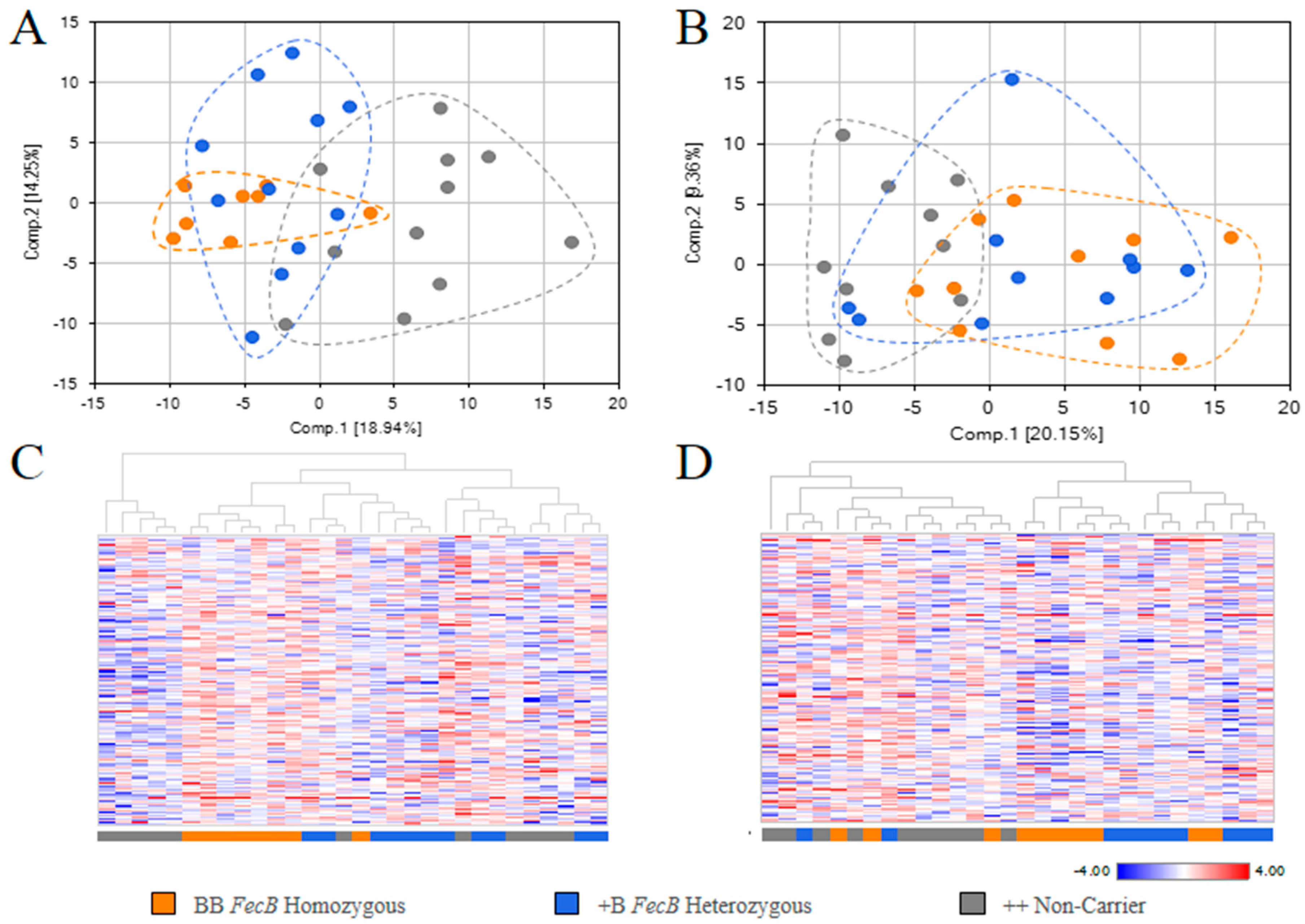
2.2. Overview of Metabolite in Pre-Ovulation FF and OVS Samples
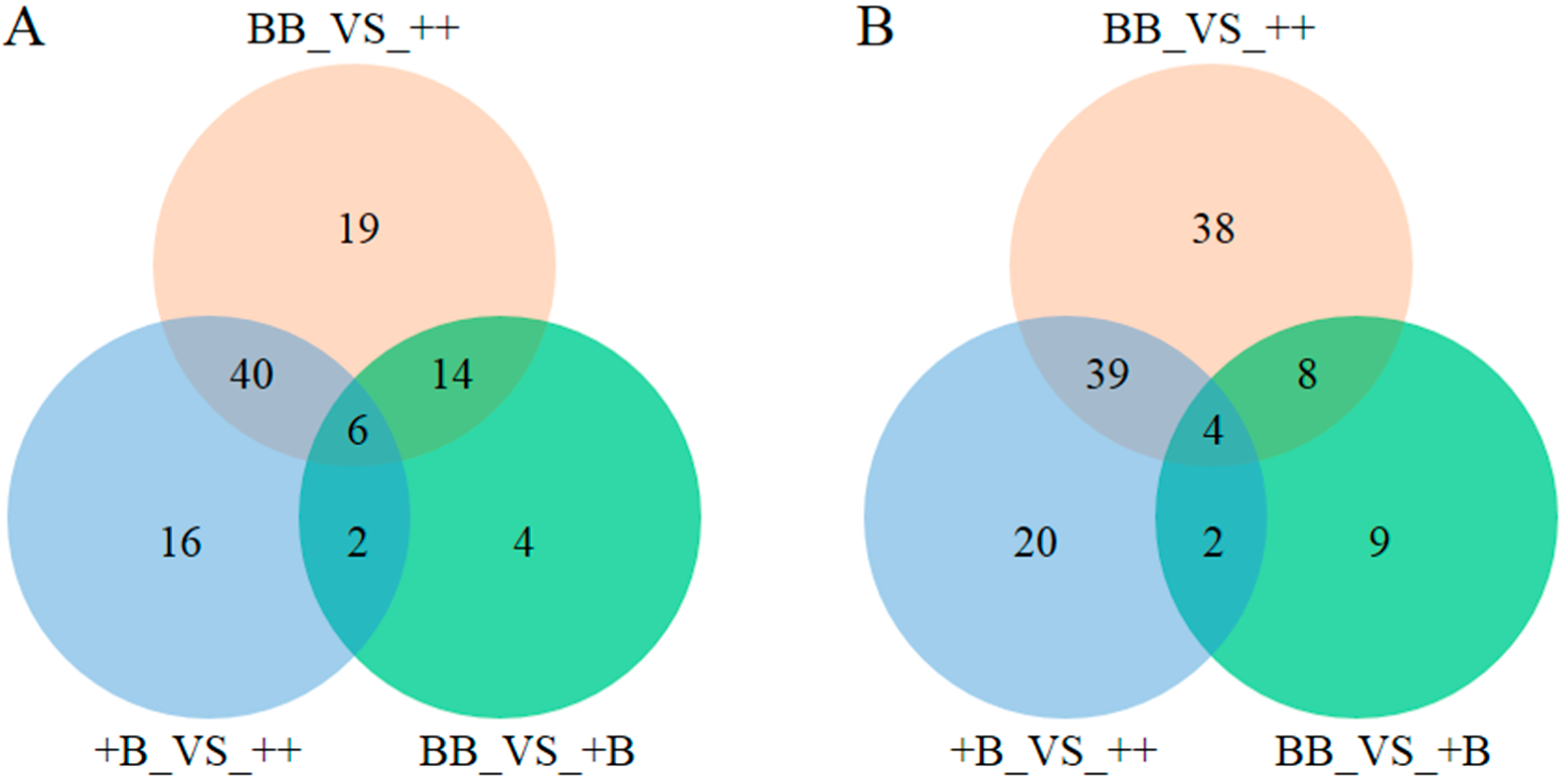
2.3. Identification of Biomarkers in FF and OVS for FecB Gene Effect
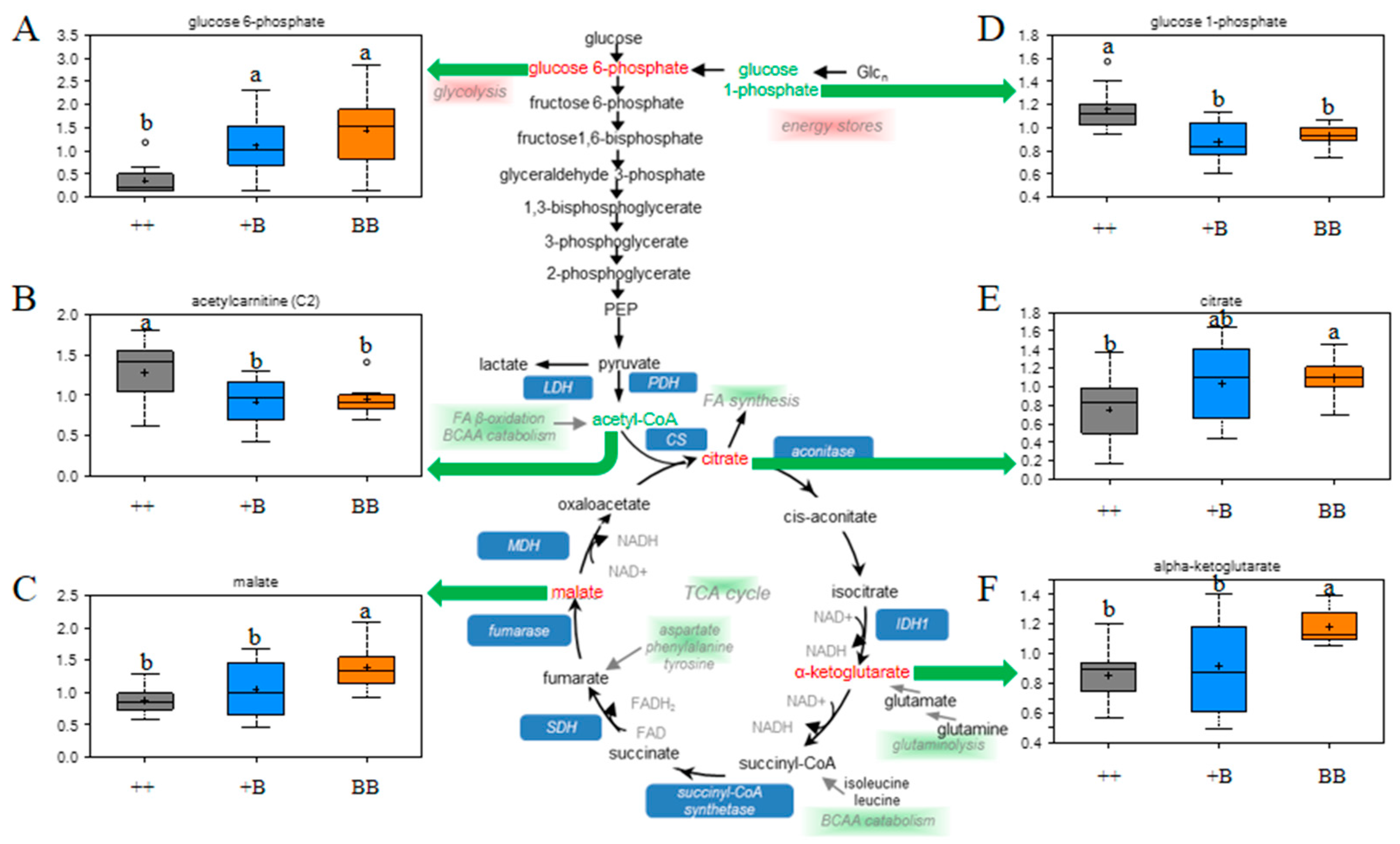
2.4. Changes in Energy Metabolism in FF
2.5. Higher Levels of Amino Acids in FF from BB Sheep
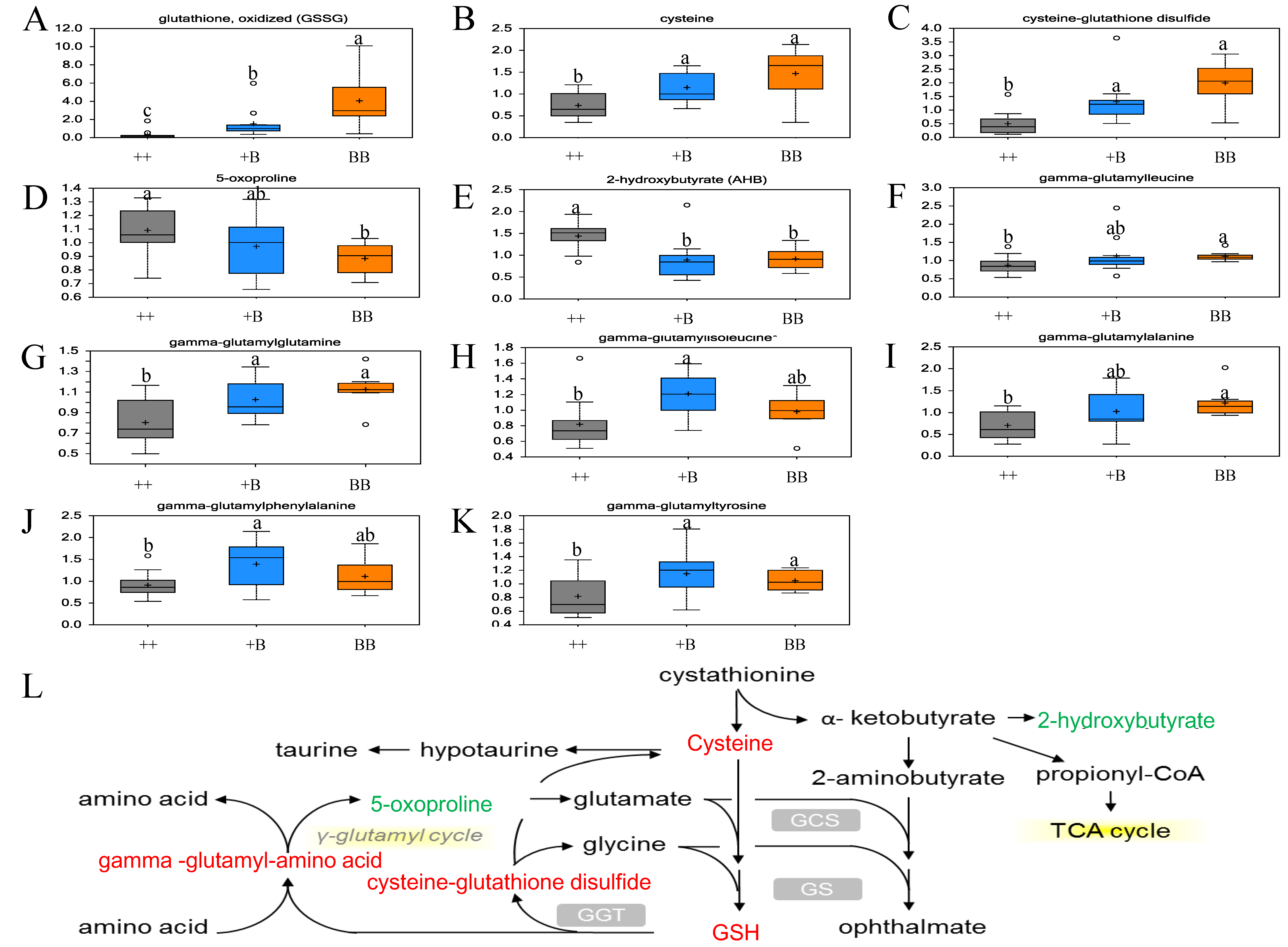
2.6. Elevation of Antioxidant Defense Capacity in FF for BB Sheep
2.7. Subtle Alterations in Steroid Hormones for OVS
2.8. The Relation Between Metabolic Changes and Ovulation Rates
3. Discussion
4. Materials and Methods
4.1. Animals and Grouping
4.2. Collecting Data of Ovulation Rate
4.3. Sample Collection and Follicle Data Measurement
4.4. LC-MS and GC-MS Analysis
4.5. Data Extraction and Compound Identification
4.6. Metabolite Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FF | follicular fluid |
| OVS | ovarian vein serum |
| STH | Small Tail Han |
| PCA | principal component analysis |
| HCA | hierarchical clustering analysis |
| GC-MS | gas chromatography-mass spectrometry |
| LC-MS | liquid chromatography-mass spectrometry |
| BFB | blood follicular barrier |
| ROS | reactive oxygen species |
| CoCs | Cumulus-oocyte complexes |
| CIDR | controlled internal drug releasing |
References
- Singh, R.V.; Sivakumar, A.; Sivashankar, S.; Das, G.; Walkden-Brown, S.W.; Werf, J.H.J.V.D.; Nimbkar, C.; Gupta, V.S. Evaluation of the Booroola (FecB) gene in Muzaffarnagari sheep. ACIAR Proc. Ser. 2009, 133, 223–224. [Google Scholar]
- Mulsant, P.; Lecerf, F.; Fabre, S.; Schibler, L.; Monget, P.; Lanneluc, I.; Pisselet, C.; Riquet, J.; Monniaux, D.; Callebaut, I. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Mérino Ewes. Proc. Natl. Acad. Sci. USA 2001, 98, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Wu, X.Y.; Juengel, J.L.; Ross, I.K.; Lumsden, J.M.; Lord, E.A.; Dodds, K.G.; Walling, G.A.; Mcewan, J.C.; O’Connell, A.R. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol. Reprod. 2001, 64, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.J.; Macdougall, C.; Macdougall, C.; Campbell, B.K.; McNeilly, A.S.; Baird, D.T. The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1 B (BMPR1B) gene. J. Endocrinol. 2001, 169, 1–6. [Google Scholar] [CrossRef]
- Davis, G.H.; Montgomery, G.W.; Allison, A.J.; Kelly, R.W.; Bray, A.R. Segregation of a major gene influencing fecundity in progeny of Booroola sheep. N. Z. J. Agric. Res. 1982, 67, 525–529. [Google Scholar] [CrossRef]
- Fogarty, N.M. A review of the effects of the Booroola gene (FecB) on sheep production. Small Rumin. Res. 2009, 85, 75–84. [Google Scholar] [CrossRef]
- Monget, P.; Fabre, S.; Mulsant, P.; Lecerf, F.; Elsen, J.M.; Mazerbourg, S.; Pisselet, C.; Monniaux, D. Regulation of ovarian folliculogenesis by IGF and BMP system in domestic animals. Domest. Anim. Endocrinol. 2002, 23, 139–154. [Google Scholar] [CrossRef]
- Richards, J.A.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Investig. 2010, 120, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.K.; Marsters, P.; Baird, D.T.; Walkdenbrown, S.W.; Werf, J.H.J.V.D.; Nimbkar, C.; Gupta, V.S. The mechanism of action of the FecB (Booroola) mutation. ACIAR Proc. Ser. 2009, 133, 46–56. [Google Scholar]
- McNatty, K.P.; Henderson, K.M. Gonadotrophins, fecundity genes and ovarian follicular function. J. Steroid Biochem. 1987, 27, 365–373. [Google Scholar] [CrossRef]
- Fry, R.C.; Clarke, I.J.; Cummins, J.T.; Bindon, B.M.; Piper, L.R.; Cahill, L.P. Induction of ovulation in chronically hypophysectomized Booroola ewes. J. Reprod. Fertil. 1988, 82, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.L.; O’Connell, A.R.; Shaw, L.; Clarke, I.J.; McNatty, K.P. Effect of exogenous FSH on ovulation rate in homozygous carriers or noncarriers of the Booroola FecB gene after hypothalamic-pituitary disconnection or after treatment with a GnRH agonist. Domest. Anim. Endocrinol. 1999, 16, 69–80. [Google Scholar] [CrossRef]
- Campbell, B.K.; Baird, D.T.; Souza, C.J.; Webb, R. The FecB (Booroola) gene acts at the ovary: In vivo evidence. Reproduction 2003, 126, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Juengel, J.L.; Davis, G.H.; McNatty, K.P. Using sheep lines with mutations in single genes to better understand ovarian function. Reproduction 2013, 146, R111–R123. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Qin, Q.L. Genome-wide transcriptome analysis of mRNAs and microRNAs in Dorset and Small Tail Han sheep to explore the regulation of fecundity. Mol. Cell. Endocrinol. 2015, 402, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Luo, Q.; Zhao, H.; Qin, X. Ovarian proteomic study reveals the possible molecular mechanism for hyperprolificacy of Small Tail Han sheep. Sci. Rep. 2016, 6, 27606. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Wallace, M.; Cottell, E.; Gibney, M.J.; Mcauliffe, F.M.; Wingfield, M.; Brennan, L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 2013, 146, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Bender, K.; Fahey, A.G.; Mamo, S.; Brennan, L.; Lonergan, P.; Fair, T. Predictive value of bovine follicular components as markers of oocyte developmental potential. Reprod. Fertil. Dev. 2014, 26, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Nadaldesbarats, L.; Gérard, N.; Dubois, A.; Holyoake, P.K.; Grupen, C.G. Differences in the metabolomic signatures of porcine follicular fluid collected from environments associated with good and poor oocyte quality. Reproduction 2013, 146, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Cottell, E.; Gibney, M.J.; Mcauliffe, F.M.; Wingfield, M.; Brennan, L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil. Steril. 2012, 97, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Huhman, D.V.; Sumner, L.W. Mass spectrometry strategies in metabolomics. J. Biol. Chem. 2011, 286, 25435–25442. [Google Scholar] [CrossRef] [PubMed]
- Aretz, I.; Meierhofer, D. Advantages and pitfalls of mass spectrometry Based metabolome profiling in systems biology. Int. J. Mol. Sci. 2016, 17, 632. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, R.; Zamani, P.; Mirhoseini, S.; Ghavi Hossein-Zadeh, N.; Nadri, S. A review on prolificacy genes in sheep. Reprod. Domest. Anim. 2016, 51, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Notter, D.R. Genetic aspects of reproduction in sheep. Reprod. Domest. Anim. 2008, 43, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.H.; Yang, L.G. A review of research progress of FecB gene in Chinese breeds of sheep. Anim. Reprod. Sci. 2009, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Juengel, J.L.; Hudson, N.L.; Whiting, L.; McNatty, K.P. Effects of immunization against bone morphogenetic protein 15 and growth differentiation factor 9 on ovulation rate, fertilization, and pregnancy in ewes. Biol. Reprod. 2004, 70, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Jiang, J.Y.; Orisaka, S.; Kotsuji, F.; Tsang, B.K. Growth differentiation factor 9 promotes rat preantral follicle growth by up-regulating follicular androgen biosynthesis. Endocrinology 2009, 150, 2740–2748. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Laurence, D.; Camille, M.; Julien, S.; Kamila, T.; Philippe, B.; Florent, W.; Jérome, L.; Grégoire, H.; Catherine, V.; Danielle, M. The highly prolific phenotype of Lacaune sheep is associated with an ectopic expression of the B4GALNT2 gene within the ovary. PLoS Genet. 2013, 9, e1003809. [Google Scholar]
- Fiky, Z.A.E.; Hassan, G.M.; Nassar, M.I. Genetic polymorphism of growth differentiation factor 9 (GDF9) gene related to fecundity in two Egyptian sheep breeds. J. Assist. Reprod. Genet. 2017, 34, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Pas, M.F.W.T.; Madsen, O.; Calus, M.P.L.; Smits, M.A. The importance of endophenotypes to evaluate the relationship between genotype and external phenotype. Int. J. Mol. Sci. 2017, 18, 472. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; Delle, P.L.; Simona, C.; Emanuela, M.; Marco, M.; Paolo, R. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar]
- Siu, M.K.Y.; Cheng, C.Y. The blood-follicle barrier (BFB) in disease and in ovarian function. Adv. Exp. Med. Biol. 2012, 763, 186–192. [Google Scholar] [PubMed]
- Campbell, B.K.; Souza, C.J.; Skinner, A.J.; Webb, R.; Baird, D.T. Enhanced response of granulosa and theca cells from sheep carriers of the FecB mutation in vitro to gonadotropins and bone morphogenic protein-2, -4, and -6. Endocrinology 2006, 147, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.E.; Lapolt, P.S.; Yoon, B.S.; Chen, J.Y.; Lu, J.K.; Lyons, K.M. The type I BMP receptor BmprIB is essential for female reproductive function. Proc. Natl. Acad. Sci. USA 2001, 98, 7994–7999. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kumar, V.G.; Manjunatha, B.M.; Gupta, P.S. Biochemical composition of ovine follicular fluid in relation to follicle size. Dev. Growth Differ. 2007, 49, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.; Wang, X.; De, V.M.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 2016, 152, R143–R157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Suttonmcdowall, M.L.; Wang, X.; Sugimura, S.; Thompson, J.G.; Gilchrist, R.B. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016, 31, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Elnashar, S.A.; Goldberg, J.M.; Sharma, R.; Mascha, E.J.; Arrigain, S.; Agarwal, A.; Falcone, T. Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. Gynecol. Endocrinol. 2012, 28, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Palini, S.; Benedetti, S.; Tagliamonte, M.C.; De, S.S.; Primiterra, M.; Polli, V.; Rocchi, P.; Catalani, S.; Battistelli, S.; Canestrari, F. Influence of ovarian stimulation for IVF/ICSI on the antioxidant defence system and relationship to outcome. Reprod. Biomed. Online 2014, 29, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, E.B.; Agarwal, A.; Sharma, R.K.; Izzo, V.M.; Pinotti, J.A.; Joshi, N.J.; Rose, B.I. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil. Steril. 2004, 81, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, G.; Grieco, N.; Di, F.M.; Cappiello, F.; Topo, E.; D’Aniello, E.; Ronsini, S. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum. Reprod. 2007, 22, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, A.; Di, F.M.; Fisher, G.H.; Milone, A.; Seleni, A.; D’Aniello, S.; Perna, A.F.; Ingrosso, D. Occurrence of d-aspartic acid and N-methyl-d-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J. 2000, 14, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Ulloaleal, C.; Valderrama, X.P.; Bogle, O.A.; Adams, G.P.; Ratto, M.H. Nerve growth factor from seminal plasma origin (spβ-NGF) increases CL vascularization and level of mRNA expression of steroidogenic enzymes during the early stage of corpus luteum development in llamas. Theriogenology 2017, 103, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Foroughinia, G.; Fazileh, A.; Eghbalsaied, S. Expression of genes involved in BMP and estrogen signaling and AMPK production can be important factors affecting total number of antral follicles in ewes. Theriogenology 2017, 91, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.C.; Gr, V.D.B.; Bleuming, S.A.; Ballester, I.; Jm, V.D.B.; Keller, J.J.; Offerhaus, G.J.; van Deventer, S.J.; Peppelenbosch, M.P. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology 2004, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Alimi, A.; Weethfeinstein, L.A.; Stettner, A.; Caldera, F.; Weiss, J.M. Juvenile polyposis syndrome. Am. J. Med. Genet. A 2013, 161, 1012–1018. [Google Scholar]
- Allaire, J.M.; Roy, S.A.B.; Ouellet, C.; Lemieux, É.; Jones, C.; Paquet, M.; Boudreau, F.; Perreault, N. Bmp signaling in colonic mesenchyme regulates stromal microenvironment and protects from polyposis initiation. Int. J. Cancer 2016, 138, 2700–2712. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.E.; Ayres, H.; Oliveira, L.G.; Barros, F.F.; Oba, E.; Bicudo, S.D.; Bartlewski, P.M.; Fonseca, J.F.; Vicente, W.R. Effects of season and ovarian status on the outcome of long-term progesterone-based estrus synchronization protocols and ovulatory follicle development in Santa Ines ewes under subtropical conditions. Theriogenology 2015, 85, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.L.; Heath, D.A.; Reader, K.L.; Quirke, L.D.; Hudson, N.L.; Juengel, J.L.; McNatty, K.P. Oocytes in sheep homozygous for a mutation in bone morphogenetic protein receptor 1B express lower mRNA levels of bone morphogenetic protein 15 but not growth differentiation factor 9. Reproduction 2011, 142, 53–61. [Google Scholar] [CrossRef] [PubMed]
- McNatty, K.P.; Lun, S.; Heath, D.A.; Ball, K.; Smith, P.; Hudson, N.L.; Mcdiarmid, J.; Gibb, M.; Henderson, K.M. Differences in ovarian activity between Booroola X Merino ewes which were homozygous, heterozygous and non-carriers of a major gene influencing their ovulation rate. J. Reprod. Fertil. 1986, 77, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; Dehaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Dehaven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010, 2, 9. [Google Scholar] [CrossRef] [PubMed]

| Genotype | ++ | +B | BB |
|---|---|---|---|
| Ovulation rate (Statistics number) | 1.06 ± 0.06 c (16) | 2.29 ± 0.17 b (17) | 3.06 ± 0.26 a (18) |
| Pre-ovulation follicle number (Statistics number) | 1.29 ± 0.10 c (21) | 2.32 ± 0.15 b (19) | 3.00 ± 0.38 a (16) |
| Pre-ovulation follicle diameter (Statistics number) | 7.56 ± 0.52 a (27) | 5.32 ± 0.25 b (31) | 4.20 ± 0.15 c (36) |
| Biochemical Name | Fold of Change | Statistical Values (p-Value) | ||||
|---|---|---|---|---|---|---|
| BB/++ | +B/++ | BB/+B | BB/++ | +B/++ | BB/+B | |
| serine | 1.39 | 1.30 | 1.07 | 0.0572 | 0.0919 | 0.7046 |
| threonine | 1.62 | 1.34 | 1.21 | 0.0333 | 0.0752 | 0.5902 |
| alanine | 1.56 | 1.13 | 1.38 | 0.0174 | 0.7135 | 0.0372 |
| aspartate | 2.39 | 1.67 | 1.43 | 0.0002 | 0.0117 | 0.067 |
| glutamate | 1.45 | 1.15 | 1.27 | 0.0001 | 0.1000 | 0.0065 |
| lysine | 1.53 | 1.92 | 0.79 | 0.0081 | 0.0003 | 0.3325 |
| tyrosine | 1.24 | 1.33 | 0.93 | 0.0471 | 0.0071 | 0.558 |
| tryptophan | 1.25 | 1.16 | 1.08 | 0.0476 | 0.1342 | 0.5157 |
| isoleucine | 1.24 | 1.20 | 1.04 | 0.0293 | 0.0787 | 0.5377 |
| valine | 1.23 | 1.18 | 1.04 | 0.0356 | 0.101 | 0.5185 |
| methionine | 1.20 | 1.25 | 0.95 | 0.1794 | 0.072 | 0.7364 |
| cysteine | 1.99 | 1.55 | 1.28 | 0.0051 | 0.0191 | 0.4534 |
| arginine | 1.28 | 1.22 | 1.05 | 0.009 | 0.0177 | 0.6233 |
| proline | 1.20 | 1.18 | 1.02 | 0.039 | 0.0531 | 0.7566 |
| Biochemical Name | Fold of Change | Statistical Values (p-Value) | ||||
|---|---|---|---|---|---|---|
| BB/++ | +B/++ | BB/+B | BB/++ | +B/++ | BB/+B | |
| glutathione, oxidized (GSSG) | 12.87 | 4.88 | 2.64 | 0.0000 | 0.0001 | 0.0484 |
| cysteine | 1.99 | 1.55 | 1.28 | 0.0051 | 0.0191 | 0.4534 |
| cysteine-glutathione disulfide | 3.98 | 2.61 | 1.53 | 0.0000 | 0.0001 | 0.1558 |
| γ-glutamylalanine | 1.73 | 1.45 | 1.19 | 0.0100 | 0.0944 | 0.2479 |
| γ-glutamylglutamine | 1.40 | 1.28 | 1.10 | 0.0020 | 0.0111 | 0.3621 |
| γ-glutamylisoleucine | 1.20 | 1.48 | 0.81 | 0.1416 | 0.0023 | 0.1261 |
| γ-glutamylleucine | 1.28 | 1.29 | 0.99 | 0.0508 | 0.0886 | 0.6756 |
| γ-glutamylphenylalanine | 1.22 | 1.53 | 0.80 | 0.2842 | 0.0253 | 0.2896 |
| γ-glutamyltyrosine | 1.28 | 1.41 | 0.91 | 0.0413 | 0.0093 | 0.6723 |
| 2-hydroxybutyrate (AHB) | 0.64 | 0.62 | 1.03 | 0.0082 | 0.0008 | 0.5389 |
| 5-oxoproline | 0.81 | 0.89 | 0.91 | 0.0286 | 0.1369 | 0.3734 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, X.; Di, R.; Liu, Q.; Hu, W.; He, X.; Yu, J.; Zhang, X.; Zhang, J.; Broniowska, K.; et al. Metabolic Effects of FecB Gene on Follicular Fluid and Ovarian Vein Serum in Sheep (Ovis aries). Int. J. Mol. Sci. 2018, 19, 539. https://doi.org/10.3390/ijms19020539
Guo X, Wang X, Di R, Liu Q, Hu W, He X, Yu J, Zhang X, Zhang J, Broniowska K, et al. Metabolic Effects of FecB Gene on Follicular Fluid and Ovarian Vein Serum in Sheep (Ovis aries). International Journal of Molecular Sciences. 2018; 19(2):539. https://doi.org/10.3390/ijms19020539
Chicago/Turabian StyleGuo, Xiaofei, Xiangyu Wang, Ran Di, Qiuyue Liu, Wenping Hu, Xiaoyun He, Jiarui Yu, Xiaosheng Zhang, Jinlong Zhang, Katarzyna Broniowska, and et al. 2018. "Metabolic Effects of FecB Gene on Follicular Fluid and Ovarian Vein Serum in Sheep (Ovis aries)" International Journal of Molecular Sciences 19, no. 2: 539. https://doi.org/10.3390/ijms19020539





