S1P Signalling Differentially Affects Migration of Peritoneal B Cell Populations In Vitro and Influences the Production of Intestinal IgA In Vivo
Abstract
:1. Introduction
2. Results
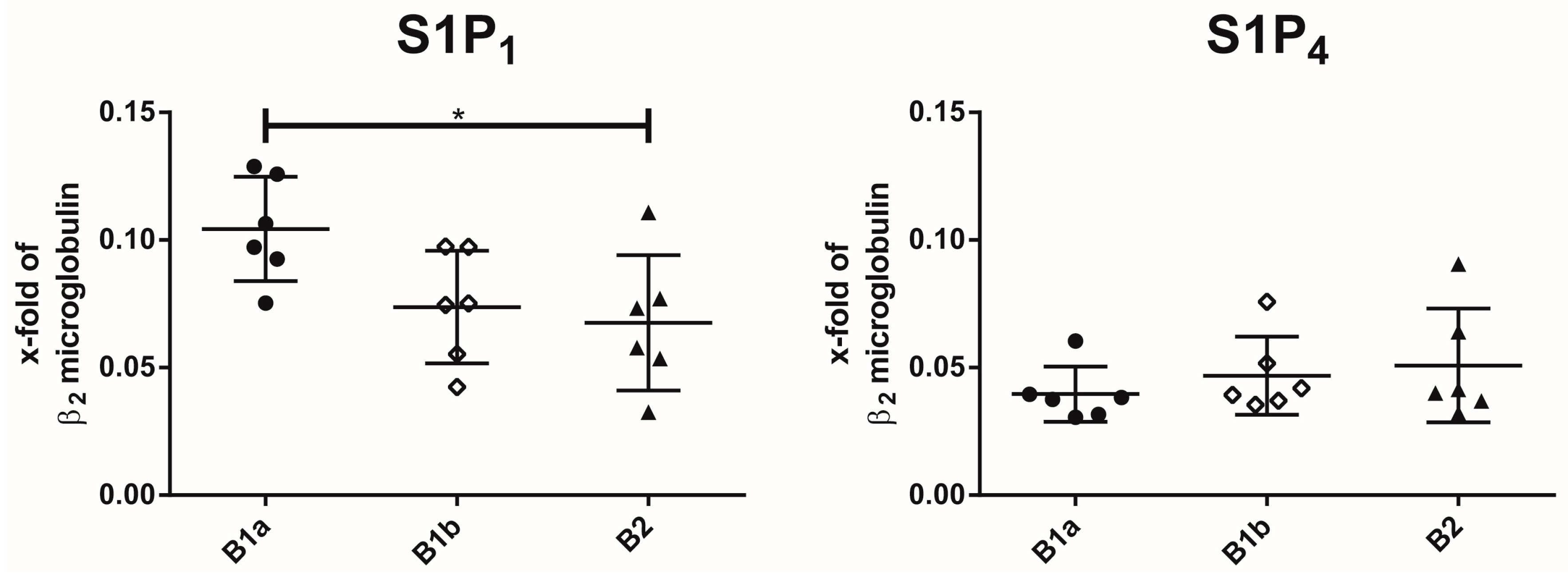
2.1. Expression of S1P Receptor Subtypes on Peritoneal B Cell Subpopulations
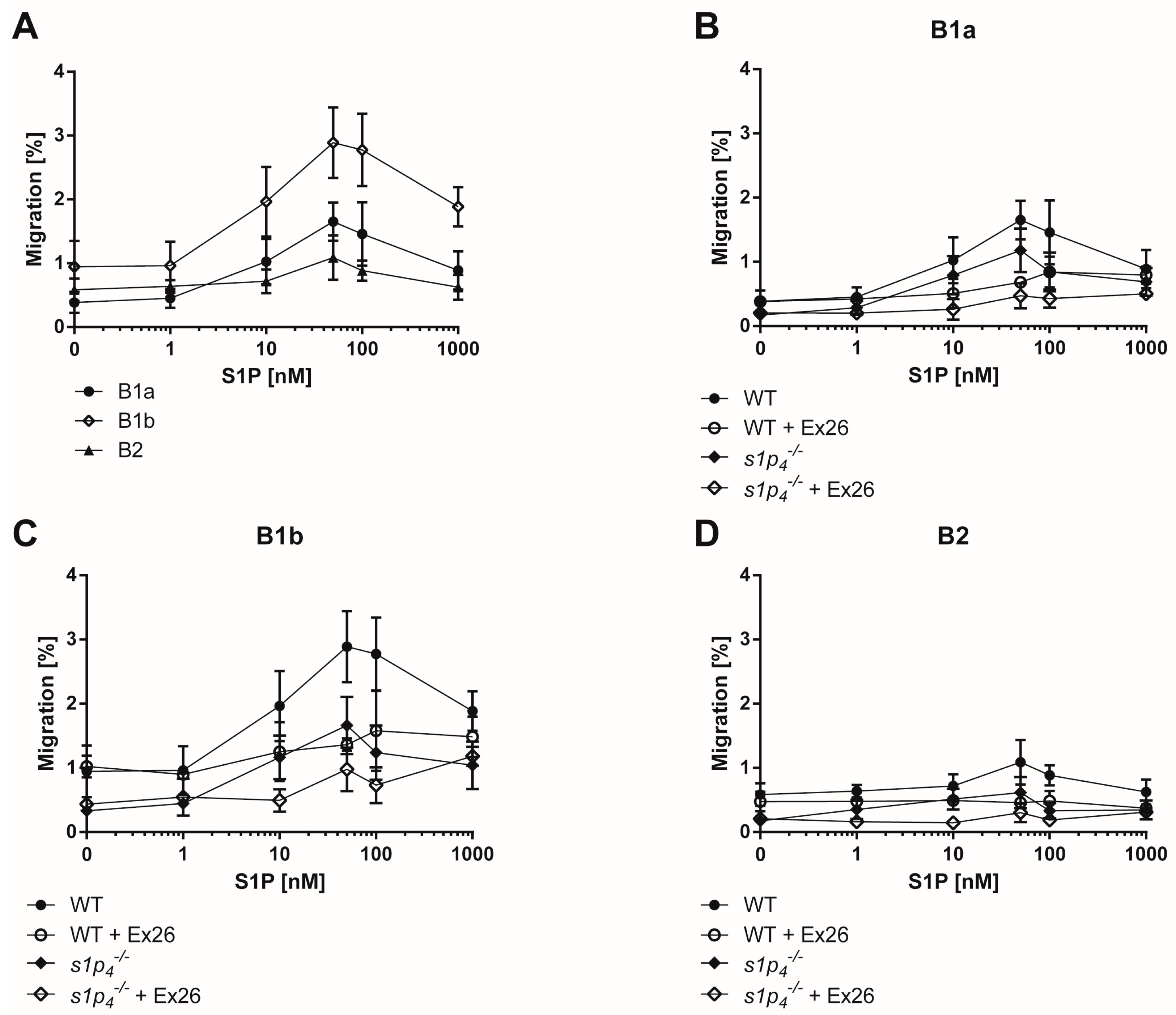
2.2. S1P-Induced Chemotaxis Is Mediated Synergistically via S1P1 and S1P4
2.3. S1P4 Deficiency Induced Profound Changes in Peritoneal B Cell Populations
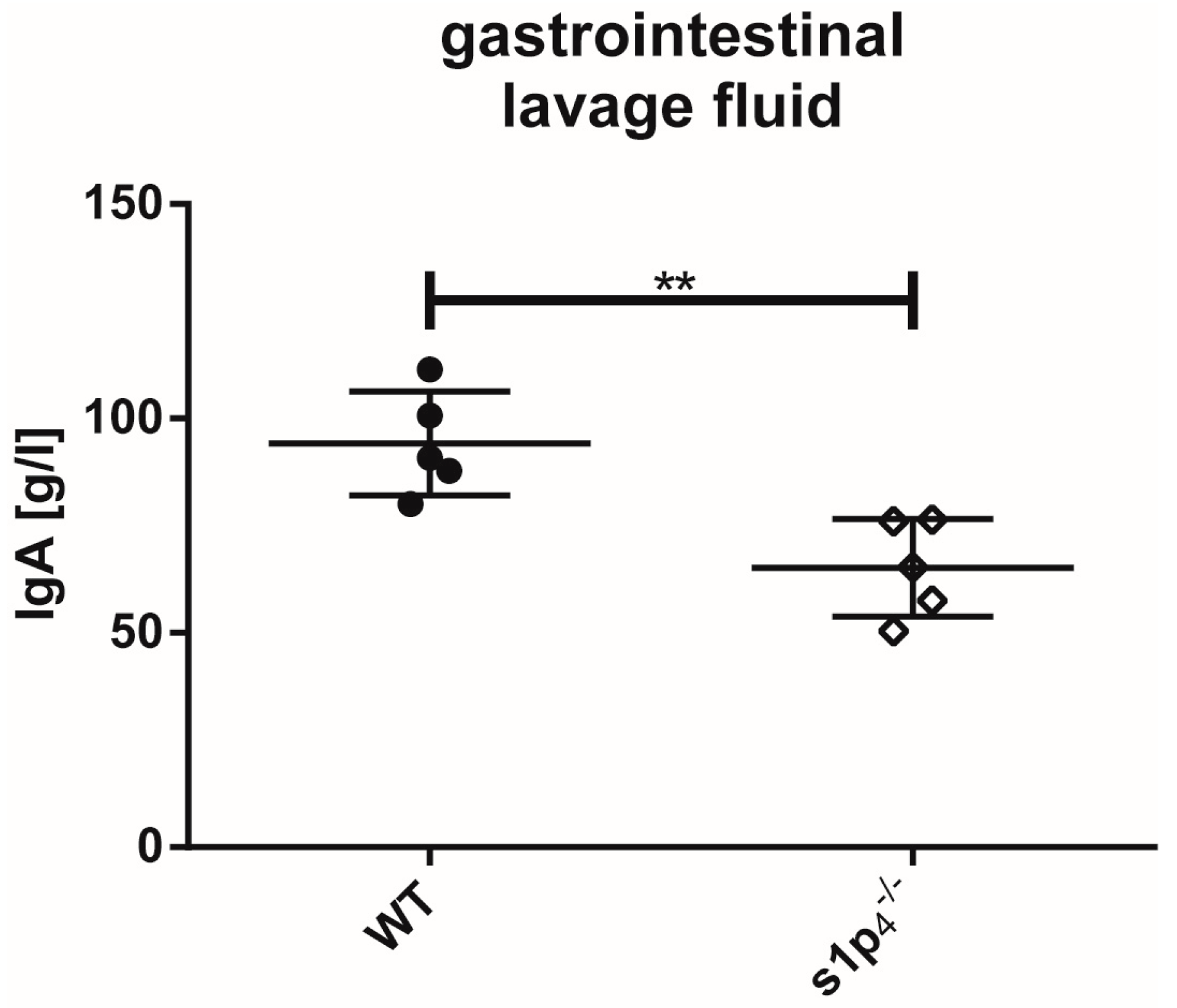
2.4. Influence of S1P4 Deficiency on Intestinal IgA Levels
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Isolation from the Peritoneal Cavity
4.3. Flow Cytometry and Fluorescence-Activated Cell Sorting (FACS)
4.4. RNA Isolation and qPCR
4.5. Transwell Migration Assay
4.6. Gastrointestinal Lavage
4.7. Antibody Quantification
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| S1P | Sphingosine-1-P |
| BALF | Bronchoalveolar lavage fluid |
| MZ | Marginal zone |
| Ab | Antibody |
| DC | Dendritic cells |
| PP | Peyer’s patches |
| Lp | Lamina propria |
| WT | Wild-type |
References
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 2017, 107. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklyn, J.R.; Williams, J.B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 163, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Proia, R.L.; Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008, 8, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; McNaughton, M.; Boomkamp, S.; MacRitchie, N.; Evangelisti, C.; Martelli, A.M.; Jiang, H.-R.; Ubhi, S.; Pyne, S. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv. Biol. Regul. 2016, 60, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannavo, A.; Liccardo, D.; Komici, K.; Corbi, G.; de Lucia, C.; Femminella, G.D.; Elia, A.; Bencivenga, L.; Ferrara, N.; Koch, W.J.; et al. Sphingosine Kinases and Sphingosine 1-Phosphate Receptors: Signaling and Actions in the Cardiovascular System. Front. Pharmacol. 2017, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Nofer, J.R.; Bot, M.; Brodde, M.; Taylor, P.J.; Salm, P.; Brinkmann, V.; van Berkel, T.; Assmann, G.; Biessen, E.A.L. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2007, 115, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; von Bernstorff, W.; Heidecke, C.D.; Schulze, T. Differential S1P Receptor Profiles on M1- and M2-Polarized Macrophages Affect Macrophage Cytokine Production and Migration. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Panther, E.; Corinti, S.; Morelli, A.; Ferrari, D.; Herouy, Y.; Dichmann, S.; Mockenhaupt, M.; Gebicke-Haerter, P.; Di Virgilio, F.; et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002, 16, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Czeloth, N.; Schippers, A.; Wagner, N.; Muller, W.; Kuster, B.; Bernhardt, G.; Forster, R. Sphingosine-1 Phosphate Signaling Regulates Positioning of Dendritic Cells within the Spleen. J. Immunol. 2007, 179, 5855–5863. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Matsuyuki, H.; Shimano, K.; Kataoka, H.; Sugahara, K.; Chiba, K. Migration of CD4 T Cells and Dendritic Cells toward Sphingosine 1-Phosphate (S1P) Is Mediated by Different Receptor Subtypes: S1P Regulates the Functions of Murine Mature Dendritic Cells via S1P Receptor Type 3. J. Immunol. 2007, 178, 3437–3446. [Google Scholar] [CrossRef] [PubMed]
- Graeler, M.; Goetzl, J. Activation-regulated expression and chemotctic function of shingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002, 16, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Cinamon, G.; Matloubian, M.; Lesneski, M.J.; Xu, Y.; Low, C.; Lu, T.; Proia, R.L.; Cyster, J.G. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 2004, 5, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Hammad, H.; van Nimwegen, M.; Kool, M.; Muller, T.; Soullie, T.; Willart, M.A.M.; Hijdra, D.; Hoogsteden, H.C.; Lambrecht, B.N.; et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J. Clin. Investig. 2006, 116, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Dorsam, G.; Graeler, M.H.; Seroogy, C.; Kong, Y.; Voice, J.K.; Goetzl, E.J. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J. Immunol. 2003, 171, 3500–3507. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Graeler, M.H.; Goetzl, E.J. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005, 19, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Kroese, F.G.; Butcher, E.C.; Stall, A.M.; Lalor, P.A.; Adams, S.; Herzenberg, L.A. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1989, 1, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Beagley, K.W.; Bao, S.; Ramsay, A.J.; Eldridge, J.H.; Husband, A.J. IgA production by peritoneal cavity B cells is IL-6 independent: Implications for intestinal IgA responses. Eur. J. Immunol. 1995, 25, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; von Andrian, U.H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008, 1, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Gronwall, C.; Vas, J.; Silverman, G.J. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Barbeiro, D.F.; Barbeiro, H.V.; Faintuch, J.; Ariga, S.K.; Mariano, M.; Popi, A.F.; Souza, H.P.; Velasco, I.T.; Soriano, F.G. B-1 cells temper endotoxemic inflammatory responses. Immunobiology 2011, 216, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Gao, W.; Degauque, N.; Bai, C.; Lu, Y.; Kenny, J.; Oukka, M.; Strom, T.B.; Rothstein, T.L. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur. J. Immunol. 2007, 37, 2400–2404. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Holodick, N.E.; Rothstein, T.L.; Wang, P. The role of B-1 cells in inflammation. Immunol. Res. 2015, 63, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Alugupalli, K.R.; Leong, J.M.; Woodland, R.T.; Muramatsu, M.; Honjo, T.; Gerstein, R.M. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 2004, 21, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Rauch, P.J.; Chudnovskiy, A.; Robbins, C.S.; Weber, G.F.; Etzrodt, M.; Hilgendorf, I.; Tiglao, E.; Figueiredo, J.L.; Iwamoto, Y.; Theurl, I.; et al. Innate response activator B cells protect against microbial sepsis. Science 2012, 335, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Haynes, N.M.; Xu, Y.; Nutt, S.L.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 2006, 203, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Gohda, M.; Kunisawa, J.; Miura, F.; Kagiyama, Y.; Kurashima, Y.; Higuchi, M.; Ishikawa, I.; Ogahara, I.; Kiyono, H. Sphingosine 1-Phosphate Regulates the Egress of IgA Plasmablasts from Peyer’s Patches for Intestinal IgA Responses. J. Immunol. 2008, 180, 5335–5343. [Google Scholar] [CrossRef] [PubMed]
- Cinamon, G.; Zachariah, M.A.; Lam, O.M.; Foss, F.W., Jr.; Cyster, J.G. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 2008, 9, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kurashima, Y.; Gohda, M.; Higuchi, M.; Ishikawa, I.; Miura, F.; Ogahara, I.; Kiyono, H. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood 2007, 109, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.; Golfier, S.; Tabeling, C.; Rabel, K.; Graler, M.H.; Witzenrath, M.; Lipp, M. Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011, 25, 4024–4036. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Lee, J.G.; Shin, S.H.; Kim, T.J. LPS-induced migration of peritoneal B-1 cells is associated with upregulation of CXCR4 and increased migratory sensitivity to CXCL12. J. Korean Med. Sci. 2012, 27, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ansel, K.M.; Harris, R.B.; Cyster, J.G. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 2002, 16, 67–76. [Google Scholar] [CrossRef]
- Externest, D.; Meckelein, B.; Schmidt, M.A.; Frey, A. Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage. Infect. Immun. 2000, 68, 3830–3839. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Saba, J.D. Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: The fat’s in the fire. Transl. Cancer Res. 2015, 4, 469–483. [Google Scholar] [PubMed]
- Choi, Y.S.; Dieter, J.A.; Rothaeusler, K.; Luo, Z.; Baumgarth, N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur. J. Immunol. 2012, 42, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Maseda, D.; Candando, K.M.; Smith, S.H.; Kalampokis, I.; Weaver, C.T.; Plevy, S.E.; Poe, J.C.; Tedder, T.F. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. J. Immunol. 2013, 191, 2780–2795. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, S.; Staun-Ram, E.; Miller, A. Fingolimod therapy modulates circulating B cell composition, increases B regulatory subsets and production of IL-10 and TGFbeta in patients with Multiple Sclerosis. J. Autoimmun. 2016, 70, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Itakura, A.; Szczepanik, M.; Campos, R.A.; Paliwal, V.; Majewska, M.; Matsuda, H.; Takatsu, K.; Askenase, P.W. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J. Immunol. 2005, 175, 7170–7178. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, S.M.; Gonzalez-Cabrera, P.J.; Nguyen, N.; Guerrero, M.; Cisar, E.A.; Leaf, N.B.; Brown, S.J.; Roberts, E.; Rosen, H. Sphingosine 1-phosphate receptor 1 (S1P(1)) upregulation and amelioration of experimental autoimmune encephalomyelitis by an S1P(1) antagonist. Mol. Pharmacol. 2013, 83, 316–321. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence |
|---|---|
| S1P1 forward | 5′-AAC TTT GCG AGT GAG CTG GT-3′ |
| S1P1 reverse | 5′-CTA GAG GGC GAG GTT GAG TG-3′ |
| S1P2 forward | 5′-ATA GAC CGA GCA CAG CCA AC-3′ |
| S1P2 reverse | 5′-GAG GTG GTC TCC TGC ATG TC-3′ |
| S1P3 forward | 5′-AAG CCT AGC GGG AGA GAA AC-3′ |
| S1P3 reverse | 5′-TCA GGG AAC AAT TGG GAG AG-3′ |
| S1P4 forward | 5′-GGA CTT CTC GGT CAC TCA GC-3′ |
| S1P4 reverse | 5′-GGC TTG CTG TCA TGT TCT CA-3′ |
| S1P5 forward | 5′-GGA GGG ACT CTC CTG GAT TC-3′ |
| S1P5 reverse | 5′-TTC CTC TGT AGC CAG CCA CT-3′ |
| β2 microglobulin forward | 5′-ATT CAC CCC CAC TGA GAC TG-3′ |
| β2 microglobulin reverse | 5′-GCT ATT TCT TTC TGC GTG CAT-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleinwort, A.; Lührs, F.; Heidecke, C.-D.; Lipp, M.; Schulze, T. S1P Signalling Differentially Affects Migration of Peritoneal B Cell Populations In Vitro and Influences the Production of Intestinal IgA In Vivo. Int. J. Mol. Sci. 2018, 19, 391. https://doi.org/10.3390/ijms19020391
Kleinwort A, Lührs F, Heidecke C-D, Lipp M, Schulze T. S1P Signalling Differentially Affects Migration of Peritoneal B Cell Populations In Vitro and Influences the Production of Intestinal IgA In Vivo. International Journal of Molecular Sciences. 2018; 19(2):391. https://doi.org/10.3390/ijms19020391
Chicago/Turabian StyleKleinwort, Annabel, Felix Lührs, Claus-Dieter Heidecke, Martin Lipp, and Tobias Schulze. 2018. "S1P Signalling Differentially Affects Migration of Peritoneal B Cell Populations In Vitro and Influences the Production of Intestinal IgA In Vivo" International Journal of Molecular Sciences 19, no. 2: 391. https://doi.org/10.3390/ijms19020391





