Comparative Transcriptome Analyses Uncover Key Candidate Genes Mediating Flight Capacity in Bactrocera dorsalis (Hendel) and Bactrocera correcta (Bezzi) (Diptera: Tephritidae)
Abstract
:1. Introduction
2. Results
2.1. Flight Capacity Differs Significantly between Two Bactrocera Species
2.2. Qualitative Description for Assembly and Annotation of Transcriptomes
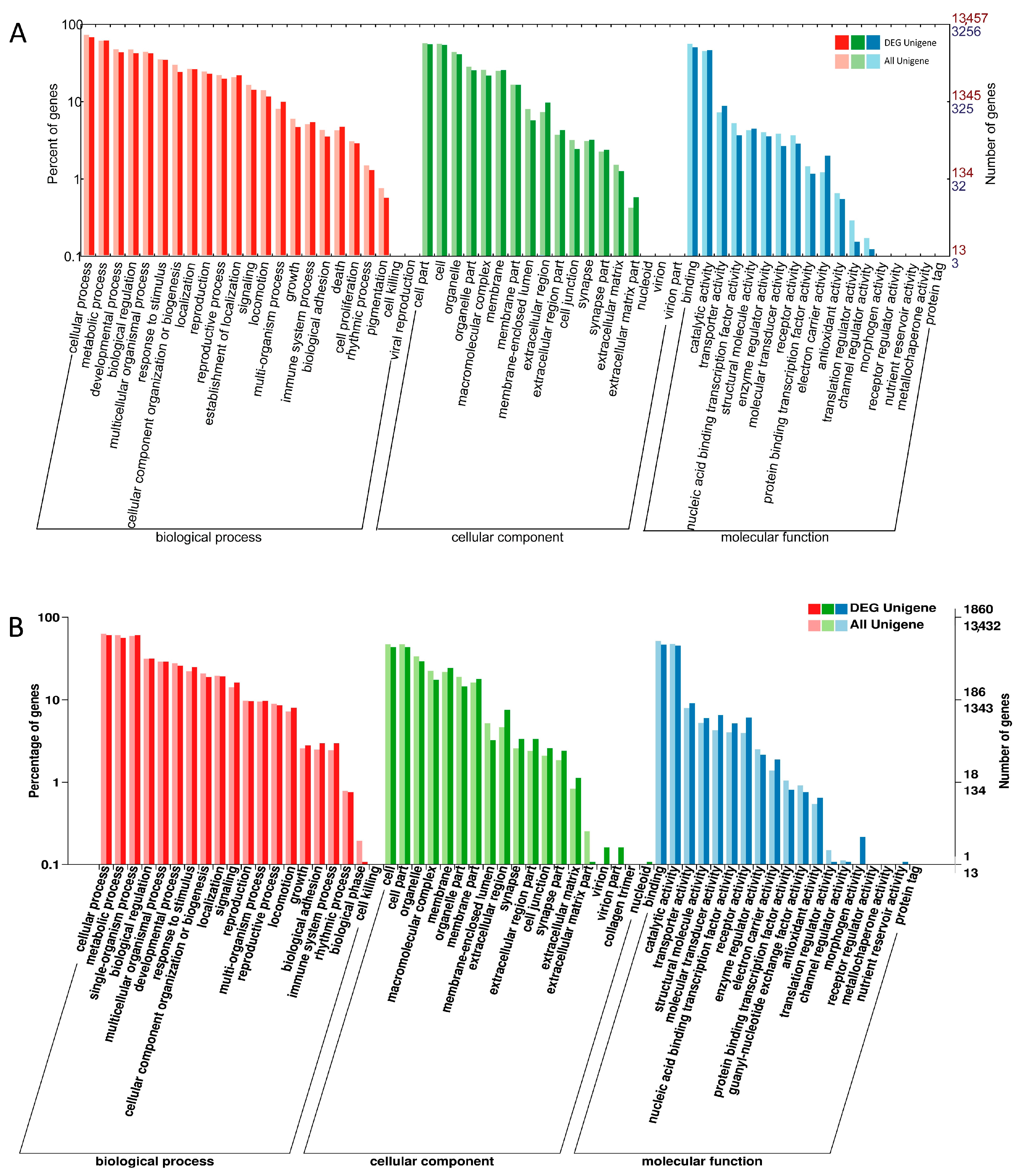
2.3. DEGs Annotation in GO, COG and KEGG Databases
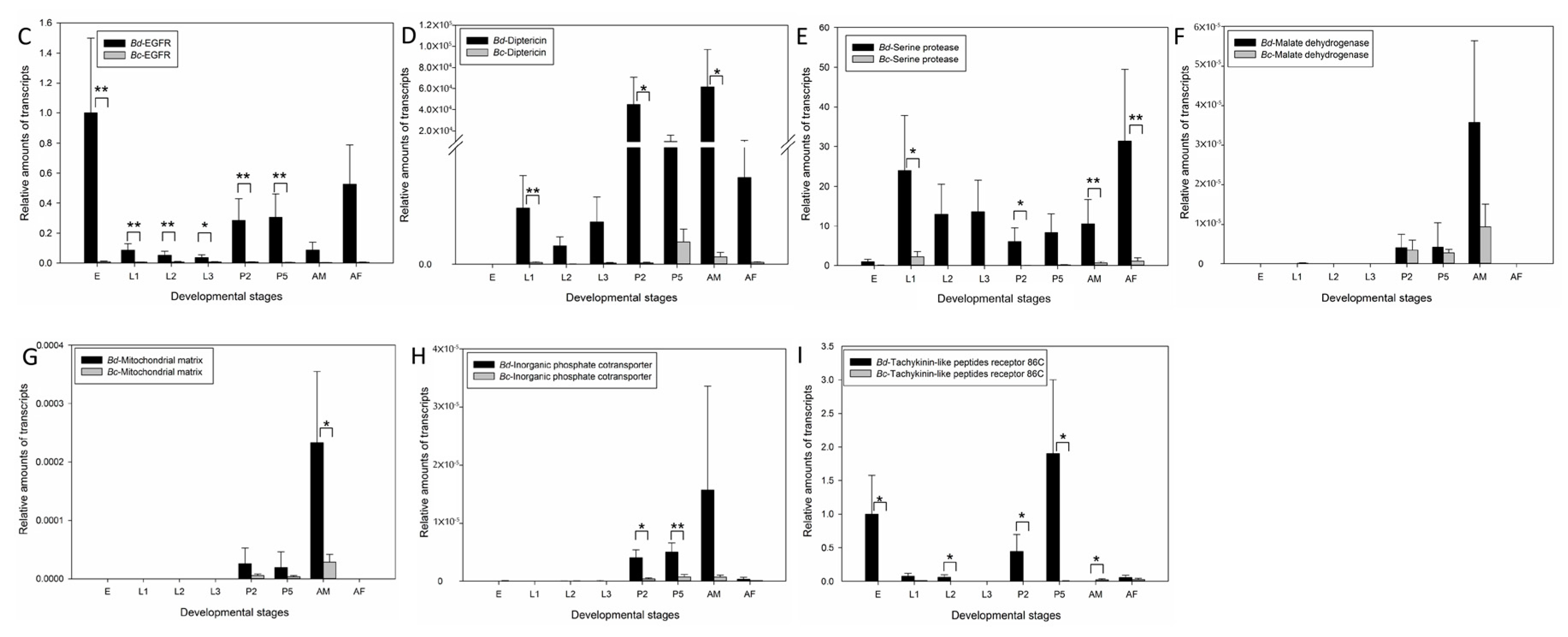
2.4. Candidate Genes Involved in Flight Capacity
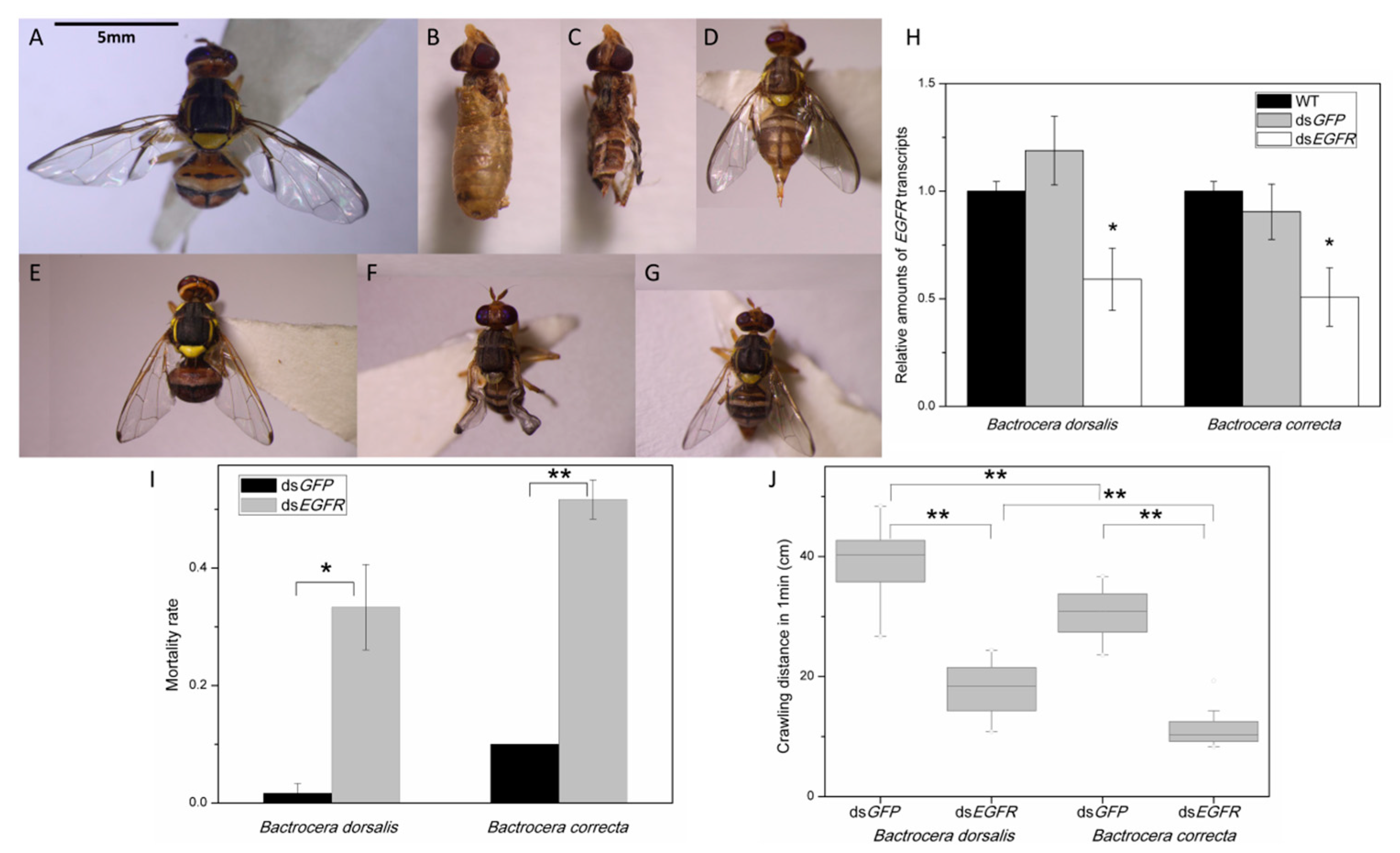
2.5. Suppression of EGFR via RNAi Markedly Inhibits Flight
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. Flight Capacity Tests
4.3. RNA-Seq
4.4. Assembly and Annotation of the Transcriptome
4.5. Differential Gene Expression Analysis
4.6. Synthesis of dsRNA and RNA Interference
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Ricciardi, A. Are modern biological invasions an unprecedented form of global change? Conserv. Biol. 2007, 21, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.A.I.; Hooper, G.H.S.; Bateman, M.A. Economic Fruit Flies of the South Pacific Region; Oriental Fruit Fly Working Party, Standing Committee on Agriculture: Canberra, Australia, 1978; ISBN 0-7242-0889-5. [Google Scholar]
- Li, Z.H.; Jiang, F.; Ma, X.L.; Fang, Y.; Sun, Z.Z.; Qin, Y.J.; Wang, Q.L. Review on prevention and control techniques of Tephritidae invasion. Plant Quar. 2013, 27, 1–10. [Google Scholar]
- Vargas, B.I.; Walsh, W.A.; Jang, E.B.; Armstrong, J.W.; Kanehisa, D.T. Survival and development of immature stages of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Ann. Entomol. Soc. Am. 1996, 89, 64–69. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit flies of economic significance: Their identification and bionomics. CAB International: Wallingford, WA, USA, 1992; ISBN 0-85198-790-7. [Google Scholar]
- Deng, Z.S. Research progress in control of oriental fruit fly Dacus (Bactrocera) dorsalis (Hendal) in tropical orchard. Chin. J. Trop. Crop. 2001, 22, 91–95. [Google Scholar]
- Chen, J.H.; Zhang, N.N.; Ji, Q.E.; Yang, J.Q.; Zheng, M.L. Genetic structure analysis of three kinds of fruit fly of economic important in China. In Proceedings of the Ninth International Symposium on Fruit Flies of Economic Importance (9th ISFFEI), Bangkok, Thailand, 12–16 May 2014; ISBN 978-616-358-207-2. [Google Scholar]
- Qu, H.X.; Sun, J.S. Observation of the living habit of Bactrocera dorsalis. Chin. Hortic. Abstr. 2013, 2, 51. [Google Scholar]
- Liu, X.F.; Jin, Y.; Ye, H. Recent spread and climatic ecological niche of the invasive guava fruit fly, Bactrocera correcta, in mainland China. J. Pest. Sci. 2013, 86, 449–458. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.B.; Yin, J. Comparative studies on the flight ability of the social type and scattered type of Locusta migratoria manilensis. Plant Prot. 2007, 33, 34–37. [Google Scholar]
- Yang, F. The Study on Flight Ability among Geographic Populations of Asian Gypsy Moth, Lymantria dispar in China. Master’s Thesis, Beijing Forestry University, Beijing, China, 2013. [Google Scholar]
- Wang, F.Y.; Zhang, X.X.; Zhai, B.P. Flight and re-migration capacity of the rice leaf folder moth, Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Crambidae). Acta Entomol. Sin. 2010, 53, 1265–1272. [Google Scholar]
- Wang, Y.K.; Zhai, B. Re-migration capacity of the white-backed planthopper, Sogatella furcifera (Horvath). Acta Entomol. Sin. 2004, 47, 467–473. [Google Scholar]
- Yue, R.Q. The Study on the Relation between the Development and Flight Capacity of Colorado Potato Beetle (Leptinotarsa decemlineata). Master’s Thesis, Shihezi University, Shihezi, China, 2013. [Google Scholar]
- Liang, F.; Wu, J.J.; Liang, G.Q. The first report of the test on the flight ability of oriental fruit fly. Acta Agric. Univ. Jiangxiensis 2001, 23, 259–260. [Google Scholar]
- Yuan, R.L.; Yang, S.; Wang, X.W.; Chen, P. Test on flight ability of Bactrocera dorsalis. J. West China For. Sci. 2014, 43, 66–71. [Google Scholar]
- Chen, M.; Chen, P.; Ye, H.; Yuan, R.L.; Wang, X.W.; Xu, J. Flight capacity of Bactrocera dorsalis (Diptera: Tephritidae) adult females based on flight mill studies and flight muscle ultrastructure. J. Insect Sci. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.S. The biology of dacine fruit flies. Annu. Rev. Entomol. 1987, 32, 115–144. [Google Scholar] [CrossRef]
- Sharp, J.L.; Chambers, D.L.; Haramoto, F.H. Flight mill and stroboscopic studies of oriental fruit flies and melon flies, including observations of mediterranean fruit flies. Proc. Hawaii. Entomol. Soc. 1975, 22, 137–144. [Google Scholar]
- Steiner, L.F.; Mitchell, W.C.; Baum1iover, A.H. Progress of fruit fly control by irradiation sterilization in Hawaii and the Mariana Islands. Int. J. Appl. Radiat. Isot. 1962, 13, 427–434. [Google Scholar] [CrossRef]
- Hou, M.Z.; Shen, G.M.; Wei, D.; Li, Y.L.; Dou, W.; Wang, J.J. Characterization of Bactrocera dorsalis serine proteases and evidence for their indirect role in insecticide tolerance. Int. J. Mol. Sci. 2014, 15, 3272–3286. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.W.; Peng, T.; He, W.; Zhang, H.Y. High-throughput sequencing to reveal genes involved in reproduction and development in Bactrocera dorsalis (Diptera: Tephritidae). PLoS ONE 2012, 7, e36463. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Yuan, G.R.; Cong, L.; Xie, Y.F.; Wang, J.J. De novo cloning and annotation of genes associated with immunity, detoxification and energy metabolism from the fat body of the oriental fruit fly, Bactrocera dorsalis. PLoS ONE 2014, 9, e94470. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.G.; Peng, H.; Yao, Q.; Chen, H.X.; Xie, Q.; Tang, B.; Zhang, W.Q. Developmental control of a Lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 2009, 4, e6225. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.H.; Jiang, H.B.; Xu, L.; Pei, Y.X.; Liu, X.Q.; Smagghe, G.; Wang, J.J. Role of a tachykinin-related peptide and its receptor in modulating the olfactory sensitivity in the oriental fruit fly, Bactrocera dorsalis (Hendel). Insect Biochem. Mol. Biol. 2017, 80, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.L.; Jiang, H.B.; Gui, S.H.; Chen, E.H.; Wei, D.D.; Li, H.M.; Wang, J.J.; Smagghe, G. A role of corazonin receptor in larval-pupal transition and pupariation in the oriental fruit fly Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Front. Physiol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Lu, X.P.; Meng, L.W.; Huang, Y.; Wei, D.; Jiang, H.B.; Smagghe, G.; Wang, J.J. Functional characterization of an α-esterase gene involving malathion detoxification in Bactrocera dorsalis (Hendel). Pestic. Biochem. Physiol. 2016, 130, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.L.; Li, Q.J.; Zhang, H.Y. The noa gene is functionally linked to the activation of the Toll/Imd signaling pathways in Bactrocera dorsalis (Hendel). Dev. Comp. Immunol. 2016, 55, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Hou, M.Z.; Shen, G.M.; Lu, X.P.; Wang, Z.; Jia, F.X.; Wang, J.J.; Dou, W. Functional analysis of five trypsin-like protease genes in the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Pestic. Biochem. Physiol. 2017, 136, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Greener, M.J.; Roberts, R.G. Conservation of components of the dystrophin complex in Drosophila. FEBS Lett. 2000, 482, 13–18. [Google Scholar] [CrossRef]
- Sanada, Y.; Yasogawa, N.; Katunuma, N. Effect of a serine protease on isolated myofibrils. J. Biochem. 1979, 85, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, M.; Osatomi, K.; Hara, K.; Nozaki, Y.; Aranishi, F.; Ishihara, T. Purification and characterization of myofibril-bound serine protease from crucian carp Carassius giberio langsdorfi muscle. Fish. Sci. 2004, 70, 1183–1185. [Google Scholar] [CrossRef]
- Kronert, W.A.; Melkani, G.C.; Melkani, A.; Bernstein, S.I. Mutating the converter-relay interface of Drosophila myosin perturbs ATPase activity, actin motility, myofibril stability and flight ability. J. Mol. Biol. 2010, 398, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Beller, M.; Riedel, D.; Jansch, L.; Dieterich, G.; Wehland, J.; Jackle, H.; Kuhnlein, R.P. Characterization of the Drosophila lipid droplet subproteome. Mol. Cell. Proteom. 2006, 5, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Otsuka, M.; Morimoto, R.; Hirota, S.; Yatsushiro, S.; Takeda, J.; Yamamoto, A.; Moriyama, Y. Differentiation-associated Na+-dependent Inorganic Phosphate Cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. J. Biol. Chem. 2001, 276, 43400–43406. [Google Scholar] [CrossRef] [PubMed]
- Glinn, M.; Ni, B.; Paul, S.M. Characterization of Na+-dependent phosphate uptake in cultured fetal rat cortical neurons. J. Neurochem. 1995, 65, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Sanburg, L.L.; Kramer, K.J.; Kézdy, F.J.; Law, J.H.; Oberlander, H. Role of juvenile-hormone esterases and carrier proteins in insect development. Nature 1975, 253, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Broeck, J.V. Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster. Peptides 2001, 22, 241–254. [Google Scholar] [CrossRef]
- Neumann, C.; Cohen, S. Morphogens and pattern formation. Bioessays 1997, 19, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Strigini, M.; Cohen, S.M. Formation of morphogen gradients in the Drosophila wing. Semin. Cell Dev. Biol. 1999, 10, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Klein, T. Wing disc development in the fly: The early stages. Curr. Opin. Genet. Dev. 2001, 11, 470–475. [Google Scholar] [CrossRef]
- Morata, G. How Drosophila appendages develop. Nat. Rev. Mol. Cell Biol. 2001, 2, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.M. The development and evolution of crossveins in insect wings. J. Anat. 2001, 199, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.K.; Murakami, M.S.; Cleghon, V. Protein kinases and phosphatases in the Drosophila genome. J. Cell Biol. 2000, 150, F57–F62. [Google Scholar] [CrossRef] [PubMed]
- Strecker, T.R.; Yip, M.L.R.; Lipshitz, H.D. Zygotic genes that mediate torso receptor tyrosine kinase functions in the Drosophila melanogaster embryo. Proc. Natl. Acad. Sci. USA 1991, 88, 5824–5828. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, R.; Shilo, B.Z. A thousand and one roles for the Drosophila EGF receptor. Trends Genet. 1997, 13, 191–196. [Google Scholar] [CrossRef]
- De Celis, J.F. Pattern formation in the Drosophila wing: The development of the veins. Bioessays 2003, 25, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, I.; Gibson, G. Epidermal growth factor receptor and transforming growth factor-β signaling contributes to variation for wing shape in Drosophila melanogaster. Genetics 2006, 173, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.H.; Tu, M.S. The function of dipteran flight muscle. Comp. Biochem. Phys. A 1997, 116, 223–238. [Google Scholar] [CrossRef]
- Anderson, M.; Finlayson, L.H. Effect of exercise on growth of mitochondria and myofibrils in flight muscles of tsetse-fly, Glossina morsitans. J. Morphol. 1976, 150, 321–326. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Qiu, Y.; Zhang, Q.F.; Li, D. Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br. J. Nutr. 2014, 112, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Osada, Y.; Kimura, F.; Shirakawa, H.; Fujimoto, K. Effects of Japanese torreya (Torreya nucifera) seed oil on the activities and mRNA expression of lipid metabolism-related enzymes in rats. Biosci. Biotechnol. Biochem. 2006, 22, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen: London, UK, 1969. [Google Scholar]
- Lin, X.; Yao, Y.; Wang, B.; Emlen, D.J.; Lavine, L.C. Ecological trade-offs between migration and reproduction are mediated by the nutrition-sensitive insulin-signaling pathway. Int. J. Biol. Sci. 2016, 12, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Grönke, S.; Partridge, L. The functions of insulin-like peptides in insects. In IGFs: Local Repair and Survival Factors Throughout Life Span; Springer: Berlin, Germany, 2010; pp. 105–124. ISBN 978-3-642-04301-7. [Google Scholar] [CrossRef]
- Davenport, R.J. Big Mac and Flies: Signaling pathway tells insects when food’s on the table (Insulin-related signaling). Science 2002, 8, 28. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Kong, Q.; Xiao, C.; Yang, S.S.; Sun, W.; Zhang, J.B.; Li, Z.Y. Introduction to two kinds of artificial diets for mass rearing of adult Bactrocera dorsalis (Hendel). J. Huazhong Agric. Univ. 2006, 25, 371–374. [Google Scholar]
- Cheng, D.; Tian, Z.; Sun, J.; Ni, H.; Li, G.A. Computer monitored flight mill system for tiny insects such as aphids. Acta Entomol. Sin. 1997, 40, 172–179. [Google Scholar]
- Rowley, W.A.; Graham, C.L.; Williams, R.E. A flight mill system for the laboratory study of mosquito flight. Ann. Entomol. Soc. Am. 1968, 61, 1507–1514. [Google Scholar] [CrossRef]
- Schumacher, P.; Weyeneth, A.; Weber, D.C.; Dorn, S. Long flights in Cydia pomonella L. (Lepidoptera: Tortricidae) measured by a flight mill: Influence of sex, mated status and age. Physiol. Entomol. 1997, 22, 149–160. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Huang, X.Q.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids. Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Li, J.Q.; Wu, S.F.; Zhu, Y.P.; Chen, Y.W.; He, F.C. Integrated nr database in protein annotation system and its localization. Computer Eng. 2006, 32, 71–74. [Google Scholar]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.Z.; Lopez, R.; Magrane, M.; et al. UniProt: The Universal Protein knowledgebase. Nucleic Acids. Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 2073. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Zwinderman, A.H. On the Benjamini-Hochberg method. Ann. Stat. 2006, 34, 1827–1849. [Google Scholar] [CrossRef]
- You, S.S.; Cai, Q.; Zheng, Y.; He, B.C.; Shen, J.; Yang, W.T.; Yin, M.Z. Perylene-cored star-shaped polycations for fluorescent gene vectors and bioimaging. ACS Appl. Mater. Interfaces 2014, 6, 16327–16334. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Zhao, Z.; Liu, L.; Li, Z.; Shen, J. Comparative Transcriptome Analyses Uncover Key Candidate Genes Mediating Flight Capacity in Bactrocera dorsalis (Hendel) and Bactrocera correcta (Bezzi) (Diptera: Tephritidae). Int. J. Mol. Sci. 2018, 19, 396. https://doi.org/10.3390/ijms19020396
Guo S, Zhao Z, Liu L, Li Z, Shen J. Comparative Transcriptome Analyses Uncover Key Candidate Genes Mediating Flight Capacity in Bactrocera dorsalis (Hendel) and Bactrocera correcta (Bezzi) (Diptera: Tephritidae). International Journal of Molecular Sciences. 2018; 19(2):396. https://doi.org/10.3390/ijms19020396
Chicago/Turabian StyleGuo, Shaokun, Zihua Zhao, Lijun Liu, Zhihong Li, and Jie Shen. 2018. "Comparative Transcriptome Analyses Uncover Key Candidate Genes Mediating Flight Capacity in Bactrocera dorsalis (Hendel) and Bactrocera correcta (Bezzi) (Diptera: Tephritidae)" International Journal of Molecular Sciences 19, no. 2: 396. https://doi.org/10.3390/ijms19020396





