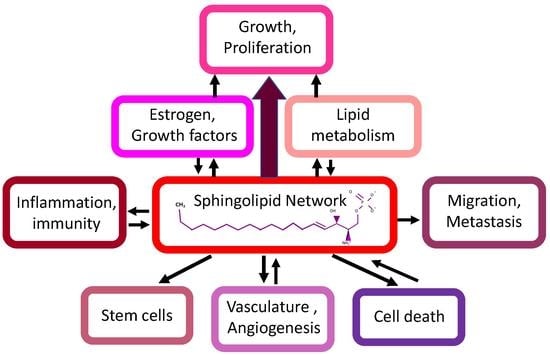
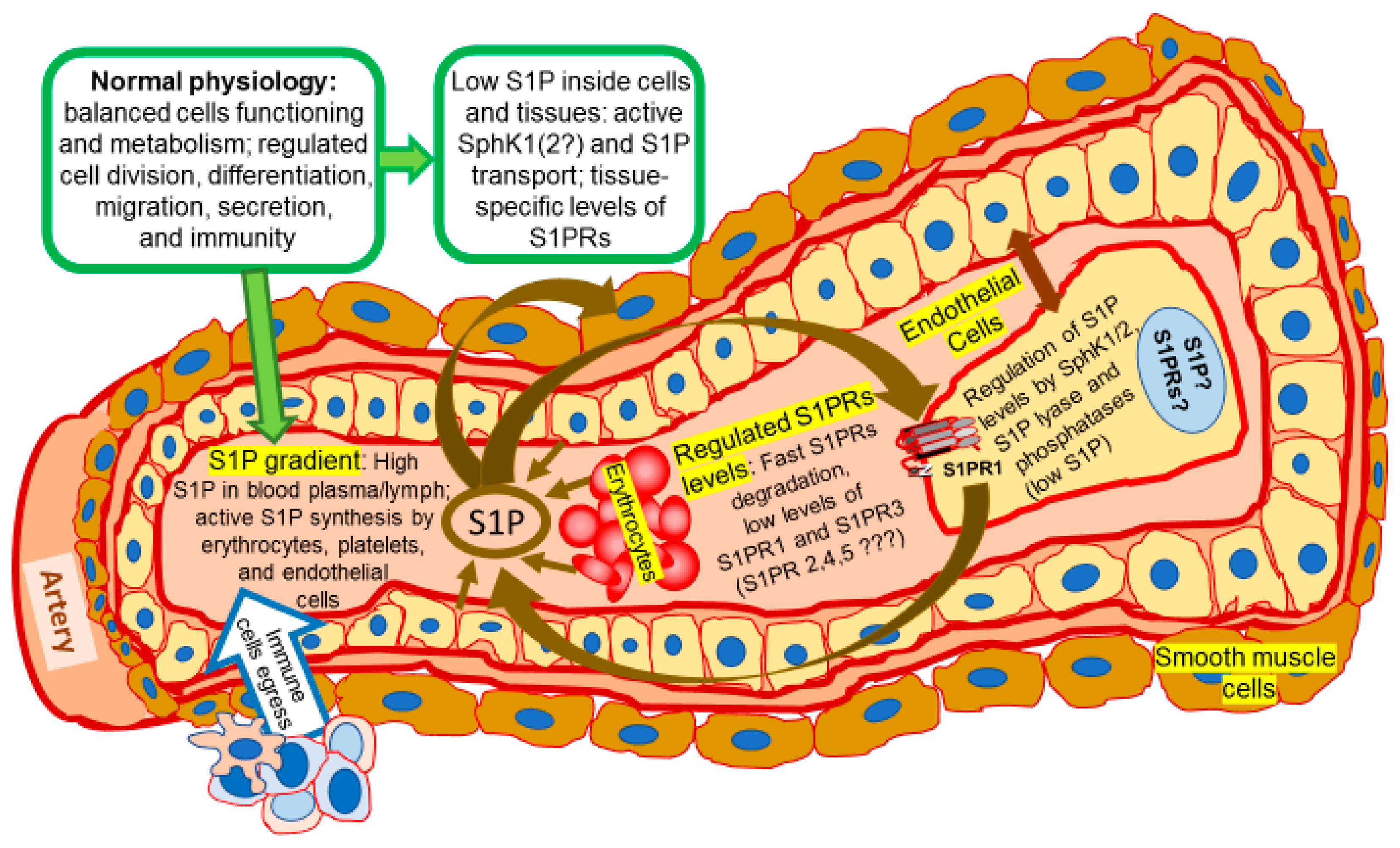
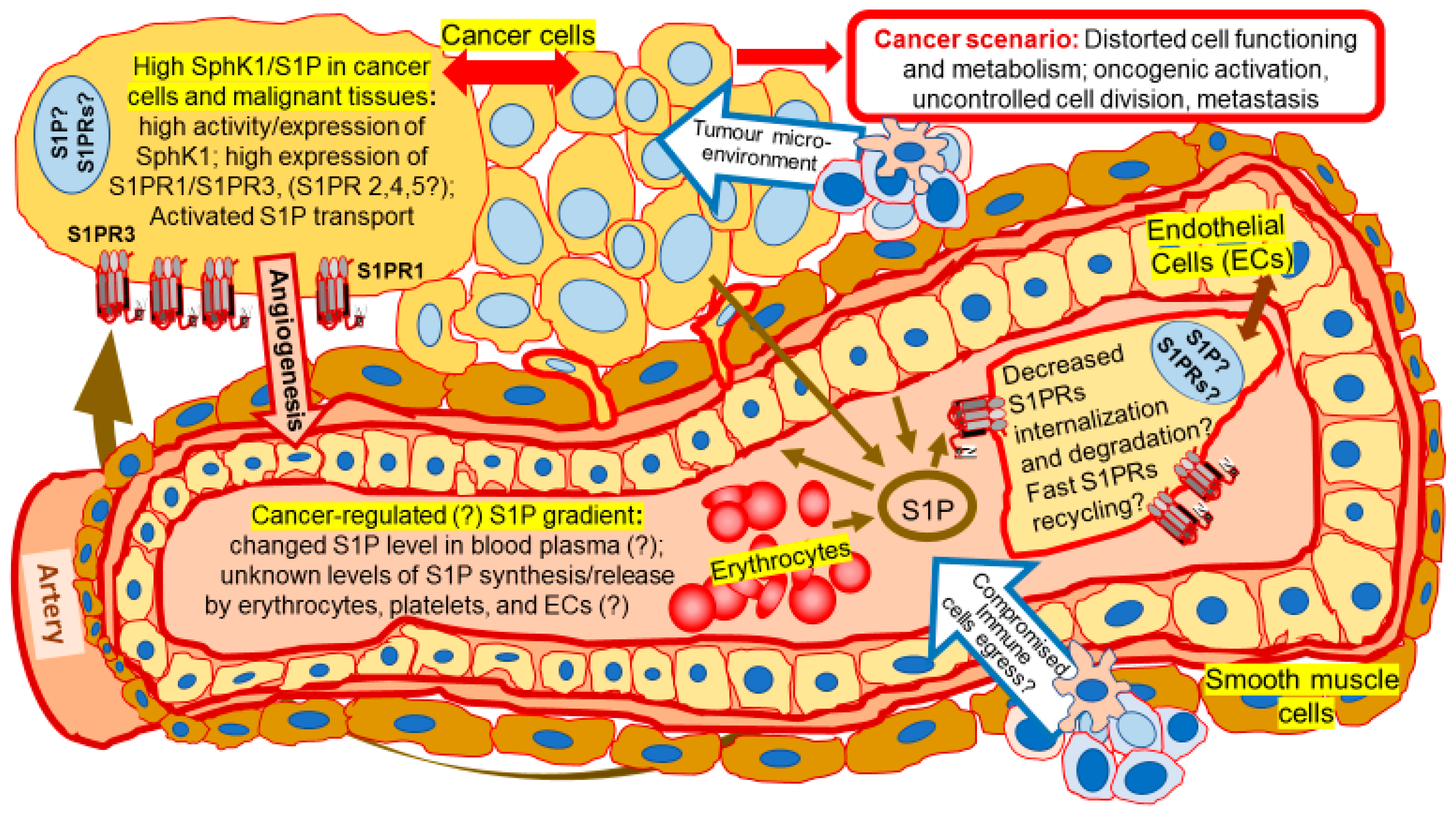
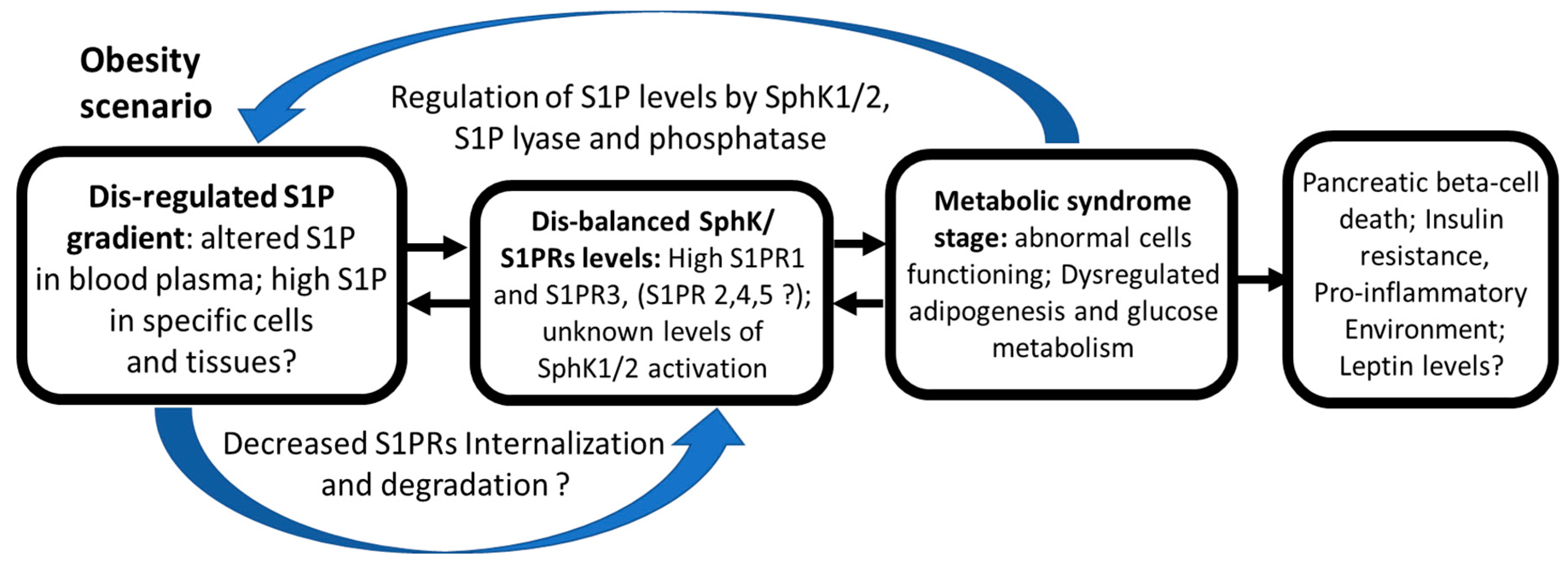
Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming
Abstract
:1. Introduction
2. Sphingosine Kinases, Sphingosine-1-Phosphate, and Membrane Metabolism
3. Estrogen Signaling Network: A Brief Overview
4. Contribution of Sphingolipids in Estrogen and Growth Factor Interactive Effects in Mammary Carcinomas
5. Role of Sphingolipids in Development of Drug Resistance
6. S1P Signaling in Stem Cells
7. Is SphK Intracellular Localization Connected to Its Functions?
8. Involvement of SphK/S1P in the Regulation of Apoptosis and Autophagy
9. Regulation of Cell Shape, Mobility, and Metastasis by Sphingolipids
10. Sphingolipid Signaling in Terminally Differentiated Cells: What Can Be Used in Cancer Prevention?
11. Future Perspectives of Sphingolipid Anti-Cancer Strategy
Acknowledgments
Conflicts of Interest
Abbreviations
| EGFR | Epidermal growth factor receptors |
| SphK | Sphingosine kinase |
| S1P | Sphingosine-1-phosphate |
| S1PRn (n = 1–5) | Sphingosine-1-phosphate receptor |
| S1PR1 | S1P receptor 1 |
| S1PR3 | S1P receptor 3 |
| S1PR2 | S1P receptor 2 |
| S1PR4 | S1P receptor 4 |
| S1PR5 | S1P receptor 5 |
| IHC | Immunohistochemistry |
| MAPK | Mitogen-activated protein kinase |
| Erk1/2 | Extracellular signal-regulated kinase 1/2 |
| ER | Estrogen Receptor |
| Ca2+ | Calcium ions |
| TAM | Tamoxifen |
| MPA | Medroxy-progesterone acetate |
| GPR | G-protein coupled receptor |
| hTERT | human telomerase reverse transcriptase |
References
- Katzenellenbogen, B.S.; Choi, I.; Delage-Mourroux, R.; Ediger, T.R.; Martini, P.G.; Montano, M.; Sun, J.; Weis, K.; Katzenellenbogen, J.A. Molecular mechanisms of estrogen action: Selective ligands and receptor pharmacology. J. Steroid Biochem. Mol. Biol. 2000, 74, 279–2785. [Google Scholar] [CrossRef]
- Osborne, C.K.; Schiff, R.; Fuqua, S.A.; Shou, J. Estrogen receptor: Current understanding of its activation and modulation. Clin. Cancer Res. 2001, 7, 4338–4342. [Google Scholar]
- Leitman, D.C.; Paruthiyil, S.; Yuan, C.; Herber, C.B.; Olshansky, M.; Tagliaferri, M.; Cohen, I.; Speed, T.P. Tissue-specific regulation of genes by estrogen receptors. Semin. Reprod. Med. 2012, 30, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Schiff, R.; Massarweh, S.; Shou, J.; Osborne, C.K. Breast cancer endocrine resistance: How growth factor signaling and estrogen receptor coregulators modulate response. Clin. Cancer Res. 2003, 9 Pt 2, 447S–454S. [Google Scholar] [PubMed]
- Sukocheva, O.A.; Wang, L.; Albanese, N.; Pitson, S.M.; Vadas, M.A.; Xia, P. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol. Endocrinol. 2003, 17, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Döll, F.; Pfeilschifter, J.; Huwiler, A. Prolactin upregulates sphingosine kinase-1 expression and activity in the human breast cancer cell line MCF7 and triggers enhanced proliferation and migration. Endocr. Relat. Cancer 2007, 14, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Takabe, K.; Kapitonov, D.; Milstien, S.; Spiegel, S. Targeting SphK1 as a new strategy against cancer. Curr. Drug Targets 2008, 9, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Vadas, M.; Xia, P.; McCaughan, G.; Gamble, J. The role of sphingosine kinase 1 in cancer: Oncogene or non-oncogene addiction? Biochim. Biophys. Acta 2008, 1781, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wang, L.; Verrier, E.; Vadas, M.A.; Xia, P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology 2009, 150, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Long, J.S.; Orange, C.; Tannahill, C.L.; Mallon, E.; McGlynn, L.M.; Pyne, S.; Pyne, N.J.; Edwards, J. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am. J. Pathol. 2010, 177, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; White, M.D.; Driver, J.L.; Burow, M.E.; Beckman, B.S. Sphingosine kinase isoforms as a therapeutic target in endocrine therapy resistant luminal and basal-A breast cancer. Exp. Biol. Med. (Maywood) 2012, 237, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; White, M.D.; Burow, M.E.; Beckman, B.S. Dual inhibition of sphingosine kinase isoforms ablates TNFα-induced drug resistance. Oncol. Rep. 2012, 27, 1779–1786. [Google Scholar] [PubMed]
- Levin, E.R. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol. Endocrinol. 2003, 17, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wadham, C.; Holmes, A.; Albanese, N.; Verrier, E.; Feng, F.; Bernal, A.; Derian, C.K.; Ullrich, A.; Vadas, M.A.; et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: The role of sphingosine kinase-1. J. Cell Biol. 2006, 173, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hait, N.C.; Sarkar, S.; Le Stunff, H.; Mikami, A.; Maceyka, M.; Milstien, S.; Spiegel, S. Role of sphingosine kinase 2 in cell migration toward epidermal growth factor. J. Biol. Chem. 2005, 280, 29462–29469. [Google Scholar] [CrossRef] [PubMed]
- Paugh, B.S.; Paugh, S.W.; Bryan, L.; Kapitonov, D.; Wilczynska, K.M.; Gopalan, S.M.; Rokita, H.; Milstien, S.; Spiegel, S.; Kordula, T. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 2008, 22, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wadham, C.; Xia, P. Estrogen defines the dynamics and destination of transactivated EGF receptor in breast cancer cells: Role of S1P₃ receptor and Cdc42. Exp. Cell Res. 2013, 319, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Hirata, N.; Yamada, S.; Shoda, T.; Kurihara, M.; Sekino, Y.; Kanda, Y. Sphingosine-1-phosphate promotes expansion of cancer stem cells via S1PR3 by a ligand-independent Notch activation. Nat. Commun. 2014, 5, 4806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Tsai, C.F.; Chuang, H.L.; Chang, Y.C.; Chen, H.S.; Lee, J.N.; Tsai, E.M. Benzyl butyl phthalate promotes breast cancer stem cell expansion via SPHK1/S1P/S1PR3 signaling. Oncotarget 2016, 7, 29563–29576. [Google Scholar] [CrossRef] [PubMed]
- Vouret-Craviari, V.; Bourcier, C.; Boulter, E.; van Obberghen-Schilling, E. Distinct signals via Rho GTPases and Src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells. J. Cell Sci. 2002, 115 Pt 12, 2475–2484. [Google Scholar] [PubMed]
- Sukocheva, O.; Wadham, C.; Gamble, J.; Xia, P. Sphingosine-1-phosphate receptor 1 transmits estrogens’ effects in endothelial cells. Steroids 2015, 104, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Nagahashi, M.; Yamada, A.; Takabe, K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat. Res. Biol. 2012, 10, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.X.; Ernst, A.M.; Haberkant, P.; Björkholm, P.; Lindahl, E.; Gönen, B.; Tischer, C.; Elofsson, A.; von Heijne, G.; Thiele, C.; et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 2012, 481, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M. Sphingolipid lysosomal storage disorders. Nature 2014, 510, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Thangada, S.; Michaud, J.; Oo, M.L.; Ai, Y.; Lee, Y.M.; Wu, M.; Parikh, N.S.; Khan, F.; Proia, R.L.; et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem. J. 2006, 397, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, D.; Wilkins, M.R.; Lay, A.J.; Kaczorowski, D.C.; Hatoum, D.; Bajan, S.; Hutvagner, G.; Lai, J.H.; Wu, W.; Martiniello-Wilks, R.; et al. Sphingosine kinase 1 isoform-specific interactions in breast cancer. Mol. Endocrinol. 2014, 28, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Ding, G.; Sonoda, H.; Kajimoto, T.; Haga, Y.; Khosrowbeygi, A.; Gao, S.; Miwa, N.; Jahangeer, S.; Nakamura, S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J. Biol. Chem. 2005, 280, 36318–36325. [Google Scholar] [CrossRef] [PubMed]
- Pitson, S.M.; Moretti, P.A.; Zebol, J.R.; Lynn, H.E.; Xia, P.; Vadas, M.A.; Wattenberg, B.W. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003, 22, 5491–5500. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.R.; Iyer, S.S.; Melrose, N.; VanOosten, R.; Johnson, K.; Pitson, S.M.; Obeid, L.M.; Kusner, D.J. Sphingosine kinase 1 (SK1) is recruited to nascent phagosomes in human macrophages: Inhibition of SK1 translocation by Mycobacterium tuberculosis. J. Immunol. 2005, 174, 3551–3561. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Giordano, F.; Wu, Y.; Chan, J.; Zhu, C.; Milosevic, I.; Wu, X.; Yao, K.; Chen, B.; Baumgart, T.; et al. Coupling between endocytosis and sphingosine kinase 1 recruitment. Nat. Cell Biol. 2014, 16, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Li, P.Y.; Wada, A.; Mitsutake, S.; Igarashi, Y. Identification of functional nuclear export sequences in human sphingosine kinase 1. Biochem. Biophys. Res. Commun. 2003, 311, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ohotski, J.; Edwards, J.; Elsberger, B.; Watson, C.; Orange, C.; Mallon, E.; Pyne, S.; Pyne, N.J. Identification of novel functional and spatial associations between sphingosine kinase 1, sphingosine 1-phosphate receptors and other signaling proteins that affect prognostic outcome in estrogen receptor-positive breast cancer. Int. J. Cancer 2013, 132, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mao, J.; Redfield, S.; Mo, Y.; Lage, J.M.; Zhou, X. Systemic distribution, subcellular localization and differential expression of sphingosine-1-phosphate receptors in benign and malignant human tissues. Exp. Mol. Pathol. 2014, 97, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Sonoda, H.; Yu, H.; Kajimoto, T.; Goparaju, S.K.; Jahangeer, S.; Okada, T.; Nakamura, S. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J. Biol. Chem. 2007, 282, 27493–27502. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, N.; Okada, T.; Hayashi, S.; Fujita, T.; Jahangeer, S.; Nakamura, S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 2003, 278, 46832–46839. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Sasaki, T.; Kawai, H.; Olivera, A.; Mi, Y.; van Echten-Deckert, G.; Hajdu, R.; Rosenbach, M.; Keohane, C.A.; Mandala, S.; et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004, 279, 52487–52492. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef] [PubMed]
- Le Stunff, H.; Giussani, P.; Maceyka, M.; Lepine, S.; Milstien, S.; Spiegel, S. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J. Biol. Chem. 2007, 282, 34372–34380. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Kihara, A.; Gokoh, M.; Igarashi, Y. Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2. J. Biol. Chem. 2003, 278, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Saba, J.D. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem. Biophys. Res. Commun. 1998, 242, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Ohkuni, A.; Kitamura, T.; Abe, K.; Naganuma, T.; Ohno, Y.; Zoeller, R.A.; Kihara, A. The Sjogren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol. Cell 2012, 46, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Allende, M.L.; Lee, B.G.; Chen, W.; Amar, M.J.; Remaley, A.T.; Saba, J.D.; Proia, R.L. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 2010, 285, 10880–10889. [Google Scholar] [CrossRef] [PubMed]
- Dobrosotskaya, I.Y.; Seegmiller, A.C.; Brown, M.S.; Goldstein, J.L.; Rawson, R.B. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science 2002, 296, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Holthuis, J.C.; Pomorski, T.; Raggers, R.J.; Sprong, H.; Van Meer, G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 2001, 81, 1689–1723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Byun, H.S.; Bittman, R.; Saba, J.D. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell. Signal. 2011, 23, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Sato, K.; Kon, J.; Tomura, H.; Okajima, F. Quantitative measurement of sphingosine 1-phosphate by radioreceptor-binding assay. Anal. Biochem. 2000, 282, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yatomi, Y.; Igarashi, Y.; Yang, L.; Hisano, N.; Qi, R.; Asazuma, N.; Satoh, K.; Ozaki, Y.; Kume, S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997, 121, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Sato, K.; Kon, J.; Tomura, H.; Yanagita, M.; Kuwabara, A.; Ui, M.; Okajima, F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 2000, 352 Pt 3, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Kobayashi, N.; Yamaguchi, A.; Nishi, T. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J. Biol. Chem. 2009, 284, 21192–21200. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Hisano, Y.; Kobayashi, N.; Yamaguchi, A.; Nishi, T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE 2012, 7, e38941. [Google Scholar] [CrossRef] [PubMed]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.W.; Huang, Y.; Cyster, J.G.; et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Nishi, T.; Hirata, T.; Kihara, A.; Sano, T.; Igarashi, Y.; Yamaguchi, A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J. Lipid Res. 2006, 47, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Yatomi, Y.; Ozaki, Y.; Ohmori, T.; Igarashi, Y. Sphingosine 1-phosphate: Synthesis and release. Prostaglandins 2001, 64, 107–122. [Google Scholar] [CrossRef]
- Takabe, K.; Kim, R.H.; Allegood, J.C.; Mitra, P.; Ramachandran, S.; Nagahashi, M.; Harikumar, K.B.; Hait, N.C.; Milstien, S.; Spiegel, S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 2010, 285, 10477–10486. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Nishi, T.; Hisano, Y.; Fukui, H.; Yamaguchi, A.; Mochizuki, N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 2009, 323, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Simmons, S.; Kawamura, S.; Inoue, A.; Orba, Y.; Tokudome, T.; Sunden, Y.; Arai, Y.; Moriwaki, K.; Ishida, J.; et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Investig. 2012, 122, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Jonnalagadda, D.; Sunkara, M.; Morris, A.J.; Whiteheart, S.W. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim. Biophys. Acta 2014, 1841, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, P.; Sukocheva, O.A.; Wang, T.; Mayne, G.C.; Watson, D.I.; Hussey, D.J. Effects of chemotherapy agents on Sphingosine-1-Phosphate receptors expression in MCF-7 mammary cancer cells. Biomed. Pharmacother. 2016, 81, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, L.; Moretti, P.A.; Albanese, N.; Chai, F.; Pitson, S.M.; D’Andrea, R.J.; Gamble, J.R.; Vadas, M.A. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-α signaling. J. Biol. Chem. 2002, 277, 7996–8003. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wadham, C.; Xia, P. Role of sphingolipids in the cytoplasmic signaling of estrogens. Steroids 2009, 74, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wadham, C. Role of sphingolipids in oestrogen signaling in breast cancer cells: An update. J. Endocrinol. 2014, 220, R25–R35. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, L.; Gamble, J.R.; Vadas, M.A. Activation of sphingosine kinase by tumor necrosis factor-α inhibits apoptosis in human endothelial cells. J. Biol. Chem. 1999, 274, 34499–34505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Dai, L.; Qi, Y.; Di, W.; Xia, P. Combination of FTY720 with cisplatin exhibits antagonistic effects in ovarian cancer cells: Role of autophagy. Int. J. Oncol. 2013, 42, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.L.; Wadham, C.; Sukocheva, O.A. The role of sphingolipid signaling in diabetes-associated pathologies (Review). Int. J. Mol. Med. 2017, 39, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yu, Y.; Zhang, N.; Cui, Y.; Zhai, L.; Li, H.; Zhang, Y.; Li, F.; Kan, Y.; Qin, S. Higher level of plasma bioactive molecule sphingosine 1-phosphate in women is associated with estrogen. Biochim. Biophys. Acta 2014, 1841, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Bernier, S.; Michel, T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 2001, 276, 12420–12426. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Wu, W.; Mosteller, R.D.; Broek, D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol. Cell. Biol. 2002, 22, 7758–7768. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Takuwa, N.; Yoshioka, K.; Okamoto, Y.; Gonda, K.; Sugihara, K.; Fukamizu, A.; Asano, M.; Takuwa, Y. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010, 70, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Stilhano, R.S.; To, V.P.; Tran, L.; Wong, K.; Silva, E.A. Hypoxia augments outgrowth endothelial cell (OEC) sprouting and directed migration in response to sphingosine-1-phosphate (S1P). PLoS ONE 2015, 10, e0123437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurenzana, A.; Cencetti, F.; Serratì, S.; Bruno, G.; Japtok, L.; Bianchini, F.; Torre, E.; Fibbi, G.; Del Rosso, M.; Bruni, P.; et al. Endothelial sphingosine kinase/SPNS2 axis is critical for vessel-like formation by human mesoangioblasts. J. Mol. Med. 2015, 93, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef] [PubMed]
- Weichand, B.; Weis, N.; Weigert, A.; Grossmann, N.; Levkau, B.; Brune, B. Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur. J. Immunol. 2013, 43, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar]
- Watson, D.G.; Tonelli, F.; Alossaimi, M.; Williamson, L.; Chan, E.; Gorshkova, I.; Berdyshev, E.; Bittman, R.; Pyne, N.J.; Pyne, S. The roles of sphingosine kinases 1 and 2 in regulating the Warburg effect in prostate cancer cells. Cell. Signal. 2013, 25, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Ma, Y.J.; Han, L.; Wang, Y.J.; Han, J.A.; Zhu, Y. Role of sphingosine 1-phosphate in human pancreatic cancer cells proliferation and migration. Int. J. Clin. Exp. Med. 2015, 8, 20349–20354. [Google Scholar] [PubMed]
- Long, J.; Xie, Y.; Yin, J.; Lu, W.; Fang, S. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumour Biol. 2016, 37, 6831–6836. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Li, L.; Wang, X.H.; Wen, X.Z.; Ji, K.; Ye, L.; Cai, J.; Jiang, W.G.; Ji, J.F. Inhibition of sphingosine-1-phosphate phosphatase 1 promotes cancer cells migration in gastric cancer: Clinical implications. Oncol. Rep. 2015, 34, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.M.; Li, L.; Jing, B.Q.; Zhao, Y.S.; Wang, C.L.; Feng, L.; Xie, Y.E. Effect of S1P5 on proliferation and migration of human esophageal cancer cells. World J. Gastroenterol. 2010, 16, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Zondag, G.; Postma, F.; Etten, I.; Verlaan, I.; Moolenaar, W. Sphingosine-1-phosphate signaling through the G-protein-coupled receptor Edg-1. Biochem. J. 1998, 330, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Siehler, S.; Wang, Y.; Fan, X.; Windh, R.; Manning, D.R. Sphingosine 1-phosphate activates nuclear factor-κB through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J. Biol. Chem. 2001, 276, 48733–48739. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Sphingosine kinase 1 enables communication between melanoma cells and fibroblasts that provides a new link to metastasis. Oncogene 2014, 33, 3361–3363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 78. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Manetsch, M.; Quante, T.; Rahman, M.M.; Patel, B.S.; Ge, Q.; Ammit, A.J. Sphingosine 1-phosphate induces MKP-1 expression via p38 MAPK- and CREB-mediated pathways in airway smooth muscle cells. Biochim. Biophys. Acta 2012, 1823, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007, 115, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Bondesson, M.; Hao, R.; Lin, C.Y.; Williams, C.; Gustafsson, J.Å. Estrogen receptor signaling during vertebrate development. Biochim. Biophys. Acta 2015, 1849, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ariazi, E.A.; Jordan, V.C. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr. Top. Med. Chem. 2006, 6, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J.Å. Estrogen receptors: Therapies targeted to receptor subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Barkhem, T.; Nilsson, S.; Gustafsson, J.A. Molecular mechanisms, physiological consequences and pharmacological implications of estrogen receptor action. Am. J. Pharmacogenomics 2004, 4, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Krucken, J.; Benten, W.P.; Wunderlich, F. Estradiol-induced nongenomic calcium signaling regulates genotropic signaling in macrophages. J. Biol. Chem. 2002, 277, 7044–7050. [Google Scholar] [CrossRef] [PubMed]
- Keshamouni, V.G.; Mattingly, R.R.; Reddy, K.B. Mechanism of 17-β-estradiol-induced Erk1/2 activation in breast cancer cells. A role for HER2 AND PKC-delta. J. Biol. Chem. 2002, 277, 22558–22565. [Google Scholar] [CrossRef] [PubMed]
- Bergelin, N.; Blom, T.; Heikkilä, J.; Löf, C.; Alam, C.; Balthasar, S.; Slotte, J.P.; Hinkkanen, A.; Törnquist, K. Sphingosine kinase as an oncogene: Autocrine sphingosine 1-phosphate modulates ML-1 thyroid carcinoma cell migration by a mechanism dependent on protein kinase C-α and ERK1/2. Endocrinology 2009, 150, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.B.; Huang, J.A.; Liu, S.Q.; Tang, G.D.; Jiang, H.X. Inhibition of SPHK1 suppresses phorbol 12-myristate 13-acetate-induced metastatic phenotype in colorectal cancer HT-29 cells. Oncol. Res. 2011, 19, 573–582. [Google Scholar] [CrossRef] [PubMed]
- El-Shewy, H.M.; Johnson, K.R.; Lee, M.H.; Jaffa, A.A.; Obeid, L.M.; Luttrell, L.M. Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors. J. Biol. Chem. 2006, 281, 31399–31407. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Appleton, K.M.; El-Shewy, H.M.; Sorci-Thomas, M.G.; Thomas, M.J.; Lopes-Virella, M.F.; Luttrell, L.M.; Hammad, S.M.; Klein, R.L. S1P in HDL promotes interaction between SR-BI and S1PR1 and activates S1PR1-mediated biological functions: Calcium flux and S1PR1 internalization. J. Lipid Res. 2017, 58, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Hobson, J.P.; Rosenfeldt, H.M.; Barak, L.S.; Olivera, A.; Poulton, S.; Caron, M.G.; Milstien, S.; Spiegel, S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science 2001, 291, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- Auge, N.; Garcia, V.; Maupas-Schwalm, F.; Levade, T.; Salvayre, R.; Negre-Salvayre, A. Oxidized LDL-induced smooth muscle cell proliferation involves the EGF receptor/PI-3 kinase/Akt and the sphingolipid signaling pathways. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qi, Y.; Chen, J.; Kaczorowski, D.; Di, W.; Wang, W.; Xia, P. Sphingosine kinase(SphK) 1 and SphK2 play equivalent roles in mediating insulin’s mitogenic action. Mol. Endocrinol. 2014, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Inoue, S.; Yoshida, Y.; Kodaka, A.; Tsuji, T.; Tsuiji, M. Sphingosine kinase 1 is upregulated with lysophosphatidic acid receptor 2 in human colorectal cancer. World J. Gastroenterol. 2016, 22, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, T.; Jin, Z.G.; Berk, B.C. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine-1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J. Biol. Chem. 2002, 277, 42997–43001. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Song, W.K.; Kim, J.H.; Chun, J.S. Sphingosine 1-phosphate activates Erk-1/-2 by transactivating epidermal growth factor receptor in rat-2 cells. IUBMB Life 2000, 50, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, Y.I.; Shin, K.O.; Seo, H.S.; Kim, J.Y.; Mann, T.; Oda, Y.; Lee, Y.M.; Holleran, W.M.; Elias, P.M. The dietary ingredient, genistein, stimulates cathelicidin antimicrobial peptide expression through a novel S1P-dependent mechanism. J. Nutr. Biochem. 2014, 25, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, H.; Krell, J.; Frampton, A.E.; Waxman, J.; Blyuss, O.; Zaikin, A.; Winkler, M.; Stebbing, J.; Yagüe, E.; Pchejetski, D. Leptin induces upregulation of sphingosine kinase 1 in oestrogen receptor-negative breast cancer via Src family kinase-mediated, janus kinase 2-independent pathway. Breast Cancer Res. 2014, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maruvada, R.; Morris, A.J.; Liu, J.O.; Wolfgang, M.J.; Baek, D.J.; Bittman, R.; Kim, K.S. Sphingosine 1-Phosphate Activation of EGFR As a Novel Target for Meningitic Escherichia coli Penetration of the Blood-Brain Barrier. PLoS Pathog. 2016, 12, e1005926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Urtz, N.; Gaertner, F.; Legate, K.R.; Petzold, T.; Lorenz, M.; Mazharian, A.; Watson, S.P.; Massberg, S. Sphingosine kinase 2 (Sphk2) regulates platelet biogenesis by providing intracellular sphingosine 1-phosphate (S1P). Blood 2013, 122, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, A.; Piccolo, D.; Castoria, G.; Di Domenico, M.; Bilancio, A.; Lombardi, M.; Gong, W.; Beato, M.; Auricchio, F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998, 17, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Baran, Y.; Salas, A.; Senkal, C.E.; Gunduz, U.; Bielawski, J.; Obeid, L.M.; Ogretmen, B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J. Biol. Chem. 2007, 282, 10922–10934. [Google Scholar] [CrossRef] [PubMed]
- Ruckhaberle, E.; Karn, T.; Denkert, C.; Loibl, S.; Ataseven, B.; Reimer, T.; Becker, S.; Holtrich, U.; Rody, A.; Darb-Esfahani, S. Predictive value of sphingosine kinase 1 expression in neoadjuvant treatment of breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; Meacham, W.D.; Bratton, M.R.; Slaughter, E.M.; Rhodes, L.V.; Ashe, H.B.; Wiese, T.E.; Burow, M.E.; Beckman, B.S. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. J. Mol. Endocrinol. 2011, 46, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Du, W.; Kaneko, E.; Okamoto, Y.; Yoshioka, K.; Takuwa, Y. Tumor-suppressive sphingosine-1-phosphate receptor-2 counteracting tumor-promoting sphingosine-1-phosphate receptor-1 and sphingosine kinase 1—Jekyll Hidden behind Hyde. Am. J. Cancer Res. 2011, 1, 460–481. [Google Scholar] [PubMed]
- Yamamoto, S.; Yako, Y.; Fujioka, Y.; Kajita, M.; Kameyama, T.; Kon, S.; Ishikawa, S.; Ohba, Y.; Ohno, Y.; Kihara, A.; et al. A role of the sphingosine-1-phosphate (S1P)-S1P receptor 2 pathway in epithelial defense against cancer (EDAC). Mol. Biol. Cell 2016, 27, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kitayama, J.; Shida, D.; Yamaguchi, H.; Mori, K.; Osada, M.; Aoki, S.; Yatomi, Y.; Takuwa, Y.; Nagawa, H. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: Differential regulation on the migration and proliferation. J. Surg. Res. 2006, 130, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Kitayama, J.; Takuwa, N.; Arikawa, K.; Inoki, I.; Takehara, K.; Nagawa, H.; Takuwa, Y. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem. J. 2003, 374 Pt 3, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklyn, J.R.; Williams, J.B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 163, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Avni, D.; Yamada, A.; Nagahashi, M.; Aoyagi, T.; Aoki, H.; Dumur, C.I.; Zelenko, Z.; Gallagher, E.J.; Leroith, D.; et al. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERα expression and enhances hormonal therapy for breast cancer. Oncogenesis 2015, 4, e156. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Hung, M.C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signaling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, K.; Grandal, M.V.; Henriksen, L.; Knudsen, S.L.; Lerdrup, M.; Grøvdal, L.; Willumsen, B.M.; van Deurs, B. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 2009, 10, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.S.; Wu, W.J. Cdc42: An effector and regulator of ErbB1 as a strategic target in breast cancer therapy. Expert Rev. Anticancer Ther. 2007, 7, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Edwards, J.; Watson, C.; Tovey, S.; Mair, K.M.; Schiff, R.; Natarajan, V.; Pyne, N.J.; Pyne, S. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol. Cell. Biol. 2010, 30, 3827–3841. [Google Scholar] [CrossRef] [PubMed]
- Ohotski, J.; Long, J.S.; Orange, C.; Elsberger, B.; Mallon, E.; Doughty, J.; Pyne, S.; Pyne, N.J.; Edwards, J. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br. J. Cancer 2012, 106, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, J.; Nagahashi, M.; Nakajima, M.; Moro, K.; Tatsuda, K.; Ramanathan, R.; Takabe, K.; Wakai, T. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J. Surg. Res. 2016, 205, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Furuya, H.; Tamashiro, P.M.; Iino, K.; Chan, O.T.M.; Goodison, S.; Pagano, I.; Hokutan, K.; Peres, R.; Loo, L.W.M.; et al. Genetic deletion of Sphingosine Kinase 1 suppresses mouse breast tumor development in a HER2 transgenic model. Carcinogenesis 2017. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Dev, K.K. The structure and function of the S1P1 receptor. Trends Pharmacol. Sci. 2013, 34, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Sassoli, C.; Nuti, F.; Martinesi, M.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L.; Meacci, E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: A role for a gap junction-dependent and -independent function. Mol. Biol. Cell 2006, 17, 4896–4910. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Bini, F.; Battistini, C. Sphingosine-1-phosphate signaling in skeletal muscle cells. Methods Mol. Biol. 2012, 874, 155–165. [Google Scholar] [PubMed]
- Mendelson, K.; Evans, T.; Hla, T. Sphingosine 1-phosphate signaling. Development 2014, 141, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Formigli, L.; Bini, F.; Tani, A.; Squecco, R.; Battistini, C.; Zecchi-Orlandini, S.; Francini, F.; Meacci, E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J. Cell. Mol. Med. 2011, 15, 2498–2511. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.C.; Leong, W.I.; Carlson, M.E.; Oskouian, B.; Kumar, A.; Fyrst, H.; Zhang, M.; Proia, R.L.; Hoffman, E.P.; Saba, J.D. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE 2012, 7, e37218. [Google Scholar] [CrossRef]
- Sassoli, C.; Frati, A.; Tani, A.; Anderloni, G.; Pierucci, F.; Matteini, F.; Chellini, F.; Zecchi-Orlandini, S.; Formigli, L.; Meacci, E. Mesenchymal stromal cell secreted sphingosine 1-phosphate (S1P) exerts a stimulatory effect on skeletal myoblast proliferation. PLoS ONE 2014, 9, e108662. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Cencetti, F.; Pertici, I.; Japtok, L.; Bernacchioni, C.; Donati, C.; Bruni, P. CTGF/CCN2 exerts profibrotic action in myoblasts via the up-regulation of sphingosine kinase-1/S1P3 signaling axis: Implications in the action mechanism of TGFβ. Biochim. Biophys. Acta 2015, 1851, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Baek, Y.B.; Shin, M.S.; Park, J.H.; Park, S.H.; Lee, J.H.; Han, H.J. Sphingosine-1phosphate-induced Flk-1 transactivation stimulates mouse embryonic stem cell proliferation through S1P1/S1P3-dependent b-arrestin/c-Src pathways. Stem Cell Res. 2014, 12, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Balthasar, S.; Bergelin, N.; Lo¨f, C.; Vainio, M.; Andersson, S.; Törnquist, K. Interactions between sphingosine-1-phosphate and vascular endothelial growth factor signaling in ML-1 follicular thyroid carcinoma cells. Endocr. Relat. Cancer 2008, 15, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Hla, T.; Ferrer, F. Sphingolipid modulation of angiogenic factor expression in neuroblastoma. Cancer Prev. Res. 2011, 4, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Juarez, J.G.; Harun, N.; Thien, M.; Welschinger, R.; Baraz, R.; Pena, A.D.; Pitson, S.M.; Rettig, M.; DiPersio, J.F.; Bradstock, K.F.; et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood 2012, 119, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Schneider, G.; Abdel-Latif, A.; Mierzejewska, K.; Sunkara, M.; Borkowska, S.; Ratajczak, J.; Morris, A.J.; Kucia, M.; Ratajczak, M.Z. Ceramide-1-phosphate regulates migration of multipotent stromal cells and endothelial progenitor cells: Implications for tissue regeneration. Stem Cells 2013, 31, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Rochwalsky, U.; Reinhold, J.; Seeger, F.; Aicher, A.; Urbich, C.; Spyridopoulos, I.; Chun, J.; Brinkmann, V.; Keul, P.; et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Sanchez, T.; Yamase, H.; Hla, T.; Oo, M.L.; Pappalardo, A.; Lynch, K.R.; Lin, C.Y.; Ferrer, F. S1P/S1P1 signaling stimulates cell migration and invasion in Wilms tumor. Cancer Lett. 2009, 276, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, J.; Lee, J.F.; Gartung, A.; Jawadi, H.; Zhang, W.; Lominadze, D.; Lee, M.J. 3-amino-4-(3-hexylphenylamino)-4-oxobutyl phosphonic acid (W146), a selective antagonist of sphingosine-1-phospahte receptor subtype 1, enhances AMD3100-stimulated mobilization of hematopoietic stem progenitor cells in animals. J. Biochem. Pharmacol. Res. 2013, 1, 197–203. [Google Scholar] [PubMed]
- Shen, H.; Zhou, E.; Wei, X.; Fu, Z.; Niu, C.; Li, Y.; Pan, B.; Mathew, A.V.; Wang, X.; Pennathur, S.; et al. High density lipoprotein promotes proliferation of adipose-derived stem cells via S1P1 receptor and Akt, ERK1/2 signal pathways. Stem Cell Res. Ther. 2015, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Poitevin, S.; Cussac, D.; Leroyer, A.S.; Albinet, V.; Sarlon-Bartoli, G.; Guillet, B.; Hubert, L.; Andrieu-Abadie, N.; Couderc, B.; Parini, A.; et al. Sphingosine kinase 1 expressed by endothelial colony-forming cells has a critical role in their revascularization activity. Cardiovasc. Res. 2014, 103, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Matsuzaki, E.; Higashi, K.; Takahashi-Yanaga, F.; Takano, A.; Hirata, M.; Nishimura, F. Sphingosine-1-phosphate inhibits differentiation of C3H10T1/2 cells into adipocyte. Mol. Cell. Biochem. 2015, 401, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Göthert, J.R.; Gomez, R.A.; Sequeira-Lopez, M.L. Hemovascular Progenitors in the Kidney Require Sphingosine-1-Phosphate Receptor 1 for Vascular Development. J. Am. Soc. Nephrol. 2016, 27, 1984–1995. [Google Scholar] [CrossRef] [PubMed]
- Svetlov, S.I.; Sautin, Y.Y.; Crawford, J.M. EDG receptors and hepatic pathophysiology of LPA and S1P: EDG-ology of liver injury. Biochim. Biophys. Acta 2002, 1582, 251–256. [Google Scholar] [CrossRef]
- Salas, A.; Ponnusamy, S.; Senkal, C.E.; Meyers-Needham, M.; Selvam, S.P.; Saddoughi, S.A.; Apohan, E.; Sentelle, R.D.; Smith, C.; Gault, C.R.; et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 2011, 117, 5941–5952. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, A.; Chesini, G.P.; Taylor, A.E.; Sussman, M.A.; Brown, J.H.; Purcell, N.H. Sphingosine 1-phosphate elicits RhoA-dependent proliferation and MRTF-A mediated gene induction in CPCs. Cell. Signal. 2016, 28, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Terai, K.; Nakajima, H.; Chiba, A.; Fukuhara, S.; Mochizuki, N. S1P-Yap1 signaling regulates endoderm formation required for cardiac precursor cell migration in zebrafish. Dev. Cell 2014, 31, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-H.; Sanchez, T.; Pappalardo, A.; Lynch, K.R.; Hla, T.; Ferrer, F. Induction of antiproliferative connective tissue growth factor expression in Wilms’ tumor cells by sphingosine-1-phosphate receptor 2. Mol. Cancer Res. 2008, 6, 1649–1656. [Google Scholar] [PubMed]
- Sugimoto, N.; Takuwa, N.; Okamoto, H.; Sakurada, S.; Takuwa, Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine- 1-phosphate receptor isoform. Mol. Cell. Biol. 2003, 23, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Boehmler, A.M.; Seitz, G.; Kuci, S.; Wiesner, T.; Brinkmann, V.; Kanz, L.; Möhle, R. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood 2004, 103, 4478–4486. [Google Scholar] [CrossRef] [PubMed]
- Stessin, A.M.; Gursel, D.B.; Schwartz, A.; Parashar, B.; Kulidzhanov, F.G.; Sabbas, A.M.; Boockvar, J.; Nori, D.; Wernicke, A.G. FTY720, sphingosine 1-phosphate receptor modulator, selectively radioprotects hippocampal neural stem cells. Neurosci. Lett. 2012, 516, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Takahara, S.; Ichimaru, N.; Wang, J.D.; Itoh, Y.; Otsuki, Y.; Morimoto, J.; Fukui, R.; Hoshiga, M.; Ishihara, T.; et al. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002, 62, 1410–1419. [Google Scholar] [PubMed]
- Ghosh, T.K.; Bian, J.; Gill, D.L. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J. Biol. Chem. 1994, 269, 22628–22635. [Google Scholar] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Panneer Selvam, S.; De Palma, R.M.; Oaks, J.J.; Oleinik, N.; Peterson, Y.K.; Stahelin, R.V.; Skordalakes, E.; Ponnusamy, S.; Garrett-Mayer, E.; Smith, C.D.; et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci. Signal. 2015, 8, ra58. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.E.; Harikumar, K.B.; Hait, N.C.; Allegood, J.; Strub, G.M.; Kim, E.Y.; Maceyka, M.; Jiang, H.; Luo, C.; Kordula, T.; et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 2010, 465, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, Y.; Otsuki, K.; Fujita, T.; Uesato, S. Effects of phosphorylation of immunomodulatory agent FTY720 (fingolimod) on antiproliferative activity against breast and colon cancer cells. Biol. Pharm. Bull. 2008, 31, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Bhosle, V.K.; Chemtob, S. Subcellular G-protein coupled receptor signaling hints at greater therapeutic selectivity. Expert Opin. Ther. Targets 2015, 19, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, S.; Pfeilschifter, J.; Huwiler, A. Sphingosine-1-phosphate: A Janus-faced mediator of fibrotic diseases. Biochim. Biophys. Acta 2013, 1831, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Gellings Lowe, N.; Swaney, J.S.; Moreno, K.M.; Sabbadini, R.A. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-β-stimulated collagen production by cardiac fibroblasts. Cardiovasc. Res. 2009, 82, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphigolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Rosenthal, D.S.; Smulson, M.E.; Spiegel, S. Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide-mediated apoptosis in Jurkat T lymphocytes. J. Biol. Chem. 1998, 273, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.E.; Hobson, J.P.; Murthy, S.; Milstien, S.; Spiegel, S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp. Cell Res. 2002, 281, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Edsall, L.C.; Cuvillier, O.; Twitty, S.; Spiegel, S.; Milstien, S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J. Neurochem. 2001, 76, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Song, D.D.; Zhang, T.T.; Chen, J.L.; Xia, Y.F.; Qin, Z.H.; Waeber, C.; Sheng, R. Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain. Cell Death Dis. 2017, 8, e2912. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Pascual, C.; Klionsky, D.J. Autophagy: Machinery and regulation. Microb. Cell 2016, 3, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Lavieu, G.; Scarlatti, F.; Sala, G.; Carpentier, S.; Levade, T.; Ghidoni, R.; Botti, J.; Codogno, P. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J. Biol. Chem. 2006, 281, 8518–8527. [Google Scholar] [CrossRef] [PubMed]
- Lépine, S.; Allegood, J.C.; Park, M.; Dent, P.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ. 2011, 18, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Suszynska, M.; Borkowska, S.; Ratajczak, J.; Schneider, G. The role of sphingosine-1 phosphate and ceramide-1 phosphate in trafficking of normal stem cells and cancer cells. Expert Opin. Ther. Targets 2014, 18, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, D.; Hafler, D.A. Fingolimod for multiple sclerosis. N. Engl. J. Med. 2012, 366, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Goparaju, S.K.; Jolly, P.S.; Watterson, K.R.; Bektas, M.; Alvarez, S.; Sarkar, S.; Mel, L.; Ishii, I.; Chun, J.; Milstien, S.; et al. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol. Cell. Biol. 2005, 25, 4237–4249. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, K.; Takuwa, N.; Yamaguchi, H.; Sugimoto, N.; Kitayama, J.; Nagawa, H.; Takehara, K.; Takuwa, Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J. Biol. Chem. 2003, 278, 32841–32851. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, R.; Zhou, X.; Liang, X.; Campbell, D.J.; Zhang, X.; Zhang, L.; Shi, R.; Wang, G.; Pandak, W.M.; et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 2014, 60, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, H.; Shankar, G.; Huang, M.C.; Bikle, D.D.; Goetzl, E.J. Biochemical regulation of breast cancer cell expression of S1P2 (Edg-5) and S1P3 (Edg-3) G protein-coupled receptors for sphingosine 1-phosphate. J. Cell. Biochem. 2003, 88, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, H.; Lin, T.; Wang, S. Sphingosine-1-phosphate/S1P receptors signaling modulates cell migration in human bone marrow-derived mesenchymal stem cells. Mediat. Inflamm. 2014, 2014, 565369. [Google Scholar] [CrossRef] [PubMed]
- Filipenko, I.; Schwalm, S.; Reali, L.; Pfeilschifter, J.; Fabbro, D.; Huwiler, A.; Zangemeister-Wittke, U. Upregulation of the S1P3 receptor in metastatic breast cancer cells increases migration and invasion by induction of PGE2 and EP2/EP4 activation. Biochim. Biophys. Acta 2016, 1861, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yao, X.; Chen, L.; Yan, Z.; Liu, J.; Zhang, Y.; Feng, T.; Wu, J.; Liu, X. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/ syndecan-1/TGF-β autocrine loop. Oncotarget 2016, 7, 63324–63337. [Google Scholar] [CrossRef] [PubMed]
- Beach, J.A.; Aspuria, P.J.; Cheon, D.J.; Lawrenson, K.; Agadjanian, H.; Walsh, C.S.; Karlan, B.Y.; Orsulic, S. Sphingosine kinase 1 is required for TGF-β mediated fibroblastto- myofibroblast differentiation in ovarian cancer. Oncotarget 2016, 7, 4167–4182. [Google Scholar] [CrossRef] [PubMed]
- Canals, D.; Jenkins, R.W.; Roddy, P.; Hernández-Corbacho, M.J.; Obeid, L.M.; Hannun, Y.A. Differential effects of ceramide and sphingosine 1-phosphate on ERM phosphorylation: Probing sphingolipid signaling at the outer plasma membrane. J. Biol. Chem. 2010, 285, 32476–32485. [Google Scholar] [CrossRef] [PubMed]
- Gandy, K.A.; Canals, D.; Adada, M.; Wada, M.; Roddy, P.; Snider, A.J.; Hannun, Y.A.; Obeid, L.M. Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. Biochem. J. 2013, 449, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Orr Gandy, K.A.; Adada, M.; Canals, D.; Carroll, B.; Roddy, P.; Hannun, Y.A.; Obeid, L.M. Epidermal growth factor-induced cellular invasion requires sphingosine-1-phosphate/ sphingosine-1-phosphate 2 receptor-mediated ezrin activation. FASEB J. 2013, 27, 3155–3166. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Cha, Y.; Ham, M.; Jung, J.; Kim, S.G.; Hwang, S.; Kleemann, R.; Moon, A. Inflammatory lipid sphingosine-1-phosphate upregulates C-reactive protein via C/EBPβ and potentiates breast cancer progression. Oncogene 2014, 33, 3583–3893. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.F.; Ozaki, H.; Zhan, X.; Wang, E.; Hla, T.; Lee, M.J. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem. Cell Biol. 2006, 126, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.A.; Argraves, K.M.; Obeid, L.M. Sphingosine-1-phosphate receptors: Receptor specificity versus functional redundancy. Biochim. Biophys. Acta 2004, 1682, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Sadahira, Y.; Ruan, F.; Hakomori, S.; Igarashi, Y. Sphingosine-1-phosphate inhibits actin nucleation and pseudopodium formation to control cell motility of mouse melanoma cells. FEBS Lett. 1996, 382, 193–197. [Google Scholar] [CrossRef]
- Koh, E.; Clair, T.; Hermansen, R.; Bandle, R.W.; Schiffmann, E.; Roberts, D.D.; Stracke, M.L. Sphingosine-1-phosphate initiates rapid retraction of pseudopodia by localized RhoA activation. Cell. Signal. 2007, 19, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.G.; Sun, C.; Bittman, R.; Pyne, N.J.; Pyne, S. (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: Effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cell. Signal. 2011, 23, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Andreeva, O.E.; Shatskaya, V.A.; Krasil’nikov, M.A. The relationships between snail1 and estrogen receptor signaling in breast cancer cells. J. Cell. Biochem. 2012, 113, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, F.; Li, S.; Chang, A.K.; Jia, Z.; Chen, Y.; Xu, F.; Pan, H.; Wu, H. AIB1 cooperates with ERα to promote epithelial mesenchymal transition in breast cancer through SNAI1 activation. PLoS ONE 2013, 8, e65556. [Google Scholar] [CrossRef] [PubMed]
- Urtz, N.; Gaertner, F.; von Bruehl, M.L.; Chandraratne, S.; Rahimi, F.; Zhang, L.; Orban, M.; Barocke, V.; Beil, J.; Schubert, I.; et al. Sphingosine 1-phosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo. Circ. Res. 2015, 117, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.D.; Pepe, G.J. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int. J. Dev. Biol. 2010, 54, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Yamada, A.; Rashid, O.M.; Adams, B.J.; Huang, W.C.; Aoyagi, T.; Nagahashi, M. Twofer anti-vascular therapy targeting sphingosine-1-phosphate for breast cancer. Gland Surg. 2012, 1, 80–83. [Google Scholar] [PubMed]
- Maeda, Y.; Matsuyuki, H.; Shimano, K.; Kataoka, H.; Sugahara, K.; Chiba, K. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J. Immunol. 2007, 178, 3437–3446. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.K.; O’Hara, M.; Lacey, H.A.; Corcoran, J.; Hemmings, D.G.; Wareing, M.; Baker, P.; Taggart, M.J. Modulation of human arterial tone during pregnancy: The effect of the bioactive metabolite sphingosine-1-phosphate. Biol. Reprod. 2007, 77, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.; Naymark, M.; Sauer, L.; Muhammad, A.; Keun, H.; Sturge, J.; Stebbing, J.; Waxman, J.; Pchejetski, D. Circulating sphingosine-1-phosphate and erythrocyte sphingosine kinase-1 activity as novel biomarkers for early prostate cancer detection. Br. J. Cancer 2012, 106, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Lisec, J.; Piechulla, B.; Nebe, B. Metabolic profiling reveals sphingosine-1-phosphate kinase 2 and lyase as key targets of (phyto-) estrogen action in the breast cancer cell line MCF-7 and not in MCF-12A. PLoS ONE 2012, 7, e47833. [Google Scholar] [CrossRef] [PubMed]
- Blom, T.; Bergelin, N.; Slotte, J.P.; Törnquist, K. Sphingosine kinase regulates voltage operated calcium channels in GH4C1 rat pituitary cells. Cell. Signal. 2006, 18, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, T.; Okada, T.; Yu, H.; Goparaju, S.K.; Jahangeer, S.; Nakamura, S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol. Cell. Biol. 2007, 27, 3429–3440. [Google Scholar] [CrossRef] [PubMed]
- Pyszko, J.A.; Strosznajder, J.B. The key role of sphingosine kinases in the molecular mechanism of neuronal cell survival and death in an experimental model of Parkinson’s disease. Folia Neuropathol. 2014, 52, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, N.; Sasaki, T.; Ebinuma, I.; Osawa, S.; Isshiki, H.; Takeo, K.; Tomita, T.; Iwatsubo, T. FTY720/fingolimod, a sphingosine analogue, reduces amyloid-β production in neurons. PLoS ONE 2013, 8, e64050. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tobón, A.; Cepeda-Prado, E.; Cardona-Gómez, G.P. Decrease of Tau hyperphosphorylation by 17β estradiol requires sphingosine kinase in a glutamate toxicity model. Neurochem. Res. 2009, 34, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Iwaki, S.; Koike, K.; Yuda, Y.; Nagasaki, A.; Ohkawa, R.; Yatomi, Y.; Furumoto, T.; Tsutsui, H.; Sobel, B.E.; et al. Increased plasma sphingosine-1-phosphate in obese individuals and its capacity to increase the expression of plasminogen activator inhibitor-1 in adipocytes. Coron Artery Dis. 2013, 24, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, H.; Wang, Q.; Frampton, A.E.; Krell, J.; Waxman, J.; Winkler, M.; Stebbing, J.; Cooper, C.; Yagüe, E.; Pchejetski, D. Sphingosine kinase 1 contributes to leptin-induced STAT3 phosphorylation through IL-6/gp130 transactivation in oestrogen receptor-negative breast cancer. Breast Cancer Res. Treat. 2015, 149, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Badeanlou, L.; Bielawski, J.; Ciaraldi, T.P.; Samad, F. Sphingosine kinase 1 regulates adipose proinflammatory responses and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E756–E768. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.; Cabot, M.C. Tamoxifen regulation of sphingolipid metabolism—Therapeutic implications. Biochim. Biophys. Acta 2015, 1851, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Yang, Y.L.; He, L.; Gu, B.; Xia, J.P.; Sun, W.L.; Su, Z.L.; Chen, B.; Bi, Z.G. Increasing ceramides sensitizes genistein-induced melanoma cell apoptosis and growth inhibition. Biochem. Biophys. Res. Commun. 2012, 421, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Lucki, N.C.; Sewer, M.B. Genistein stimulates MCF-7 breast cancer cell growth by inducing acid ceramidase (ASAH1) gene expression. J. Biol. Chem. 2011, 286, 19399–19409. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukocheva, O.A. Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming. Int. J. Mol. Sci. 2018, 19, 420. https://doi.org/10.3390/ijms19020420
Sukocheva OA. Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming. International Journal of Molecular Sciences. 2018; 19(2):420. https://doi.org/10.3390/ijms19020420
Chicago/Turabian StyleSukocheva, Olga A. 2018. "Expansion of Sphingosine Kinase and Sphingosine-1-Phosphate Receptor Function in Normal and Cancer Cells: From Membrane Restructuring to Mediation of Estrogen Signaling and Stem Cell Programming" International Journal of Molecular Sciences 19, no. 2: 420. https://doi.org/10.3390/ijms19020420