Scandoside Exerts Anti-Inflammatory Effect Via Suppressing NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Results
2.1. Effects of SCA on RAW 264.7 Macrophage Viability
2.2. Effects of SCA on Inflammatory Mediators and Inflammatory Cytokines in RAW 264.7 Macrophages
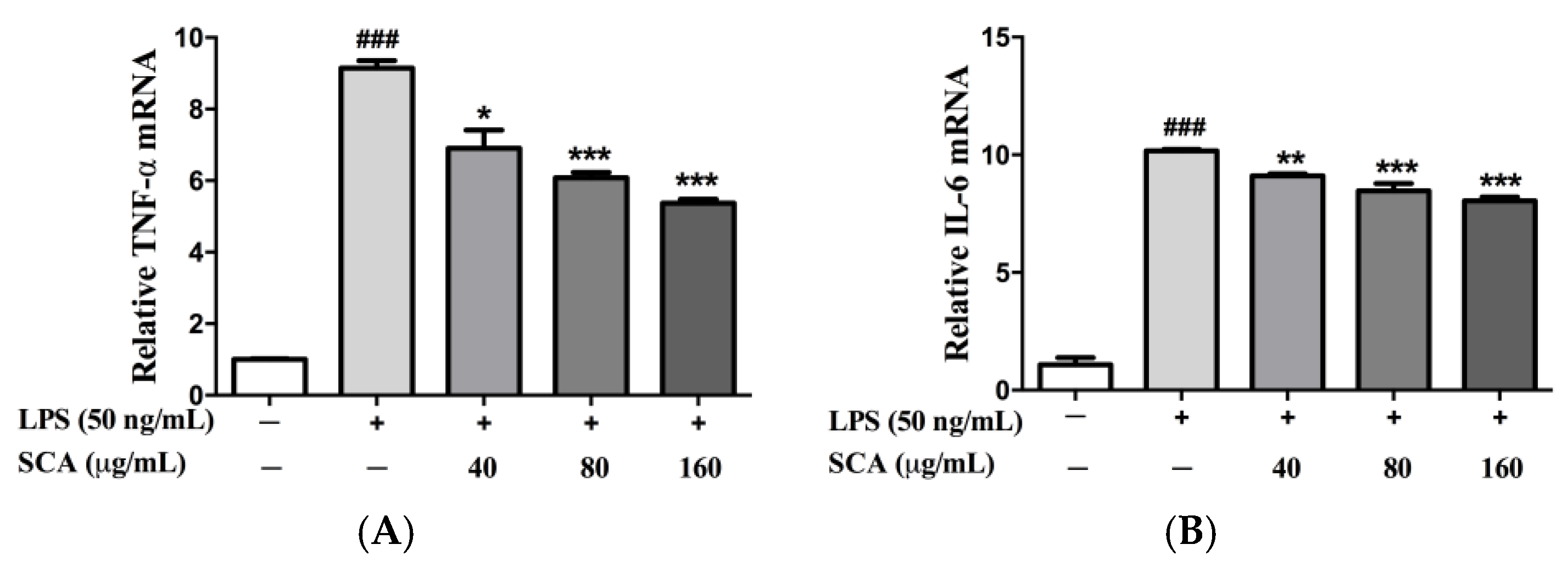
2.3. Effects of SCA on TNF-α and IL-6 Messeger RNA (mRNA) Expression in LPS-Induced RAW 264.7 Macrophages
2.4. Effects of SCA on iNOS and COX-2 Proteins and mRNA Expressions in LPS-Induced RAW 264.7 Macrophages
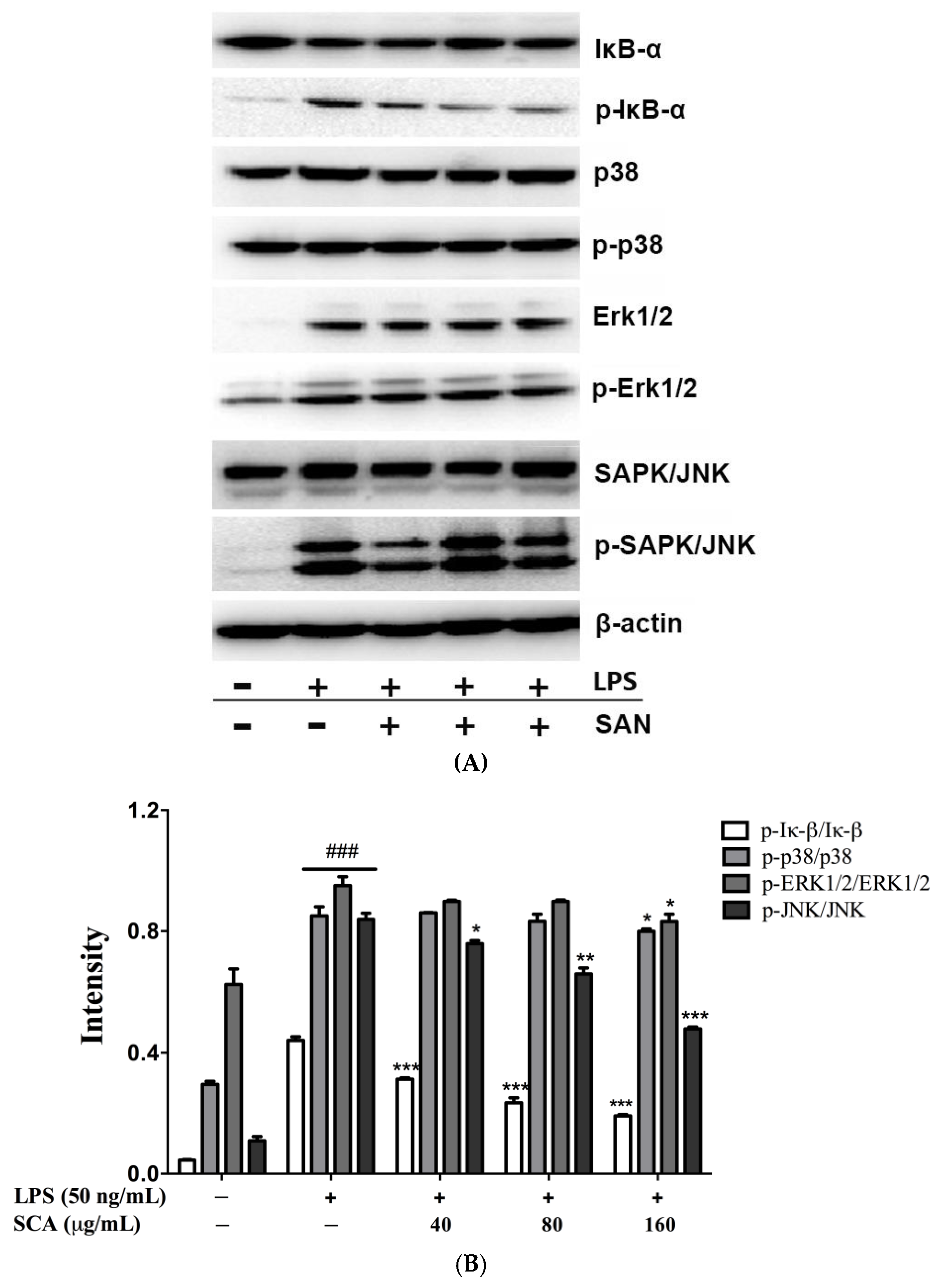
2.5. Effects of SCA on NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages
2.6. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Plant, Chemicals and Reagents
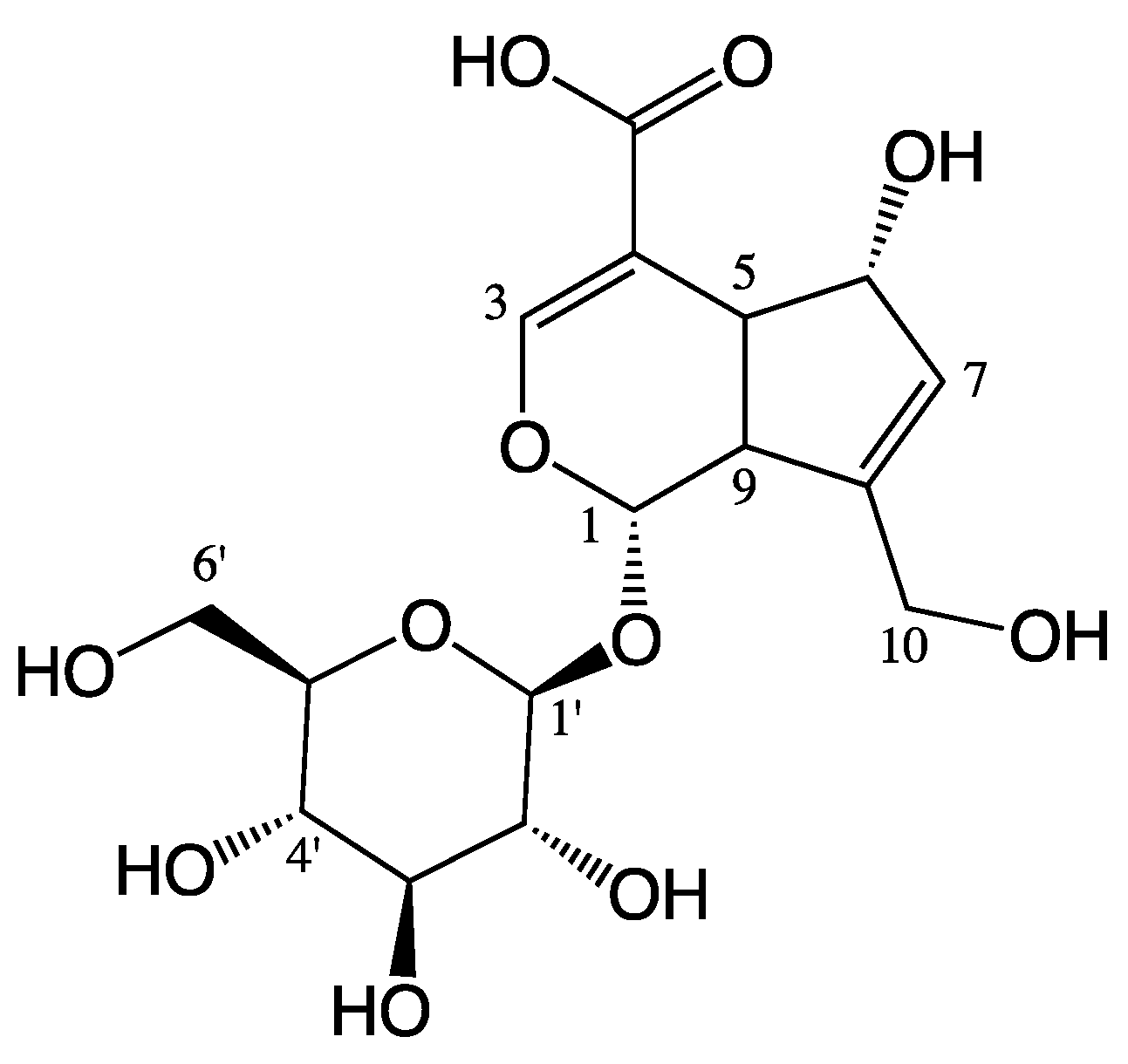
4.2. Sample Preparation
4.3. Cell Line and Culture
4.4. CCK-8 Assay for Cell Viability Evaluation
4.5. ELISA Assay of NO, PGE2, TNF-α and IL-6
4.6. RT-PCR Assay
4.7. Western Blot Analysis
4.8. Molecular Docking
4.9. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Thorn, J. The inflammatory response in humans after inhalation of bacterial endotoxin: A review. Inflamm. Res. 2001, 50, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A.K.; Hartung, T.; Huber, C.; Vollmar, A.M. Phyllanthusamarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-κB pathway. J. Hepatol. 2003, 38, 289–297. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E 2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.T.; Zhang, Z.Y.; Jiang, C.H.; Chen, J.Q.; Ye, J.Q.; Jia, X.B.; Yang, Y.; Ni, Q.; Wang, S.X.; Song, J.; et al. Nauclea officinalis inhibits inflammation in LPS-mediated RAW 264.7 macrophages by suppressing the NF-κB signaling pathway. J. Ethnopharmacol. 2016, 183, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.; Larkin, A.; Mingo, A.M.; Thuerauf, D.J.; Andrews, C.; McDonough, P.M.; Glembotski, C.C. p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 2000, 275, 23814–23824. [Google Scholar] [CrossRef] [PubMed]
- Editorial Board of Flora of China. The Chinese Academy of Sciences, Flora of China; Science Press: Beijing, China, 1999; Volume 71, p. 75. [Google Scholar]
- Nanjing University of Chinese Medicine. Dictionary of Chinese Traditional Medicine (Zhong Yao Da Ci Dian), 2nd ed.; Shanghai Scientific & Technical Publishers: Shanghai, China, 2006; pp. 1039–1041. [Google Scholar]
- Chen, R.; He, J.; Tong, X.; Tang, L.; Liu, M. The Hedyotis diffusa Willd (Rubiaceae): A review on phytochemistry, pharmacology, quality control and pharmacokinetics. Molecules 2016, 21, 710. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.J.; Kim, J.; Kim, Y.B.; Kim, S.R.; Kim, Y.C. Neuroprotective constituents from Hedyotis diffusa. J. Nat. Prod. 2001, 64, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, R.W.; Hon, P.M.; Cheng, L.; Li, L.L.; Zhou, J.R.; Shaw, P.C.; But, P.P.H. Authentication of the anti-tumor herb Baihuasheshecao with bioactive marker compounds and molecular sequences. Food Chem. 2010, 119, 1239–1245. [Google Scholar] [CrossRef]
- Ye, J.H.; Liu, M.H.; Zhang, X.L.; He, J.Y. Chemical profiles and protective effect of Hedyotis diffusa Willd in lipopolysaccharide-induced renal inflammation mice. Int. J. Mol. Sci. 2015, 16, 27252–27269. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.G.; de Campos Castilho, G.R.; da Cunha, A.L.; Miyajima, F.; de Oliveira Martins, D.T. Dilodendron bipinnatum Radlk. inhibits pro-inflammatory mediators through the induction of MKP-1 and the down-regulation of MAPKp38/JNK/NF-κB pathways and COX-2 in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2017, 202, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Xiao, Z.; Wen, X.; Luo, J.; Chen, S.; Cheng, Z.; Xiang, D.; Hu, J.; He, J. The anti-inflammatory effect of Andrographis paniculata (Burm. f.) Nees on pelvic inflammatory disease in rats through down-regulation of the NF-κB pathway. BMC Complement. Altern. Med. 2016, 16, 483. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, X.; Qu, L.; Chen, Y.; Dai, Y.; Wang, M.; Zou, W. DXXK exerts anti-inflammatory effects by inhibiting the lipopolysaccharide-induced NF-κB/COX-2 signalling pathway and the expression of inflammatory mediators. J. Ethnopharmacol. 2016, 178, 199–208. [Google Scholar] [CrossRef] [PubMed]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.J.; Zhong, Y.Q.; Lu, W.T.; Li, G.H.; Jiang, H.L.; Mao, B. Baicalin inhibits lipopolysaccharide-induced inflammation through signaling NF-κB pathway in HBE16 airway epithelial cells. Inflammation 2015, 38, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zeng, K.; Ma, X.; Song, F.; Jiang, Y.; Tu, P.; Wang, X. Resokaempferol-mediated anti-inflammatory effects on activated macrophages via the inhibition of JAK2/STAT3, NF-κB and JNK/p38 MAPK signaling pathways. Int. Immunopharmacol. 2016, 38, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Balkan, İ.A.; Akülke, A.Z.İ.; Bağatur, Y.; Telci, D.; Gören, A.C.; Kırmızıbekmez, H.; Yesilada, E. Sambulin A and B, non-glycosidic iridoids from Sambucus ebulus, exert significant in vitro anti-inflammatory activity in LPS-induced RAW 264.7 macrophages via inhibition of MAPKs’s phosphorylation. J. Ethnopharmacol. 2017, 206, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Islam, V.I.; Babu, N.P.; Pandikumar, P.; Thirugnanasambantham, K.; Chellappandian, M.; Raj, C.S.D.; Paulraj, M.G.; Ignacimuthu, S. Swertiamarin attenuates inflammation mediators via modulating NF-κB/Iκb and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. 2014, 56, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cao, J.; Fang, L.; Zhao, H.; Liu, Z.; Ran, J.; Zheng, X.; Li, X.; Zhou, Y.; Ge, D.; et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-κB, MAPK and AP-1 signaling pathways in macrophages. Int. Immunopharmacol. 2014, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.T.; Jiang, Z.H.; Leung, K.S.Y.; Zhao, Z.Z. Determination of iridoid glucosides for quality assessment of Herba Oldenlandiae by high-performance liquid chromatography. Chem. Pharm. Bull. 2006, 54, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Cao, G.S.; Li, F.; Gao, P. Contents of five iridoids in Ooldenlandia diffusa (Wind.) Roxb. based on HPLC-DAD. Chin. Hosp. Pharm. J. 2015, 35, 9–12. [Google Scholar]
- Varfolomeev, E.E.; Ashkenazi, A. Tumor necrosis factor: An apoptosis JuNKie? Cell 2004, 116, 491–497. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Neitzert, K.; Fernández, M.; Vega-Naredo, I.; Caballero, B.; García-Macía, M.; Suárez, F.M.; Rodríguez-Colunga, M.J.; Solano, J.J.; Coto-Montes, A. Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radic. Biol. Med. 2010, 49, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Wang, Y.J.; Ho, C.T. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem. Pharmacol. 2006, 72, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cisneros, M.Á.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; Rao, P.P.; Aburto-Amar, R.; Rodríguez-López, V. In vitro COX-1 and COX-2 enzyme inhibitory activities of iridoids from Penstemon barbatus, Castilleja tenuiflora, Cresentia alata and Vitex mollis. Bioorg. Med. Chem. Lett. 2015, 25, 4505–4508. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Studies on Anti-Tumor Constituents Hedyotis diffusa Willd. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2009. [Google Scholar]
- Kim, D.H.; Lee, H.J.; Oh, Y.J.; Kim, M.J.; Kim, S.H.; Jeong, T.S.; Baek, N.I. Iridoid glycosides isolated from Oldenlandia diffusa inhibit LDL-oxidation. Arch. Pharm. Res. 2005, 28, 156–1160. [Google Scholar] [CrossRef]



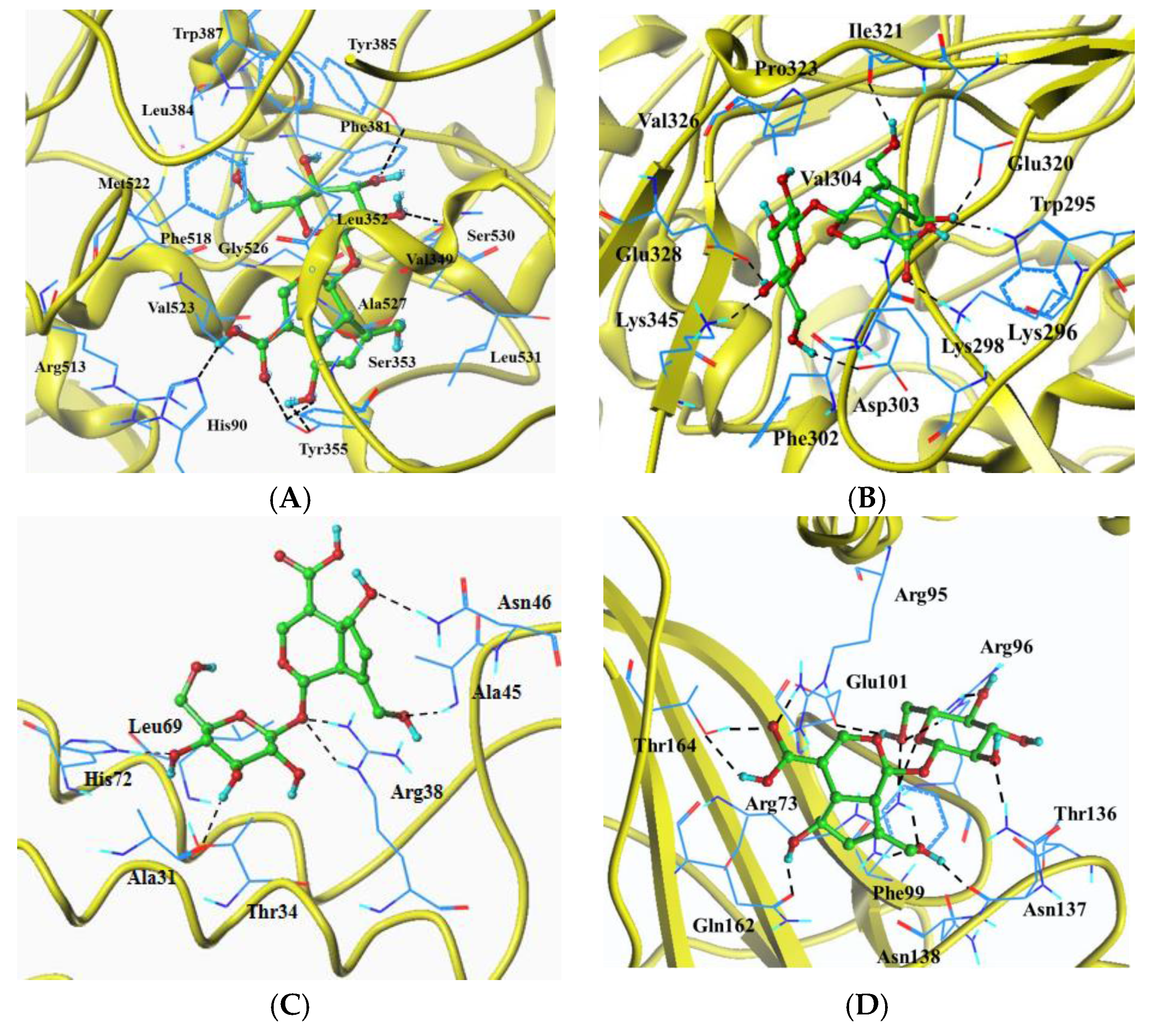
| Target Protein | Total Score 1 | Crash 2 | Polar 3 | Hydrogen Bonds | Eletrostatic Interaction | Hydrophobic Interaction (0.5 Å) |
|---|---|---|---|---|---|---|
| COX-2(1CX2) | 9.0084 | −3.0357 | 2.9740 | His90, Tyr355(3), Tyr385, Ser530 | His90, Arg513 | His90, Val349, Leu352, Ser353, Tyr355, Phe381, Leu384, Tyr385, Trp387, Phe518, Met522, Val523, Gly526, Ala527, Ser530, Leu531 |
| iNOS(4NOS) | 9.2757 | −1.4618 | 7.6190 | Trp295, Lys296, Asp303, Glu320, Ile321, Glu328, Lys345 | Lys296 | Trp295, Lys296, Lys298, Phe302, Asp303, Val304, Glu320, Ile321, Pro323, Val326, Glu328, Lys345 |
| PEG2(4AL0) | 6.2647 | −1.2700 | 5.0136 | Ala31, Arg38(2), Ala45, Asn46, His72 | Ala31, Thr34, Arg38, Ala45, Asn46, Leu69, His72 | |
| IκB(1NFI) | 9.0953 | −0.6933 | 9.9542 | Arg73(2), a Arg95, Arg96(2), Glu101, Asn137(2), Gln162, Thr164(2) | a Arg95 | Arg73, a Arg95, Arg96, Phe99, Glu101, Thr136, Asn137, Asn138, Gln162, Thr164 |
| Cytokines | Sense Primer Sequence5’-3 | Antisense Primer Sequence5’-3 |
|---|---|---|
| TNF-α | GCGACGTGGAACTGGCAGAA | CAGTAGACAGAAGAGCGTGGTG |
| IL-6 | GTTGCCTTCTTGGGACTGAT | CATTTCCACGATTTCCCAGA |
| iNOS | TGGAGCGAGTTGTGGATTGT | CTCTGCCTATCCGTCTCGTC |
| COX-2 | ACCTGGTGAACTACGACTGC | TGGTCGGTTTGATGTTACTG |
| β-actin | TGCTGTCCCTGTATGCCTCTG | GCTGTAGCCACGCTCGGTCA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Li, J.; Liu, H.; Yang, Z.; Zhou, F.; Wei, T.; Dong, Y.; Xue, H.; Tang, L.; Liu, M. Scandoside Exerts Anti-Inflammatory Effect Via Suppressing NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages. Int. J. Mol. Sci. 2018, 19, 457. https://doi.org/10.3390/ijms19020457
He J, Li J, Liu H, Yang Z, Zhou F, Wei T, Dong Y, Xue H, Tang L, Liu M. Scandoside Exerts Anti-Inflammatory Effect Via Suppressing NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages. International Journal of Molecular Sciences. 2018; 19(2):457. https://doi.org/10.3390/ijms19020457
Chicago/Turabian StyleHe, Jingyu, Jiafeng Li, Han Liu, Zichao Yang, Fenghua Zhou, Ting Wei, Yaqian Dong, Hongjiao Xue, Lan Tang, and Menghua Liu. 2018. "Scandoside Exerts Anti-Inflammatory Effect Via Suppressing NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages" International Journal of Molecular Sciences 19, no. 2: 457. https://doi.org/10.3390/ijms19020457




