Influence of Graded Levels of l-Theanine Dietary Supplementation on Growth Performance, Carcass Traits, Meat Quality, Organs Histomorphometry, Blood Chemistry and Immune Response of Broiler Chickens
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Performance
2.2. Carcass Traits
2.3. Meat Quality
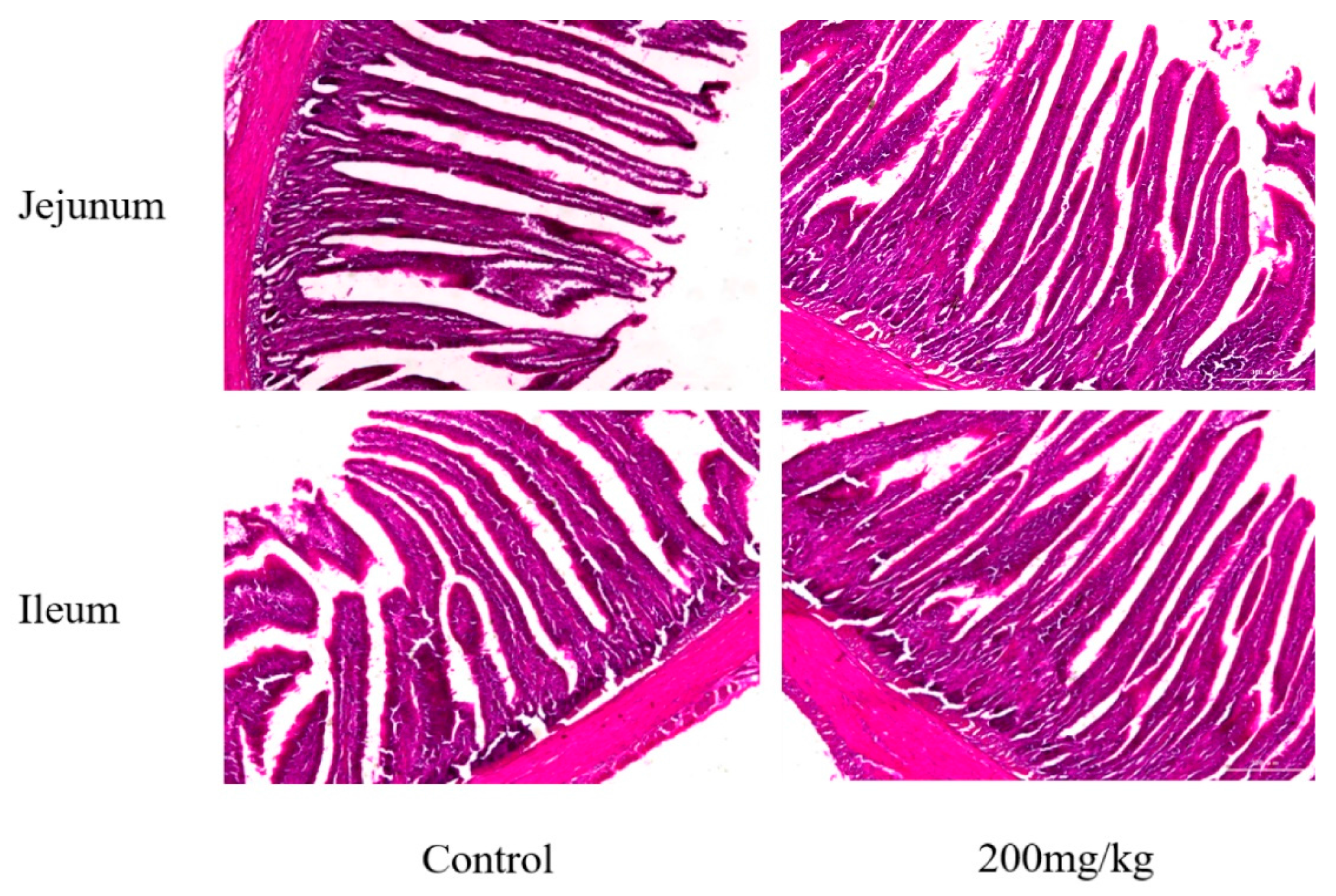
2.4. Mucosal Histomorphometry of Small Intestine
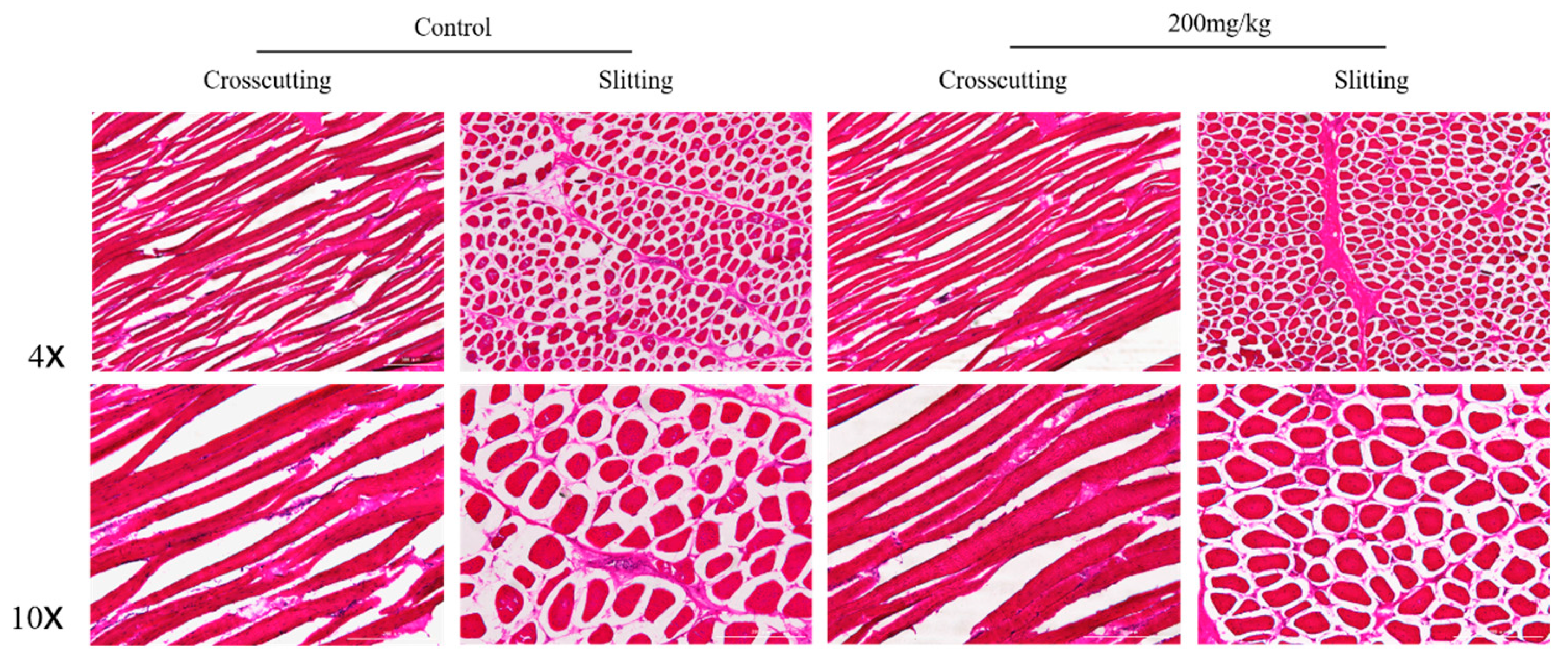
2.5. Histomorphometry of Pectoral and Gastrocnemius Muscles
2.6. Effect on Blood Chemistry
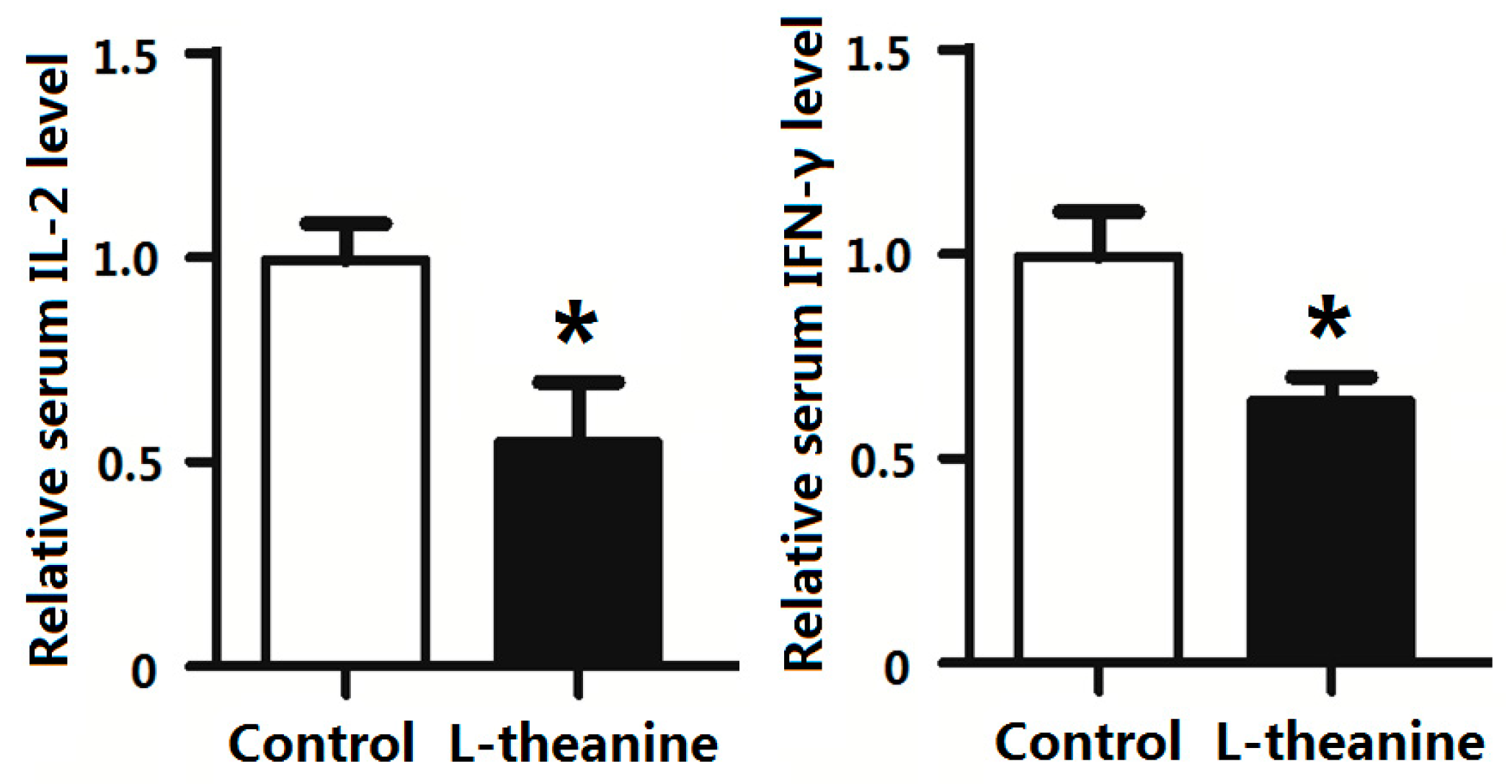
2.7. Immune Response
3. Materials and Methods
3.1. Ethical Statement
3.2. Design and Feeding Management
3.3. Data Collection
3.4. Carcass Traits
3.5. Meat Quality Assessment
3.6. Immune Response and Antioxidant Status
3.7. Blood Chemistry
3.8. The Histomorphometry of Small Intestinal Mucosa
3.9. The Histomorphometry of Pectoral and Gastrocnemius Muscles
3.10. Statistical Analysis
4. Conclusions and Application
- (1)
- l-theanine is an amino acid (the active component of green tea) which could be utilized as a natural feed additive to replace antibiotics to improve the overall performance of broilers.
- (2)
- l-theanine showed promising effects on the immune response in broilers by decreasing pro-inflammatory cytokines and enhancing the anti-oxidant capacity of the body by increasing levels of antioxidant enzymes.
- (3)
- It was concluded that supplementation of l-theanine up to 200 mg/kg can enhance meat quality (muscle color, pH and water holding capacity), anti-oxidant status, immune response, growth performance and lower total serum cholesterol in chickens.
- (4)
- The level of l-theanine at 200 mg/kg of diet was found optimum and would be advised for getting better immune and performance responses in broilers. Higher dietary levels of l-theanine may pose deleterious effects on performance and other health aspects in birds.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhatti, M. Emerging prospects of poultry production in Pakistan at the dawn of 21st century. Vet. News Views 2011, 6, 24–30. [Google Scholar]
- Dhama, K.; Latheef, S.K.; Mani, S.; Samad, H.A.; Karthik, K.; Tiwari, R.; Khan, R.U.; Alagawany, M.; Farag, M.R.; Alam, G.M. Multiple beneficial applications and modes of action of herbs in poultry health and production—A review. Int. J. Pharmacol. 2015, 11, 152–176. [Google Scholar] [CrossRef]
- Saeed, M.; Baloch, A.R.; Wang, M.; Soomro, R.N.; Baloch, A.M.; Bux, B.A.; Arian, M.A.; Faraz, S.S.; Zakriya, H.M. Use of Cichorium intybus Leaf extracts as a growth promoter, hepatoprotectant and immune modulent in broilers. J. Anim. Prod. Adv. 2015, 5, 585–591. [Google Scholar] [CrossRef]
- Ayssiwede, S.; Dieng, A.; Bello, H.; Chrysostome, C.; Hane, M.; Mankor, A.; Dahouda, M.; Houinato, M.; Hornick, J.; Missohou, A. Effects of Moringa oleifera (Lam.) leaves meal incorporation in diets on growth performances, carcass characteristics and economics results of growing indigenous senegal chicken. Pak. J. Nutr. 2011, 10, 1132–1145. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; Alagawany, M.; Farag, M.R.; Tiwari, R.; Karthik, K.; Dhama, K. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int. J. Pharmacol. 2016, 12, 232–248. [Google Scholar] [CrossRef]
- Al-Basher, G.; Ajarem, J.S.; Allam, A.A.; Mahmoud, A.M. Green tea protects against perinatal nicotine-induced histological, biochemical and hematological alterations in mice off spring. Int. J. Pharmacol. 2017, 13, 109–121. [Google Scholar]
- Lin, X.; Lin, C.-H.; Zhao, T.; Zuo, D.; Ye, Z.; Liu, L.; Lin, M.T. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and antiinflammatory mechanisms. Chem. Biol. Interact. 2017, 265, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Akinloye, O.A.; Somade, O.T.; Akindele, A.S.; Adelabu, K.B.; Elijah, F.T.; Adewumi, O.J. Anticlastogenic and hepatoprotective properties of ginger (Zingiber officinale) extract against nitrobenzene-induced toxicity in rats. Roman. J. Biochem. 2014, 51, 3–15. [Google Scholar]
- Alagawany, M.; El-Hack, M.E.A.; El-Kholy, M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. Pollut. Res. 2016, 23, 6774–6782. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Abd El-Hack, M.; Alagawany, M.; Arain, M.; Arif, M.; Mirza, M.; Naveed, M.; Chao, S.; Sarwar, M.; Sayab, M. Nutritional and Healthical Aspects of Yacon (Smallanthus sonchifolius) for Human, Animals and Poultry. Int. J. Pharmacol. 2017, 13, 361–369. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Abdo, Z.M.; Hassan, R.; El-Salam, A.A.; Helmy, S.A. Effect of adding green tea and its aqueous extract as natural antioxidants to laying hen diet on productive, reproductive performance and egg quality during storage and its content of cholesterol. Egypt. Poult. Sci. J. 2010, 30, 1121–1149. [Google Scholar]
- Kojima, S.; Yoshida, Y. Effects of green tea powder feed supplement on performance of hens in the late stage of laying. Int. J. Poult. Sci. 2008, 7, 491–496. [Google Scholar] [CrossRef]
- Eschenauer, G.; Sweet, B.V. Pharmacology and therapeutic uses of theanine. Am. J. Health Syst. Pharm. 2006, 63, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.W.; Ogita, S.; Ashihara, H. Distribution and biosynthesis of theanine in Theaceae plants. Plant Physiol. Biochem. 2010, 48, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sun, L.; Gou, L.; Ling, X.; Feng, Y.; Wang, L.; Yin, X.; Liu, Y. Protective effect of l-theanine on chronic restraint stress-induced cognitive impairments in mice. Brain Res. 2013, 1503, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Shibahara, S.; Arisaka, H.; Akiyama, Y. Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of l-cystine and l-theanine. Vet. Med. Sci. 2007, 69, 1263–1270. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Yu, H.; Yuan, R.; Yang, Y.; Pung, K.; Li, P.; Xue, L. Physiological effects of l-Theanine on Drosophila melanogaster. Molecules 2013, 18, 13175–13187. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tong, H.; Yan, Q.; Tang, S.; Han, X.; Xiao, W.; Tan, Z. l-Theanine Improves Immunity by Altering TH2/TH1 Cytokine Balance, Brain Neurotransmitters, and Expression of Phospholipase C in Rat Hearts. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 662–669. [Google Scholar] [CrossRef]
- Yin, C.; Gou, L.; Liu, Y.; Yin, X.; Zhang, L.; Jia, G.; Zhuang, X. Antidepressant-like effects of l-theanine in the forced swim and tail suspension tests in mice. Phytother. Res. 2011, 25, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tamano, H.; Suzuki, M.; Sakamoto, K.; Oku, N.; Yokogoshi, H. Unique induction of CA1 LTP components after intake of theanine, an amino acid in tea leaves and its effect on stress response. Cell. Mol. Neurobiol. 2012, 32, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Wei, S.; Zhang, S.; Hou, D.; Xiao, W.; He, X. Effects of l-theanine on performance and immune function of yellow-feathered broilers. Chin. J. Anim. Nutr. 2012, 24, 1946–1954. [Google Scholar]
- Hwang, Y.; Park, B.; Lim, J.; Kim, M.; Song, I.; Park, S.; Jung, H.; Hong, J.; Yun, H. Effects of β-Glucan from Paenibacillus polymyxa and l-theanine on Growth Performance and Immunomodulation in Weanling Piglets. Asian-Australas. J. Anim. Sci. 2008, 21, 1753–1759. [Google Scholar] [CrossRef]
- Williams, J.; Kellett, J.; Roach, P.D.; Mckune, A.; Mellor, D.; Thomas, J.; Naumovski, N. l-theanine as a functional food additive: Its role in disease prevention and health promotion. Beverages 2016, 2, 13. [Google Scholar] [CrossRef]
- Biswas, A.H.; Wakita, M. Effect of dietary Japanese green tea powder supplementation on feed utilization and carcass profiles in broilers. J. Poult. Sci. 2001, 38, 50–57. [Google Scholar] [CrossRef]
- Sarker, M.; Kim, G.; Yang, C. Effect of green tea and biotite on performance, meat quality and organ development in Ross broiler. Egypt. Poult. Sci. J. 2010, 30, 77–88. [Google Scholar]
- Erener, G.; Ocak, N.; Altop, A.; Cankaya, S.; Aksoy, H.M.; Ozturk, E. Growth performance, meat quality and caecal coliform bacteria count of broiler chicks fed diet with green tea extract. Asian-Australas. J. Anim. Sci. 2011, 24, 1128–1135. [Google Scholar] [CrossRef]
- Uuganbayar, D. A Study on the Utilization of Green Tea for Laying Hens and Broiler Chicks. Ph.D. Thesis, Sunchon National University, Suncheon, South Korea, 2004. [Google Scholar]
- Yang, C.; Yang, I.; Oh, D.; Bae, I.; Cho, S.; Kong, I.; Uuganbayar, D.; Nou, I.; Choi, K. Effect of green tea by-product on performance and body composition in broiler chicks. Asian-Australas. J. Anim. Sci. 2003, 16, 867–872. [Google Scholar] [CrossRef]
- Kamath, A.B.; Wang, L.; Das, H.; Li, L.; Reinhold, V.N.; Bukowski, J.F. Antigens in tea-beverage prime human Vγ2Vδ2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc. Natl. Acad. Sci. USA 2003, 100, 6009–6014. [Google Scholar] [CrossRef] [PubMed]
- Afsharmanesh, M.; Sadaghi, B. Effects of dietary alternatives (probiotic, green tea powder, and Kombucha tea) as antimicrobial growth promoters on growth, ileal nutrient digestibility, blood parameters, and immune response of broiler chickens. Comp. Clin. Pathol. 2014, 23, 717–724. [Google Scholar] [CrossRef]
- Bukowski, J.F.; Morita, C.T.; Brenner, M.B. Human cd T cells recognise alkylamines derived from microbes, edible plants, tea: Implications for innate immunity. Immunity 1999, 11, 57–65. [Google Scholar] [CrossRef]
- Zheng, G.; Sayama, K.; Okubo, T.; Juneja, L.R.; Oguni, I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004, 18, 55–62. [Google Scholar] [PubMed]
- Bukowski, J.F.; Percival, S.S. l-theanine intervention enhances human γδ T lymphocyte function. Nutr. Rev. 2008, 66, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complement. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Hiraoka, T.; Akutsu, M.; Sukegawa, E.; Bannai, M.; Shibahara, S. Effects of l-cystine and l-theanine supplementation on the common cold: A randomized, double-blind, and placebo-controlled trial. J. Amin. Acids 2010, 6, 307475. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Kurihara, S.; Higashi, N.; Morikawa, S.; Kase, T.; Maeda, A.; Arisaka, H.; Shibahara, S.; Akiyama, Y. Combined administration of l-cystine and l-theanine enhances immune functions and protects against influenza virus infection in aged mice. J. Vet. Med. Sci. 2010, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D. Broiler breast meat color variation, pH, and texture. Poult. Sci. 1999, 78, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Fletcher, D.; Smith, D.; Northcutt, J. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001, 80, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Stagsted, J.; Jensen, S.K.; Karlsson, A.; Henckel, P. Ascorbic acid, alpha-tocopherol, and oregano supplements reduce stress-induced deterioration of chicken meat quality. Poult. Sci. 2003, 82, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Tong, H.; Tang, S.; Tan, Z.; Han, X.; Zhou, C. l-theanine administration modulates the absorption of dietary nutrients and expression of transporters and receptors in the intestinal mucosa of rats. BioMed Res. Int. 2017, 2017, 9747256. [Google Scholar] [CrossRef] [PubMed]
- Pelícia, K.; Mendes, A.; Saldanha, E.; Pizzolante, C.; Takahashi, S.; Garcia, R.; Moreira, J.; Paz, I.; Quinteiro, R.; Komiyama, C. Probiotic and prebiotic utilization in diets for free-range broiler chickens. Revista Brasileira de Ciência Avícola 2004, 6, 99–104. [Google Scholar] [CrossRef]
- Mosleh, N.; Shomali, T.; Hamedi, S. Effects of green tea on performance, feed conversion and jejunum (histology) in broilers. Online J. Vet. Res. 2011, 15, 147–154. [Google Scholar]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Abdelqader, A.; Al-Fataftah, A.R. Effect of dietary butyric acid on performance, intestinal morphology, microflora composition and intestinal recovery of heat-stressed broilers. Livest. Sci. 2016, 183, 78–83. [Google Scholar] [CrossRef]
- Zhao, D. Review on research status of theanine. Food Sci. 2002, 23, 145–147. [Google Scholar]
- Murakami, S.; Kurihara, S.; Titchenal, C.A.; Ohtani, M. Suppression of exercise-induced neutrophilia and lymphopenia in athletes by cystine/theanine intake: A randomized, double-blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Ariana, M.; Samie, A.; Edriss, M.A.; Jahanian, R. Effects of powder and extract form of green tea and marigold, and-tocopheryl acetate on performance, egg quality and egg yolk cholesterol levels of laying hens in late phase of production. J. Med. Plant Res. 2011, 5, 2710–2716. [Google Scholar]
- Abdel-Azeem, F. Green tea flowers (Camellia sinensis) as natural anti-oxidants feed additives in growing Japanese quail diets. Egypt. Poult. Sci. J. 2005, 25, 569–588. [Google Scholar]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Zheng, G.; Bamba, K.; Okubo, T.; Raj Juneja, L.; Oguni, I.; Sayama, K. Effect of theanine, γ-glutamylethylamide, on bodyweight and fat accumulation in mice. Anim. Sci. J. 2005, 76, 153–157. [Google Scholar] [CrossRef]
- El-Deek, A.; Al-Harthi, M. Responses of modern broiler chicks to stocking density, green tea, commercial multi-enzymes and their interactions on productive performance, carcass characteristics, liver composition and plasma constituents. Int. J. Poult. Sci. 2004, 3, 635–645. [Google Scholar]
- Xu, X.; Hu, Y.; Xiao, W.; Huang, J.A.; He, X.; Wu, J.; Ryan, E.P.; Weir, T.L. Effects of fermented Camilla sinensis, Fuzhuan tea, on egg cholesterol and production performance in laying hens. Agric. Food Sci. 2012, 1, 6–10. [Google Scholar]
- Ahmad, R.S.; Butt, M.S.; Sultan, M.T.; Mushtaq, Z.; Ahmad, S.; Dewanjee, S.; de Feo, V.; Zia-Ul-Haq, M. Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J. Transl. Med. 2015, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, S.; Butt, M.; Suleria, H.; Iqbal, M. The role of green tea extract and powder in mitigating metabolic syndromes with special reference to hyperglycemia and hypercholesterolemia. Food Funct. 2014, 5, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.I.; Sang, K.N. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Tayal, V.; Kalra, B.S. Cytokines and anti-cytokines as therapeutics—An update. Eur. J. Pharmacol. 2008, 579, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ye, Y.; Kang, J.; Yao, X.; Zhang, Y.; Jiang, W.; Gao, M.; Dai, Y.; Xin, Y.; Wang, Q.; et al. l-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem. Toxicol. 2012, 50, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gao, M.; Sun, S.; Bi, A.; Xin, Y.; Han, X. Protective effect of l-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem. Biophys. Res. Commun. 2012, 422, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Pi, Z.; Wu, Y.; Liu, J. Effect of pretreatment and pelletization on nutritive value of rice straw-based total mixed ration, and growth performance and meat quality of growing Boer goats fed on TMR. Small Rumin. Res. 2005, 56, 81–88. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, H.; Gan, L.; Xu, Y.; Feng, F.; Saeed, M.; Sun, C. Reducing Smad3/ATF4 was essential for Sirt1 inhibiting ER stress-induced apoptosis in mice brown adipose tissue. Oncotarget 2017, 8, 9267–9279. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, A.; Cheema, A.; Younus, M.; Aslam, A.; Zaman, M.; Rehman, T. Histopathological and serological studies on paratuberculosis in cattle and buffaloes. Pak. Vet. J. 2012, 32, 547–551. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Zaneb, H.; Yousaf, M.S.; Ijaz, A.; Sohail, M.U.; Muti, S. Effect of dietary supplementation of prebiotics and probiotics on intestinal microarchitecture in broilers reared under cyclic heat stress. J. Anim. Physiol. Anim. Nutr. 2013, 1, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kiczorowska, B.; Al-Yasiry, A.; Samolińska, W.; Marek, A.; Pyzik, E. The effect of dietary supplementation of the broiler chicken diet with Boswellia serrata resin on growth performance, digestibility, and gastrointestinal characteristics, morphology, and microbiota. Livest. Sci. 2016, 191, 117–124. [Google Scholar] [CrossRef]
- Heywood, J.; Mcentee, G.; Stickland, N. In ovo neuromuscular stimulation alters the skeletal muscle phenotype of the chick. J. Muscle Res. Cell Motil. 2005, 26, 49–56. [Google Scholar] [CrossRef] [PubMed]

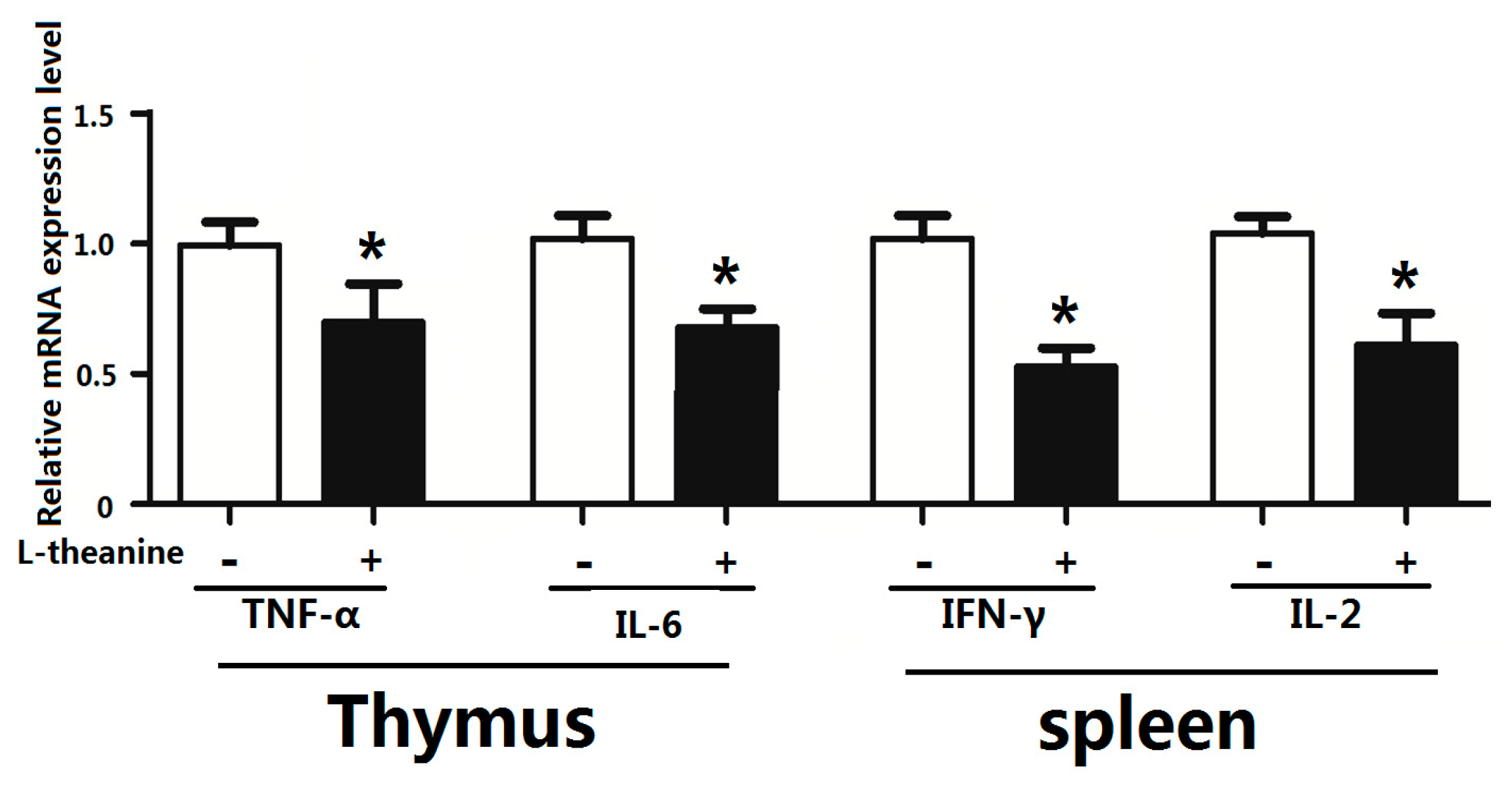
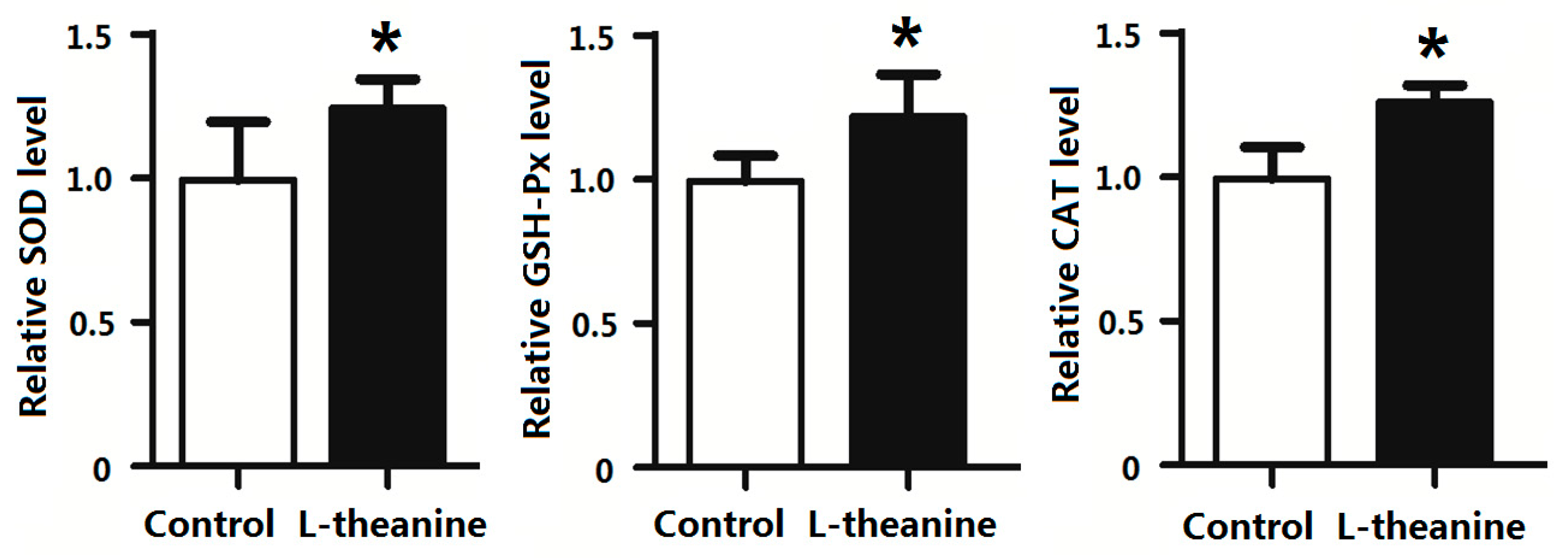
| Items | C | 100LT | 200LT | 300LT | SEM | p Value |
|---|---|---|---|---|---|---|
| 1–3 week of age | ||||||
| BWG | 922.85 b | 998.84 b | 1131.0 a | 905.78 b | 1.98 | 0.002 |
| FC | 1620.0 bc | 1566.0 c | 1757.0 a | 1690.1 ab | 1.51 | 0.015 |
| FCR | 1.7625 a | 1.5759 b | 1.5547 b | 1.8660 a | 0.47 | 0.002 |
| 3–6 week of age | ||||||
| BWG | 1423.2 c | 1511.4 bc | 1700.8 a | 1596.2 ab | 1.85 | 0.007 |
| FC | 2571.5 b | 2626.6 ab | 2750.0 a | 2680.0 ab | 1.74 | 0.121 |
| FCR | 1.8111 a | 1.7452 ab | 1.6179 b | 1.6842 ab | 0.51 | 0.185 |
| 1–6 week of age | ||||||
| BWG | 2546.1 c | 2710.3 b | 2931.8 a | 2702.0 b | 1.58 | 0.001 |
| FC | 4191.5 b | 4192.6 b | 4507.0 a | 4370.1 ab | 1.24 | 0.008 |
| FCR | 1.6462 a | 1.5469 bc | 1.5372 c | 1.6173 ab | 0.24 | 0.002 |
| Items (g) | C | 100LT | 200LT | 300LT | SEM | p Value |
|---|---|---|---|---|---|---|
| Live weight | 2525.0 d | 2810.0 b | 2925.0 a | 2650.0 c | 15.32 | 0.021 |
| Slaughter weight | 2478.8 | 2805.0 | 2760.0 | 2516.3 | 14.25 | 0.654 |
| Semi-eviscerated weight | 2350.0 | 2630.0 | 2611.3 | 2531.3 | 25.65 | 0.248 |
| Eviscerated weight | 2028.0 b | 2286.0 a | 2289.0 a | 2205.0 c | 16.35 | 0.010 |
| Leg muscles | 191.25 | 213.75 | 212.50 | 212.25 | 1.65 | 0.398 |
| Breast muscles | 306.25 | 343.75 | 368.75 | 337.50 | 4.35 | 0.214 |
| Abdominal fat | 67.60 | 70.44 | 70.80 | 64.43 | 1.35 | 0.895 |
| Liver | 35.23 | 36.07 | 35.21 | 35.10 | 2.35 | 0.542 |
| Gizzard | 16.63 b | 17.88 ab | 20.48 a | 17.10 b | 1.22 | 0.003 |
| Heart | 7.65 | 7.84 | 8.23 | 7.69 | 0.98 | 0.879 |
| Thymus | 1.77 | 2.72 | 2.06 | 2.39 | 0.42 | 0.845 |
| Spleen | 1.11 b | 1.37 ab | 1.66 a | 1.31 ab | 0.19 | 0.042 |
| Bursa | 1.32 c | 2.73 ab | 2.83 a | 1.98 bc | 0.15 | 0.021 |
| Items (g) | C | 100LT | 200LT | 300LT | SEM | p Value | |
|---|---|---|---|---|---|---|---|
| BMC | L* | 50.65 ab | 49.43 ab | 47.36 b | 52.59 a | 0.15 | 0.031 |
| a* | 5.74 c | 7.35 b | 8.68 a | 6.22 b | 0.11 | 0.036 | |
| b* | 18.19 c | 19.92 ab | 19.00 bc | 21.25 a | 0.01 | 0.025 | |
| BM pH | 45 min | 6.89 a | 6.64 b | 6.64 b | 6.68 b | 0.02 | 0.040 |
| 24 h | 6.39 | 6.37 | 6.48 | 6.48 | 0.04 | 0.065 | |
| TMC | L* | 65.48 a | 53.55 bc | 50.16 c | 58.79 b | 0.11 | 0.043 |
| a* | 5.48 b | 6.39 ab | 6.94 a | 5.78 b | 0.05 | 0.036 | |
| b* | 18.56 | 19.39 | 20.26 | 19.86 | 0.51 | 0.276 | |
| TM pH | 45 min | 6.76 a | 6.49 c | 6.63 b | 6.63 b | 0.02 | 0.031 |
| 24 h | 6.96 | 6.81 | 6.75 | 6.72 | 0.37 | 0.152 | |
| WHC (breast) | 0.33 | 0.44 | 0.46 | 0.47 | 0.25 | 0.055 | |
| WHC (thigh) | 0.42 c | 0.67 a | 0.62 ab | 0.55 b | 0.09 | 0.045 | |
| Items | Group | Villus Height (μm) | Crypt Depth (μm) | Villus Height/Crypt Depth |
|---|---|---|---|---|
| Jejunum | Control | 1212.27 ± 47.79 a | 249.78 ± 12.50 a | 4.86 ± 0.29 |
| 200 mg/kg | 1381.83 ± 36.21 b | 370.41 ± 10.03 b | 3.69 ± 0.37 | |
| Ileum | Control | 1175.01 ± 59.35 a | 213.96 ± 33.02 a | 5.58 ± 0.56 |
| 200 mg/kg | 1417.10 ± 75.12 b | 411.05 ± 45.39 b | 3.49 ± 0.51 |
| Items | Group | Mean Fibre Cross-Sectional Area (μm) |
|---|---|---|
| Gastrocnemius | Control | 107.82 |
| 200 mg/kg | 109.51 | |
| Pectoral | Control | 99.37 |
| 200 mg/kg | 99.41 |
| Items (g) | C | 100LT | 200LT | 300LT | SEM | p Value |
|---|---|---|---|---|---|---|
| ALT (U/L) | 4.38 | 3.92 | 3.75 | 4.27 | 0.85 | 0.658 |
| AST (U/L) | 238.15 | 234.28 | 205.90 | 236.5 | 4.56 | 0.956 |
| T-chol (mmol/L) | 2.54 a | 2.39 ab | 1.53 b | 2.45 ab | 0.45 | 0.020 |
| TG (mmol/L) | 0.38 | 0.320 | 0.37 | 0.40 | 0.02 | 1.325 |
| HDL-c (mmol/L) | 1.75 | 2.07 | 2.24 | 1.74 | 0.21 | 0.566 |
| LDL-c (mmol/L) | 0.54 | 0.51 | 0.45 | 0.63 | 0.03 | 0.523 |
| Ingredients g/kg | Basal Diets | |
|---|---|---|
| Starter (1–3 Weeks) | Finisher (3–6 Weeks) | |
| Yellow Corn | 571.3 | 605.3 |
| Soybean meal | 316.5 | 271.5 |
| Gluten meal | 65 | 61 |
| Di Calcium phosphate | 17 | 15 |
| Limestone | 12.4 | 11.5 |
| Vitamin Premix * | 3 | 3 |
| NaCl | 3 | 3 |
| DL Methionine | 0.5 | 0.2 |
| l-Lysine | 1.3 | 10 |
| Soybean oil | 10 | 19.5 |
| Total | 1000 g | 1000 g |
| Calculated analysis ** | ||
| CP g/kg | 230 | 210 |
| ME Kcal/kg diet | 2951 | 3099 |
| Ca g/kg | 10 | 9 |
| P (Available) g/kg | 4.5 | 4 |
| Lysine g/kg | 12 | 10.5 |
| M+C g/kg | 8.3 | 7.4 |
| CF g/kg | 35.6 | 33.1 |
| Gene | Nucleotide Sequence (5′-3′) | Amplification of Length |
|---|---|---|
| IL-6 | Forward primer: TGGTGATAAATCCCGATGAAG Reverse primer: GGCACTGAAACTCCTGGTCT | 191 |
| IFN-γ | Forward primer: ATCATACTGAGCCAGATTGTTTCG Reverse primer: TCTTTCACCTTCTTCACGCCAT | 140 |
| IL-2 | Forward primer: GCTAATGACTACAGCTTATGGAGCA Reverse primer: TGGGTCTCAGTTGGTGTGTAGAG | 135 |
| TNF-α | Forward primer: AGATGGGAAGGGAATGAACC Reverse primer: CAGAGCATCAACGCAAAAG | 268 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; Yatao, X.; Hassan, F.-u.; Arain, M.A.; Abd El-Hack, M.E.; Noreldin, A.E.; Sun, C. Influence of Graded Levels of l-Theanine Dietary Supplementation on Growth Performance, Carcass Traits, Meat Quality, Organs Histomorphometry, Blood Chemistry and Immune Response of Broiler Chickens. Int. J. Mol. Sci. 2018, 19, 462. https://doi.org/10.3390/ijms19020462
Saeed M, Yatao X, Hassan F-u, Arain MA, Abd El-Hack ME, Noreldin AE, Sun C. Influence of Graded Levels of l-Theanine Dietary Supplementation on Growth Performance, Carcass Traits, Meat Quality, Organs Histomorphometry, Blood Chemistry and Immune Response of Broiler Chickens. International Journal of Molecular Sciences. 2018; 19(2):462. https://doi.org/10.3390/ijms19020462
Chicago/Turabian StyleSaeed, Muhammad, Xu Yatao, Faiz-ul Hassan, Muhammad Asif Arain, Mohamed E. Abd El-Hack, Ahmed E. Noreldin, and Chao Sun. 2018. "Influence of Graded Levels of l-Theanine Dietary Supplementation on Growth Performance, Carcass Traits, Meat Quality, Organs Histomorphometry, Blood Chemistry and Immune Response of Broiler Chickens" International Journal of Molecular Sciences 19, no. 2: 462. https://doi.org/10.3390/ijms19020462