Comparative Membrane-Associated Proteomics of Three Different Immune Reactions in Potato
Abstract
:1. Introduction
2. Results and Discussion
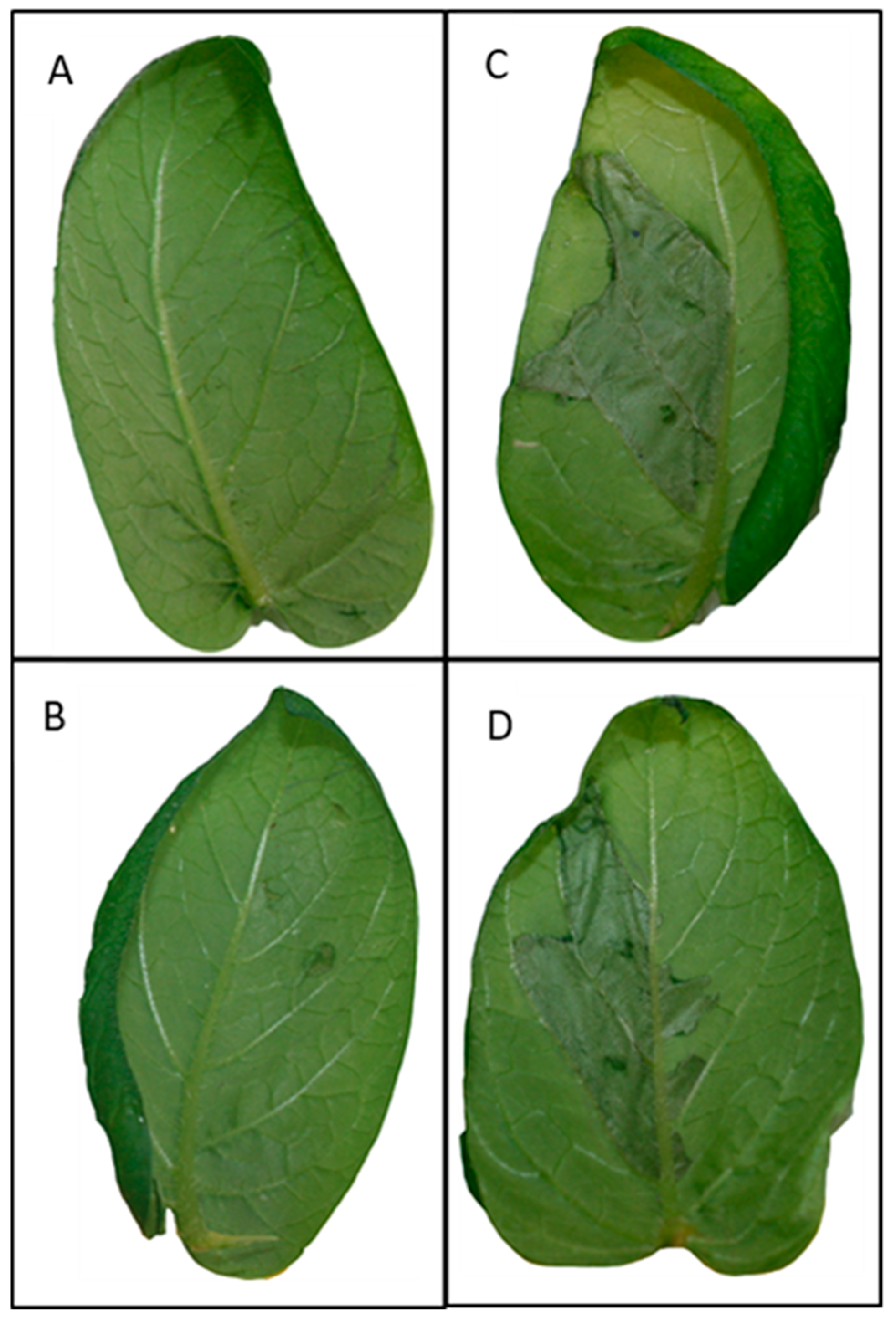
2.1. Phenotypes of PAMP-Triggered Immunity (PTI) and Effector-Triggered Immunity (ETI) Responses
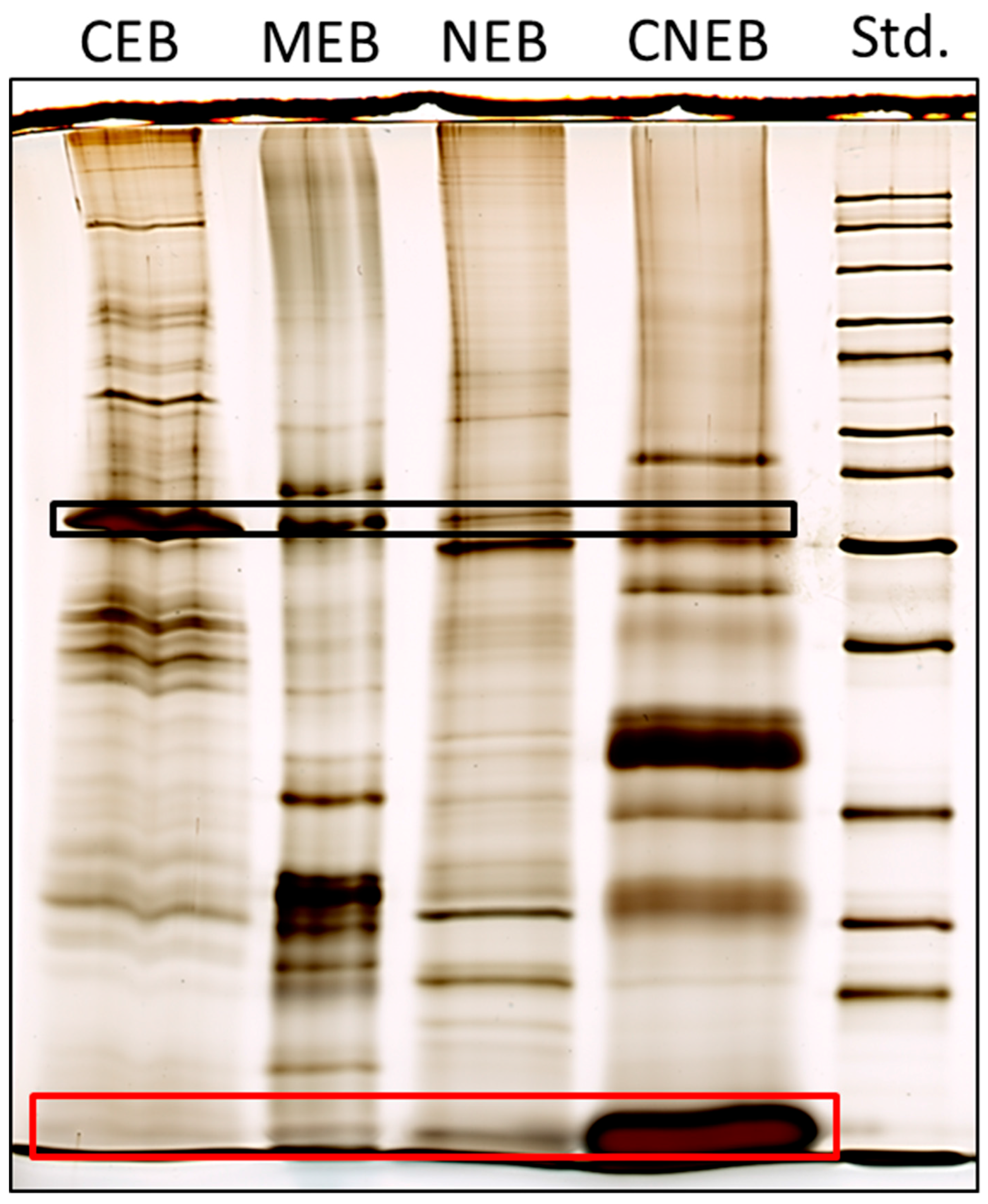
2.2. Subcellular Protein Fractionation
2.3. Membrane-Associated Proteins in the PTI Response
2.4. Proteins in ETI Responses
3. Materials and Methods
3.1. Plants and Agrobacterium Inoculation
3.2. Subcellular Protein Fractionation
3.3. Protein Concentration Determination
3.4. Silver Staining
3.5. Tryptic Digestion and Mass Spectrometry
3.6. Data Analysis
3.7. Plant Material
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PTI | PAMP triggered immunity |
| ETI | Effector triggered immunity |
References
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (pti). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011, 18, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.; Nürnberger, T.; Joosten, M.H. Of pamps and effectors: The blurred pti-eti dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Fawke, S.; Doumane, M.; Schornack, S. Oomycete interactions with plants: Infection strategies and resistance principles. Microbiol. Mol. Biol. Rev. 2015, 79, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Verzaux, E.; Chaparro-Garcia, A.; Bijsterbosch, G.; Keizer, L.P.; Zhou, J.; Liebrand, T.W.; Xie, C.; Govers, F.; Robatzek, S. Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nat. Plants 2015, 1, 15034. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Pombo, M.A.; Zheng, Y.; Fernandez-Pozo, N.; Dunham, D.M.; Fei, Z.; Martin, G.B. Transcriptomic analysis reveals tomato genes whose expression is induced specifically during effector-triggered immunity and identifies the epk1 protein kinase which is required for the host response to three bacterial effector proteins. Genome Biol. 2014, 15, 492. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Kamoun, S.; Zody, M.C.; Jiang, R.H.Y.; Handsaker, R.E.; Cano, L.M.; Grabherr, M.; Kodira, C.D.; Raffaele, S.; Torto-Alalibo, T.; et al. Genome sequence and analysis of the irish potato famine pathogen Phytophthora infestans. Nature 2009, 461, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Vleeshouwers, V.G.A.A.; Rietman, H.; Krenek, P.; Champouret, N.; Young, C.; Oh, S.-K.; Wang, M.; Bouwmeester, K.; Vosman, B.; Visser, R.G.F.; et al. Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS ONE 2008, 3, e2875. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.A.; Park, T.-H.; van Arkel, G.; Arens, M.; Ruyter-Spira, C.; Morales, J.; Whisson, S.C.; Birch, P.R.J.; Visser, R.G.F.; Jacobsen, E.; et al. Exploiting knowledge of r/avr genes to rapidly clone a new lz-nbs-lrr family of late blight resistance genes from potato linkage group iv. Mol. Plant-Microbe Interact. 2009, 22, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Lenman, M.; Ali, A.; Mühlenbock, P.; Carlson-Nilsson, U.; Liljeroth, E.; Champouret, N.; Vleeshouwers, V.A.A.; Andreasson, E. Effector-driven marker development and cloning of resistance genes against Phytophthora infestans in potato breeding clone sw93-1015. Theor. Appl. Genet. 2015, 129, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, E.M.; Breen, S.; Whisson, S.C.; Squires, J.; Hein, I.; Kaczmarek, M.; Turnbull, D.; Boevink, P.C.; Lokossou, A.; Cano, L.M.; et al. Presence/absence, differential expression and sequence polymorphisms between PIAVR2 and PIAVR2-like in Phytophthora infestans determine virulence on R2 plants. New Phytol. 2011, 191, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.G.; Breen, S.; Win, J.; Schornack, S.; Hein, I.; Bozkurt, T.O.; Champouret, N.; Vleeshouwers, V.G.; Birch, P.R.; Gilroy, E.M. Host protein bsl1 associates with Phytophthora infestans rxlr effector AVR2 and the solanum demissum immune receptor R2 to mediate disease resistance. Plant Cell 2012, 24, 3420–3434. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.-X.; Sun, Y.; Burlingame, A.L.; Wang, Z.-Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Schwanhausser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Alexandersson, E.; Sandin, M.; Resjö, S.; Lenman, M.; Hedley, P.; Levander, F.; Andreasson, E. Quantitative proteomics and transcriptomics of potato in response to Phytophthora infestans in compatible and incompatible interactions. BMC Genom. 2014, 15, 497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 3, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Erbs, G.; Silipo, A.; Aslam, S.; De Castro, C.; Liparoti, V.; Flagiello, A.; Pucci, P.; Lanzetta, R.; Parrilli, M.; Molinaro, A.; et al. Peptidoglycan and muropeptides from pathogens agrobacterium and xanthomonas elicit plant innate immunity: Structure and activity. Chem. Biol. 2008, 15, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, S.; Velásquez, A.C.; Ekengren, S.K.; Collmer, A.; Martin, G.B. Identification of Nicotiana benthamiana genes involved in pathogen-associated molecular pattern-triggered immunity. Mol. Plant-Microbe Interact. 2010, 23, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Vences-Guzmán, M.Á.; Guan, Z.; Bermúdez-Barrientos, J.R.; Geiger, O.; Sohlenkamp, C. Agrobacteria lacking ornithine lipids induce more rapid tumour formation. Environ. Microbiol. 2013, 15, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Bennett, M.H.; Forcat, S.; Huang, W.E.; Preston, G.M. Agroinfiltration reduces ABA levels and suppresses Pseudomonas syringae-elicited salicylic acid production in Nicotiana tabacum. PLoS ONE 2010, 29, e8977. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.; Goulet, C.; Goulet, M.-C.; Michaud, D. 2-DE proteomemaps for the leaf apoplast of Nicotiana benthamiana. Proteomics 2010, 10, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cox, A.M.; Kearney, C.M. Pathogenesis-related proteins induced by agroinoculation-associated cell wall weakening can be obviated by spray-on inoculation or mannitol ex vivo culture. Plant Biotechnol. Rep. 2017, 11, 1–9. [Google Scholar] [CrossRef]
- Feller, U.; Anders, I.; Mae, T. Rubiscolytics: Fate of rubisco after its enzymatic function in a cell is terminated. J. Exp. Bot. 2008, 59, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Attaran, E.; Major, I.T.; Cruz, J.A.; Rosa, B.A.; Koo, A.J.; Chen, J.; Kramer, D.M.; He, S.Y.; Howe, G.A. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiol. 2014, 165, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Charron, J.-B.F.; Ouellet, F.; Pelletier, M.; Danyluk, J.; Chauve, C.; Sarhan, F. Identification, expression, and evolutionary analyses of plant lipocalins. Plant Physiol. 2005, 139, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Charron, J.-B.F.; Ouellet, F.; Houde, M.; Sarhan, F. The plant apolipoprotein d ortholog protects arabidopsis against oxidative stress. BMC Plant Biol. 2008, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Bhatt, I.; Dietz, K.-J. Peroxiredoxins: A less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 2009, 235, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xun, Q.; Guo, Y.; Zhang, J.; Cheng, K.; Shi, T.; He, K.; Hou, S.; Gou, X.; Li, J. Genome-wide expression pattern analyses of the arabidopsis leucine-rich repeat receptor-like kinases. Mol. Plant 2015, 9, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Thomas, V.; Bennett, M.H.; Mansfield, J.; Grant, M. Modifications to the arabidopsis defense proteome occur prior to significant transcriptional change in response to inoculation with pseudomonas syringae. Plant Physiol. 2006, 142, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Eitas, T.K.; Nimchuk, Z.L.; Dangl, J.L. Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. USA 2008, 105, 6475–6480. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G. Analysis of the roles of the arabidopsis peroxisomal isocitrate dehydrogenase in leaf metabolism and oxidative stress. Environ. Exp. Bot. 2015, 114, 22–29. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class iii peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Van Aubel, G.; Buonatesta, R.; Van Cutsem, P. Cos-oga, a new oligosaccharidic elicitor that induces protection against a wide range of plant pathogens. IOBC-WPRS Bull. 2013, 89, 403–407. [Google Scholar]
- Ledoux, Q.; Van Cutsem, P.; Markό, I.E.; Veys, P. Specific localization and measurement of hydrogen peroxide in arabidopsis thaliana cell suspensions and protoplasts elicited by cos-oga. Plant Signal. Behav. 2014, 9, e28824. [Google Scholar] [CrossRef] [PubMed]
- Manara, A.; DalCorso, G.; Leister, D.; Jahns, P.; Baldan, B.; Furini, A. Atsia1 and atosa1: Two abc1 proteins involved in oxidative stress responses and iron distribution within chloroplasts. New Phytol. 2014, 201, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (sods) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Trusov, Y.; Botella, J. New faces in plant innate immunity: Heterotrimeric g proteins. J. Plant Biochem. Biotechnol. 2012, 21, 40–47. [Google Scholar] [CrossRef]
- Canonne, J.; Froidure-Nicolas, S.; Rivas, S. Phospholipases in action during plant defense signaling. Plant Signal. Behav. 2011, 6, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Marondedze, C.; Turek, I.; Parrott, B.; Thomas, L.; Jankovic, B.; Lilley, K.S.; Gehring, C. Structural and functional characteristics of cgmp-dependent methionine oxidation in arabidopsis thaliana proteins. Cell Commun. Signal. 2013, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. Ros signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Abreha, K.B.; Alexandersson, E.; Vossen, J.H.; Anderson, P.; Andreasson, E. Inoculation of transgenic resistant potato by Phytophthora infestans affects host plant choice of a generalist moth. PLoS ONE 2015, 10, e0129815. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vossen, E.; Sikkema, A.; Hekkert, B.T.L.; Gros, J.; Stevens, P.; Muskens, M.; Wouters, D.; Pereira, A.; Stiekema, W.; Allefs, S. An ancient r gene from the wild potato species solanum bulbocastanum confers broad-spectrum resistance to Phytophthora infestans in cultivated potato and tomato. Plant J. 2003, 36, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Rietman, H.; Vleeshouwers, V.G.A.A. Agroinfiltration and pvx agroinfection in potato and nicotiana benthamiana. J. Vis. Exp. JoVE 2014, e50971. [Google Scholar] [CrossRef] [PubMed]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Chawade, A.; Alexandersson, E.; Bengtsson, T.; Andreasson, E.; Levander, F. Targeted proteomics approach for precision plant breeding. J. Proteome Res. 2016, 15, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. Proteowizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, J.; Vincic, G.; Månsson, O.; Wårell, K.; Levander, F. The proteios software environment: An extensible multiuser platform for management and analysis of proteomics data. J. Proteome Res. 2009, 8, 3037–3043. [Google Scholar] [CrossRef] [PubMed]
- Consortium, P.G.S. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar]
- Elias, J.E.; Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Method 2007, 4, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Storey, J.D.; MacCoss, M.J.; Noble, W.S. Assigning significance to peptides identified by tandem mass spectrometry using decoy databases. J. Proteome Res. 2008, 7, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Sandin, M.; Krogh, M.; Hansson, K.; Levander, F. Generic workflow for quality assessment of quantitative label-free lc-ms analysis. Proteomics 2011, 11, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Teleman, J.; Chawade, A.; Sandin, M.; Levander, F.; Malmström, J. Dinosaur: A Refined Open-Source Peptide MS Feature Detector. J. Proteome Res. 2016, 15, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Sandin, M.; Ali, A.; Hansson, K.; Månsson, O.; Andreasson, E.; Resjö, S.; Levander, F. An adaptive alignment algorithm for quality-controlled label-free lc-ms. Mol. Cell. Proteom. 2013, 12, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Limma: Linear Models for Microarray Data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Chawade, A.; Alexandersson, E.; Levander, F. Normalyzer: A tool for rapid evaluation of normalization methods for omics data sets. J. Proteome Res. 2014, 13, 3114–3120. [Google Scholar] [CrossRef] [PubMed]
- Taverner, T.; Karpievitch, Y.V.; Polpitiya, A.D.; Brown, J.N.; Dabney, A.R.; Anderson, G.A.; Smith, R.D. Danter: An extensible r-based tool for quantitative analysis of -omics data. Bioinformatics 2012, 28, 2404–2406. [Google Scholar] [CrossRef] [PubMed]

| Protein ID | Protein Name | Regulation in PTI (log2) | Regulation in ETI-IpiO (log2) | Regulation in ETI-AVR2 (log2) |
|---|---|---|---|---|
| Q9LV84 | ABC transporter-like | 1.36 | 0.56 | 0.79 |
| F4KCG9 | Alternative NAD(P)H-ubiquinone oxidoreductase C1 | 1.34 | 1.32 | 1.06 |
| C0Z355 | AT1G56070 protein | 2.76 | 2.43 | 2.88 |
| PGSC0003DMP400043466 | ATP synthase 24 kDa subunit, mitochondrial | 1.19 | 0.97 | 0.87 |
| P29790 | ATP synthase gamma chain, chloroplastic (F-ATPase) | 4.58 | 4.2 | 4.43 |
| PGSC0003DMP400035579 | ATP synthase subunit b′, chloroplastic | 2.34 | 2.38 | 2.82 |
| PGSC0003DMP400010643 | ATP synthase subunit beta, mitochondrial | 1.03 | 0.48 | 0.65 |
| Q42267 | Carrier protein | 0.96 | 0.35 | 0.41 |
| PGSC0003DMP400005278 | Chaperonin 21 | 1.62 | 1.34 | 1.63 |
| PGSC0003DMP400000640 | Charged multivesicular body protein 2a | 4.53 | 5.03 | 5.57 |
| P07370 | Chlorophyll a-b binding protein 1B, chloroplastic (LHCII type I CAB-1B) | 5.43 | 4.23 | 3.76 |
| PGSC0003DMP400011729 | Conserved gene of unknown function | 2.07 | 1.49 | 1.8 |
| PGSC0003DMP400020125 | Conserved gene of unknown function | 0.81 | 0.73 | 0.48 |
| PGSC0003DMP400026692 | Conserved gene of unknown function | 4.16 | 4.27 | 5.15 |
| PGSC0003DMP400046123 | Conserved gene of unknown function | 1.39 | 0.98 | 1.22 |
| E2FAG4 | COSII_At5g14320 | 1.15 | 1.2 | 1.73 |
| Q9ZWH9 | Elongation factor 1-α | 0.78 | 0.35 | 0.48 |
| Q43775 | Glycolate oxidase (EC 1.1.3.15) | 1.01 | 0.18 | 0.67 |
| PGSC0003DMP400009092 | Glyoxisomal malate dehydrogenase | 1.65 | 1.01 | 1.34 |
| Q9LLE0 | Hexose transporter | 0.7 | 0.15 | 0.24 |
| PGSC0003DMP400035078 | Hydrolase, acting on ester bonds | 1.91 | 2.36 | 2.58 |
| B2D2G3 | Hydroxypyruvate reductase (EC 1.1.1.81) | 1.25 | 0.55 | 0.88 |
| B9JNE9 | Insertion sequence transposase protein | 2.02 | 2.43 | 2.78 |
| Q9ZU46 | Leucine-rich repeat receptor-like protein kinase | 0.67 | 0.71 | 1.12 |
| B3H4K6 | Magnesium protoporphyrin IX methyltransferase, chloroplastic | 1.39 | 1.3 | 1.48 |
| A8MQK3 | Malate dehydrogenase (EC 1.1.1.37) | 1.5 | 0.66 | 0.91 |
| PGSC0003DMP400004574 | MAR-binding filament 1 | 0.67 | 0.41 | −0.14 |
| PGSC0003DMP400020545 | NAD-malate dehydrogenase | 1.26 | 0.7 | 0.92 |
| PGSC0003DMP400002176 | Nucleolin | 1.06 | 0.33 | 1.15 |
| PGSC0003DMP400030492 | Oligopeptidase | 0.84 | 0.76 | 1.14 |
| Q9LYJ5 | Pectin lyase-like superfamily protein (Polygalacturonase-like protein) | 0.58 | 0.37 | 0.62 |
| PGSC0003DMP400001052 | Peptidyl-prolyl cis–trans isomerase | 0.82 | 0.73 | 0.47 |
| PGSC0003DMP400026173 | Peroxidase | 5.45 | 5.21 | 6.28 |
| PGSC0003DMP400013804 | Photosystem II D2 protein | 2.67 | 2.82 | 2.8 |
| PGSC0003DMP400002084 | Protein translocase subunit secA | 0.9 | 0.45 | 0.84 |
| Q30GS3 | Putative ferredoxin NADP reductase | 2.05 | 1.72 | 2.18 |
| Q38M64 | Putative uncharacterized protein | 1.11 | 1.18 | 1.39 |
| Q0WPJ1 | Putative uncharacterized protein similar to At1g65260 | 0.73 | 0.5 | 0.61 |
| Q7FIJ2 | Putative uncharacterized protein similar to AT4g09410 | 1.23 | 1.28 | 1.16 |
| Q9LMI1 | Ribosomal protein L1p/L10e family (T2D23.8 protein) | 5.74 | 5.77 | 5.61 |
| PGSC0003DMP400060292 | Saccharopine dehydrogenase family protein | 1.35 | 1.57 | 1.31 |
| PGSC0003DMP400000754 | Signal peptidase I | 0.63 | 0.43 | 0.29 |
| PGSC0003DMP400029941 | Succinic semialdehyde dehydrogenase | 1.61 | 0.46 | 0.88 |
| A7LKN1 | TAO1 | 1.28 | 1.16 | 1.26 |
| PGSC0003DMP400012430 | Transketolase 1 | 1.25 | 1.33 | 1.18 |
| PGSC0003DMP400042799 | Translationally-controlled tumor protein homolog | 1.71 | 1.95 | 2.36 |
| Q8LG76 | Zinc finger protein CONSTANS-LIKE 6 | 1.52 | 0.83 | 1.03 |
| Protein ID | Protein Name | Degree of Regulation in ETI-IpiO (log2) | Degree of Regulation in ETI-AVR2 (log2) |
|---|---|---|---|
| PGSC0003DMP400026606 | 2-deoxyglucose-6-phosphate phosphatase | 1.07 | 1.57 |
| PGSC0003DMP400026060 | 3-β hydroxysteroid dehydrogenase/isomerase family protein | 0.83 | 0.91 |
| PGSC0003DMP400002234 | 30S ribosomal protein S1, chloroplastic | 0.45 | 0.28 |
| PGSC0003DMP400021930 | 30S ribosomal protein S20 | 0.6 | 0.5 |
| Q2MI62 | 30S ribosomal protein S3, chloroplastic | 0.85 | 0.55 |
| PGSC0003DMP400051744 | 30S ribosomal protein S5 | 1.24 | 1.33 |
| P93014 | 30S ribosomal protein S5, chloroplastic | 0.75 | 0.75 |
| Q2MI54 | 30S ribosomal protein S7, chloroplastic | 0.7 | 0.96 |
| Q84P24 | 4-coumarate—CoA ligase-like 6 | 0.95 | 1.07 |
| PGSC0003DMP400008292 | 50S ribosomal protein L18, chloroplast | 0.84 | 0.79 |
| PGSC0003DMP400046774 | 50S ribosomal protein L29, chloroplastic | 0.93 | 1.03 |
| A8MQR4 | 60S acidic ribosomal protein P0 | 0.72 | 1.07 |
| PGSC0003DMP400025031 | Amino acid binding protein | 0.6 | 0.53 |
| B9DI38 | AT1G05190 protein | 0.81 | 0.7 |
| Q1H555 | At3g11510 | 1.02 | 1.37 |
| Q2MIJ9 | ATP synthase subunit a, chloroplastic (F-ATPase subunit IV) | 0.77 | 1.08 |
| Q2MIB4 | ATP synthase subunit b, chloroplastic (ATPase subunit I) | 0.77 | 1.19 |
| Q9XF89 | Chlorophyll a-b binding protein CP26, chloroplastic (LHCB5) (LHCIIc) | 0.71 | 0.6 |
| PGSC0003DMP400002042 | Chloroplast lipocalin | 1.08 | 0.92 |
| A7XZB8 | Chloroplast-localized protein | 0.67 | 0.76 |
| PGSC0003DMP400067062 | Conserved gene of unknown function | 0.59 | 0.37 |
| PGSC0003DMP400008394 | Conserved gene of unknown function | 0.53 | 0.7 |
| Q2MI70 | Cytochrome b6-f complex subunit 4 (17 kDa polypeptide) | 0.29 | 0.53 |
| Q1H537 | Divinyl chlorophyllide a 8-vinyl-reductase, chloroplastic (EC 1.3.1.75) | 0.55 | 0.57 |
| Q3HVL1 | Elongation factor-like protein | 0.44 | 0.84 |
| PGSC0003DMP400027216 | Ethylene-responsive small GTP-binding protein | 0.36 | 0.58 |
| PGSC0003DMP400045639 | FKBP-type peptidyl-prolyl cis-trans isomerase 3, chloroplastic | 0.75 | 1.02 |
| P400068995 | Glucose-1-phosphate adenylyltransferase | 0.83 | 0.7 |
| PGSC0003DMP400051213 | Glyceraldehyde-3-phosphate dehydrogenase B subunit | 1.27 | 1.66 |
| PGSC0003DMP400048842 | GrpE protein homolog | 1.01 | 0.72 |
| Q8VZ74 | GTP-binding protein Era (GTP-binding protein-like) | 1.88 | 1.73 |
| PGSC0003DMP400000783 | Heat shock protein 70-3 | 0.83 | 1.19 |
| PGSC0003DMP400030419 | Heterogeneous nuclear ribonucleoprotein A1 | 0.84 | 1.06 |
| PGSC0003DMP400026401 | Immunophilin | 1.4 | 1.56 |
| PGSC0003DMP400046332 | Isoform 2 of PsbP 2, chloroplastic | 0.36 | 0.67 |
| PGSC0003DMP400029178 | NADH dehydrogenase | 0.56 | 0.5 |
| PGSC0003DMP400026922 | NADPH:protochlorophyllide oxidoreductase | 0.59 | 1.97 |
| Q3LG51 | Nitrite reductase | 0.97 | 0.74 |
| PGSC0003DMP400034084 | OJ991214_12.13 protein | 0.19 | 0.82 |
| PGSC0003DMP400025362 | Oxygen-evolving enhancer protein 3-1, chloroplast | 0.93 | 1.33 |
| PGSC0003DMP400015048 | Peptidyl-prolyl cis–trans isomerase | 1.05 | 0.22 |
| Q9SR70 | Peptidyl-prolyl cis–trans isomerase FKBP16-4, chloroplastic | 0.87 | 0.89 |
| PGSC0003DMP400018067 | Phospholipase A1 | 0.41 | 1.09 |
| PGSC0003DMP400048121 | Photosystem I subunit XI | 0.54 | 1.07 |
| P06183 | Photosystem II 10 kDa polypeptide, chloroplastic (Light-inducible tissue-specific ST-LS1 protein) | 1.65 | 1.83 |
| PGSC0003DMP400040949 | Plastid-lipid-associated protein 13, chloroplastic | 0.71 | 0.76 |
| C7ENV4 | Polyubiquitin | 0.57 | 0.87 |
| Q7XAB8 | Protein THYLAKOID FORMATION1, chloroplastic | 0.6 | 0.87 |
| Q8S9G3 | Putative 16 kDa membrane protein | 0.73 | 0.95 |
| Q9SN01 | Putative uncharacterized protein AT4g33080 | 0.53 | 0.72 |
| Q9FR30 | Ripening regulated protein DDTFR10 | 0.96 | 1.35 |
| PGSC0003DMP400000966 | Serine-type peptidase | 0.54 | 0.29 |
| PGSC0003DMP400011690 | Serine/threonine protein kinase | 1.28 | 0.98 |
| PGSC0003DMP400012365 | Structural constituent of ribosome | 0.56 | 0.58 |
| PGSC0003DMP400047959 | Superoxide dismutase | 1.13 | 2.22 |
| PGSC0003DMP400009317 | Superoxide dismutase | 0.87 | 0.7 |
| PGSC0003DMP400016292 | Tetratricopeptide repeat protein, tpr | 0.09 | 0.59 |
| PGSC0003DMP400032278 | Thylakoid lumenal 17.4 kDa protein | 0.86 | 0.78 |
| PGSC0003DMP400014505 | Tic62 protein | 0.74 | 0.79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burra, D.D.; Lenman, M.; Levander, F.; Resjö, S.; Andreasson, E. Comparative Membrane-Associated Proteomics of Three Different Immune Reactions in Potato. Int. J. Mol. Sci. 2018, 19, 538. https://doi.org/10.3390/ijms19020538
Burra DD, Lenman M, Levander F, Resjö S, Andreasson E. Comparative Membrane-Associated Proteomics of Three Different Immune Reactions in Potato. International Journal of Molecular Sciences. 2018; 19(2):538. https://doi.org/10.3390/ijms19020538
Chicago/Turabian StyleBurra, Dharani Dhar, Marit Lenman, Fredrik Levander, Svante Resjö, and Erik Andreasson. 2018. "Comparative Membrane-Associated Proteomics of Three Different Immune Reactions in Potato" International Journal of Molecular Sciences 19, no. 2: 538. https://doi.org/10.3390/ijms19020538





