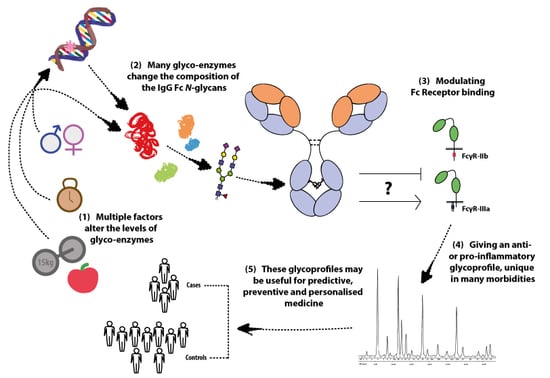
Unravelling Immunoglobulin G Fc N-Glycosylation: A Dynamic Marker Potentiating Predictive, Preventive and Personalised Medicine
Abstract
:1. Introduction
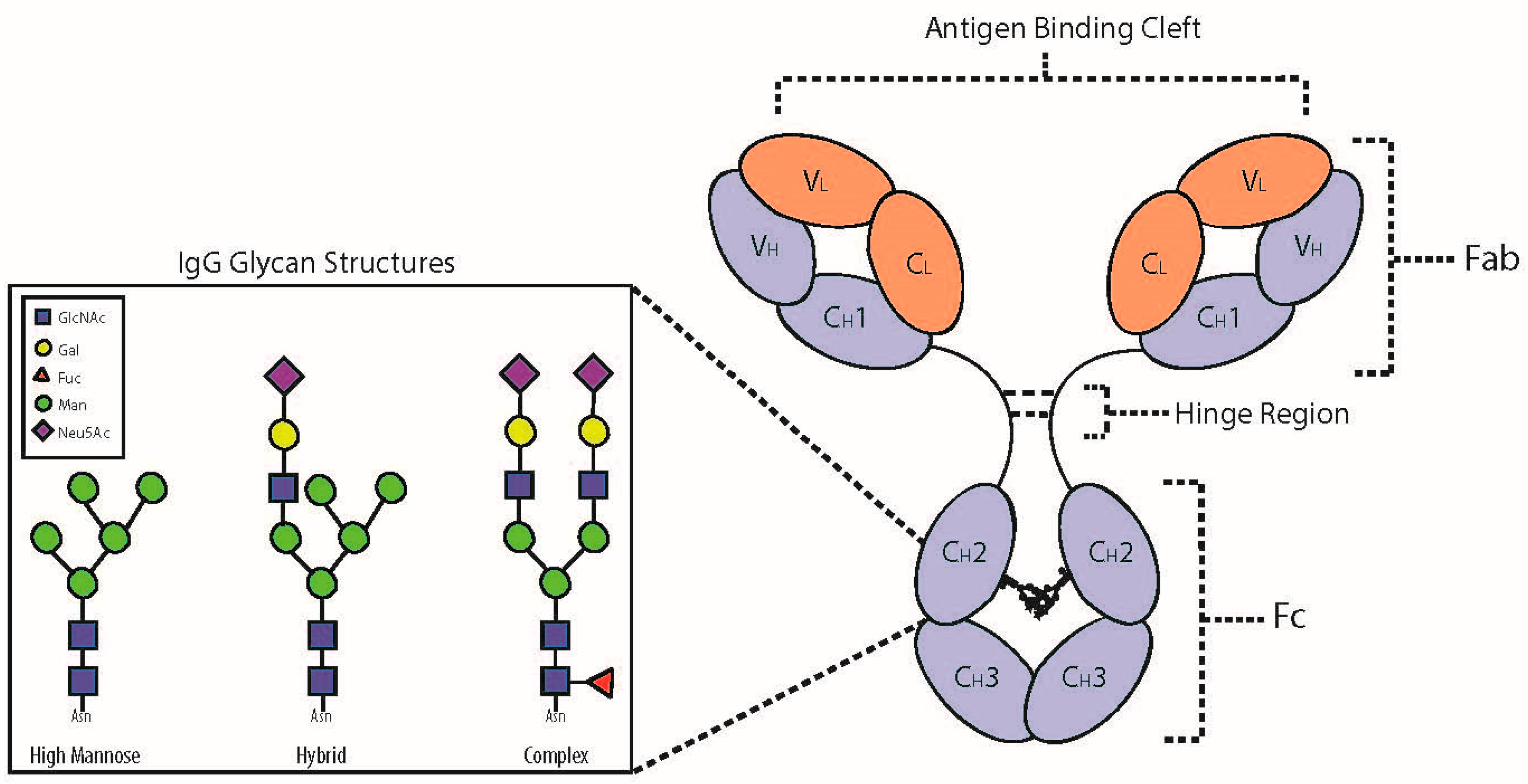
2. IgG Glycoprotein Structure and Function
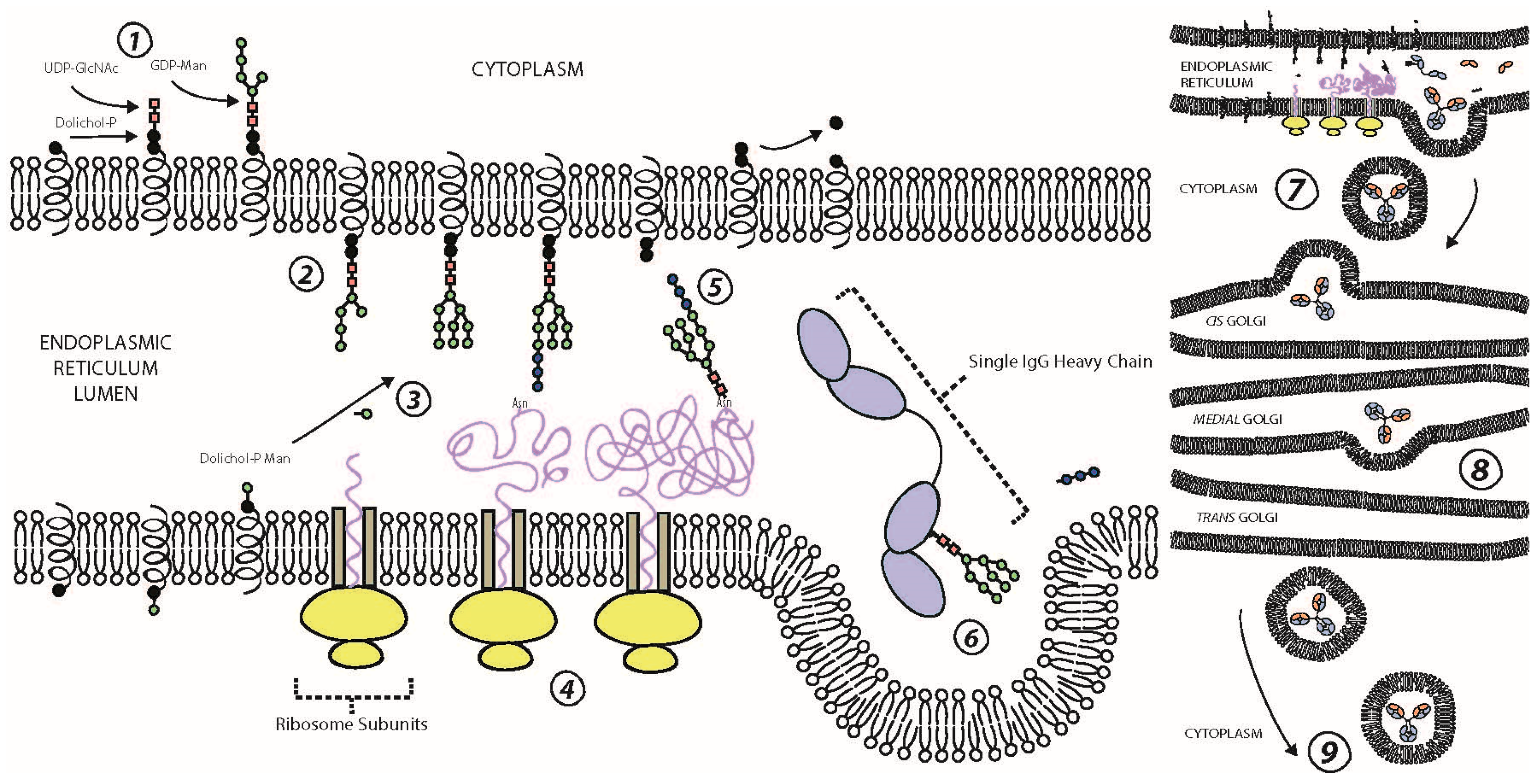
3. N-Glycosylation of the Fc Domain
4. IgG Fc Structure and Function
Coengagement of Activating and Inhibitory FcRs
5. Fc Effector Functions
6. The Complement Cascade
7. Loci Associated with Aberrant IgG Glycosylation
8. Environmental Factors Associated with Aberrant IgG Glycosylation
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IgG | Immunoglobulin G |
| FcR | Fragment crystallisable receptor |
| ADCC | Antibody-dependent cell cytotoxicity |
| MBL | Mannose-binding lectin |
| Fc | Fragment crystallisable |
| Fab | Fragment antibody-binding |
| Asn | Asparagine |
| Ser | Serine |
| Thr | Threonine |
| ER | Endoplasmic reticulum |
| Man | Mannose |
| GlcNAc | N-acetylglucosamine |
| Glc | Glucose |
| BCR | B-cell receptor |
| Neu5Ac | N-acetylneuraminic acid (aka sialic acid) |
| Fuc | Fucose |
| DC-SIGN | Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin |
| IC | Immune complex |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| SH2 | Src homology 2 |
| PI-3K | Phosphatidylinositol 3-kinase |
| MAPK | Mitogen activated protein kinase |
| PLC-γ | Phospholipase C-gamma |
| PIP2 | Phosphatidylinositol 4, 5-bisphosphate |
| IP3 | Inositol 1, 4, 5-triphosphate |
| DAG | Diacylglycerol |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| SHIP | SH2 inositol 5-phosphate |
| PTEN | Tensin homologue |
| PIP3 | Phosphatidylinositol-3, 4, 5-triphosphate |
| PKC | Protein kinase C |
| MAC | Membrane attack complex |
| BMI | Body mass index |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| TC | Total cholesterol |
| TG | Triglycerides |
| FBG | Fasting blood glucose |
References
- Subedi, G.P.; Barb, A.W. The structural role of antibody N-glycosylation in receptor interactions. Structure 2015, 23, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Krause, I.; Wu, R.; Sherer, Y.; Patanik, M.; Peter, J.; Shoenfeld, Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations—A potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transf. Med. 2002, 12, 133–139. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; de Kimpe, S.J.; Hellwig, S.M.M.; van Lent, P.L.; Hofhuis, F.M.A.; van Ojik, H.H.; Sedlik, C.; da Silveira, S.A.; Gerber, J.; de Jong, Y.F.; et al. FcγRI (CD64) Contributes Substantially to Severity of Arthritis, Hypersensitivity Responses, and Protection from Bacterial Infection. Immunity 2002, 16, 391–402. [Google Scholar] [CrossRef]
- Schwab, I.; Nimmerjahn, F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat. Rev. Immunol. 2013, 13, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2002, 2, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Quast, I.; Lünemann, J.D. Fc glycan-modulated immunoglobulin G effector functions. J. Clin. Immunol. 2014, 34, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Wormald, M.R.; Rudd, P.M.; Fischer, P.B.; Dwek, R.A.; Sim, R.B. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1995, 1, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Pučić, M.; Knežević, A.; Vidič, J.; Adamczyk, B.; Novokmet, M.; Polašek, O.; Gornik, O.; Šupraha-Goreta, S.; Wormald, M.R.; Redžić, I. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteom. 2011, 10, M111.010090. [Google Scholar] [CrossRef] [PubMed]
- Lauc, G.; Huffman, J.E.; Pučić, M.; Zgaga, L.; Adamczyk, B.; Mužinić, A.; Novokmet, M.; Polašek, O.; Gornik, O.; Krištić, J. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013, 9, e1003225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Balog, C.I.; Stavenhagen, K.; Koeleman, C.A.; Scherer, H.U.; Selman, M.H.; Deelder, A.M.; Huizinga, T.W.; Toes, R.E.; Wuhrer, M. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell. Proteom. 2011, 10, M110.004655. [Google Scholar] [CrossRef] [PubMed]
- Krištić, J.; Vučković, F.; Menni, C.; Klarić, L.; Keser, T.; Beceheli, I.; Pučić-Baković, M.; Novokmet, M.; Mangino, M.; Thaqi, K. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Adua, E.; Russell, A.; Roberts, P.; Wang, Y.; Song, M.; Wang, W. Innovation Analysis on Postgenomic Biomarkers: Glycomics for Chronic Diseases. OMICS 2017, 21, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Wilson, R.K.; Hood, L.E. Physical maps of the mouse and human immunoglobulin-like loci. Adv. Immunol. 1989, 46, 1–59. [Google Scholar] [PubMed]
- Pincetic, A.; Bournazos, S.; DiLillo, D.J.; Maamary, J.; Wang, T.T.; Dahan, R.; Fiebiger, B.-M.; Ravetch, J.V. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 2014, 15, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Quast, I.; Peschke, B.; Lünemann, J.D. Regulation of antibody effector functions through IgG Fc N-glycosylation. Cell. Mol. Life Sci. 2017, 74, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Krapp, S.; Mimura, Y.; Jefferis, R.; Huber, R.; Sondermann, P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 2003, 325, 979–989. [Google Scholar] [CrossRef]
- Van de Bovenkamp, F.S.; Hafkenscheid, L.; Rispens, T.; Rombouts, Y. The emerging importance of IgG Fab glycosylation in immunity. J. Immunol. 2016, 196, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Plomp, R.; Dekkers, G.; Rombouts, Y.; Visser, R.; Koeleman, C.A.M.; Kammeijer, G.S.M.; Jansen, B.C.; Rispens, T.; Hensbergen, P.J.; Vidarsson, G.; et al. Hinge-Region O-Glycosylation of Human Immunoglobulin G3 (IgG3). Mol. Cell. Proteom. 2015, 14, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Keser, T.; Vučković, F.; Barrios, C.; Zierer, J.; Wahl, A.; Akinkuolie, A.O.; Štambuk, J.; Nakić, N.; Pavić, T.; Periša, J. Effects of statins on the immunoglobulin G glycome. Biochim. Biophys. Acta 2017, 1861, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Anthony, R.M.; Ravetch, J.V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. USA 2007, 104, 8433–8437. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, P.D. Structural mechanism of high affinity FcγRI recognition of immunoglobulin G. Immunol. Rev. 2015, 268, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F.; Sazinsky, S.L.; Houde, D.; DiLillo, D.J.; Bird, J.; Li, K.K.; Cheng, G.T.; Qiu, H.; Engen, J.R.; Ravetch, J.V.; et al. Engineering Aglycosylated IgG Variants with Wild-Type or Improved Binding Affinity to Human Fc Gamma RIIA and Fc Gamma RIIIAs. J. Mol. Biol. 2017, 429, 2528–2541. [Google Scholar] [CrossRef] [PubMed]
- Tomiya, N.; Narang, S.; Lee, Y.C.; Betenbaugh, M.J. Comparing N-glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj. J. 2004, 21, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, E.S. The contribution of N-glycans and their processing in the endoplasmic reticulum to glycoprotein biosynthesis. Glycobiology 2003, 13, 77R–91R. [Google Scholar] [CrossRef] [PubMed]
- Subedi, G.P.; Barb, A.W. The Immunoglobulin G1 N-glycan Composition Affects Binding to Each Low Affinity Fc γ Receptor. mAbs 2016, 8, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Barb, A.W. Intramolecular N-Glycan/Polypeptide Interactions Observed at Multiple N-Glycan Remodeling Steps through [13C,15N]-N-Acetylglucosamine Labeling of Immunoglobulin G1. Biochemistry 2015, 54, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Troelsen, L.N.; Jacobsen, S.; Abrahams, J.L.; Royle, L.; Rudd, P.M.; Narvestad, E.; Heegaard, N.H.; Garred, P. IgG glycosylation changes and MBL2 polymorphisms: Associations with markers of systemic inflammation and joint destruction in rheumatoid arthritis. J. Rheumatol. 2012, 39, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Alzain, M.A.; Asweto, C.O.; Song, H.; Cui, L.; Yu, X.; Ge, S.; Dong, H.; Rao, P.; Wang, H. Glycan biomarkers for rheumatoid arthritis and its remission status in han Chinese patients. OMICS 2016, 20, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lemmers, R.F.; Vilaj, M.; Urda, D.; Agakov, F.; Šimurina, M.; Klaric, L.; Rudan, I.; Campbell, H.; Hayward, C.; Wilson, J.F. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim. Biophys. Acta 2017, 1861, 2240–2249. [Google Scholar] [CrossRef] [PubMed]
- Vučković, F.; Krištić, J.; Gudelj, I.; Teruel, M.; Keser, T.; Pezer, M.; Pučić-Baković, M.; Štambuk, J.; Trbojević-Akmačić, I.; Barrios, C. Association of Systemic Lupus Erythematosus with Decreased Immunosuppressive Potential of the IgG Glycome. Arthritis Rheumatol. 2015, 67, 2978–2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Klaric, L.; Yu, X.; Thaqi, K.; Dong, J.; Novokmet, M.; Wilson, J.; Polasek, O.; Liu, Y.; Krištic, J.; et al. The Association between Glycosylation of Immunoglobulin G and Hypertension: A Multiple Ethnic Cross-Sectional Study. Medicine 2016, 95, e3379. [Google Scholar] [CrossRef] [PubMed]
- Meany, D.L.; Chan, D.W. Aberrant glycosylation associated with enzymes as cancer biomarkers. Clin. Proteom. 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Lundström, S.L.; Yang, H.; Lyutvinskiy, Y.; Rutishauser, D.; Herukka, S.-K.; Soininen, H.; Zubarev, R.A. Blood plasma IgG Fc glycans are significantly altered in Alzheimer’s disease and progressive mild cognitive impairment. J. Alzheimers Dis. 2013, 38, 567–579. [Google Scholar]
- Wuhrer, M.; Selman, M.H.; McDonnell, L.A.; Kümpfel, T.; Derfuss, T.; Khademi, M.; Olsson, T.; Hohlfeld, R.; Meinl, E.; Krumbholz, M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflamm. 2015, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Šimurina, M.; Garcia, M.; Novokmet, M.; Wang, Y.; Rudan, I.; Campbell, H.; Lauc, G.; Thomas, M.; Wang, W. The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson’s disease. Glycobiology 2017, 27, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef] [PubMed]
- Quast, I.; Keller, C.W.; Maurer, M.A.; Giddens, J.P.; Tackenberg, B.; Wang, L.-X.; Münz, C.; Nimmerjahn, F.; Dalakas, M.C.; Lünemann, J.D. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Investig. 2015, 125, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.B.; Oswald, D.M.; Joshi, S.; Whiteheart, S.W.; Orlando, R.; Cobb, B.A. B-cell-independent sialylation of IgG. Proc. Natl. Acad. Sci. USA 2016, 113, 7207–7212. [Google Scholar] [CrossRef] [PubMed]
- Harre, U.; Lang, S.C.; Pfeifle, R.; Rombouts, Y.; Frühbeißer, S.; Amara, K.; Bang, H.; Lux, A.; Koeleman, C.A.; Baum, W. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat. Commun. 2015, 6, 6651. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, C.; Grau, S.; Jäger, C.; Sondermann, P.; Brünker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Ochiai, H.; Huang, W.; Yang, Q.; Li, C.; Wang, L.-X. Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor. J. Am. Chem. Soc. 2011, 133, 18975–18991. [Google Scholar] [CrossRef] [PubMed]
- Maupin, K.A.; Liden, D.; Haab, B.B. The fine specificity of mannose-binding and galactose-binding lectins revealed using outlier motif analysis of glycan array data. Glycobiology 2012, 22, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Uh, H.-W.; Beekman, M.; Koeleman, C.; Hokke, C.H.; Westendorp, R.; Wuhrer, M.; Houwing-Duistermaat, J.J.; Slagboom, P.E.; Deelder, A.M. Decreased levels of bisecting GlcNAc glycoforms of IgG are associated with human longevity. PLoS ONE 2010, 5, e12566. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Y.; Kristic, J.; Dong, J.; Chu, X.; Ge, S.; Wang, H.; Fang, H.; Gao, Q.; Liu, D. Profiling IgG N-glycans as potential biomarker of chronological and biological ages: A community-based study in a Han Chinese population. Medicine 2016, 95, e4112. [Google Scholar] [CrossRef] [PubMed]
- Peschke, B.; Keller, C.W.; Weber, P.; Quast, I.; Lunemann, J.D. Fc-galactosylation of human IgG isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 2017, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Karsten, C.M.; Köhl, J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology 2012, 217, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Bolland, S. Igg fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, P.; Pincetic, A.; Maamary, J.; Lammens, K.; Ravetch, J.V. General mechanism for modulating immunoglobulin effector function. Proc. Natl. Acad. Sci. USA 2013, 110, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Hirano, T.; Taga, T.; Kishimoto, T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990, 4, 2860–2867. [Google Scholar] [CrossRef] [PubMed]
- Daëron, M. Fc receptor biology. Annu. Rev. Immunol. 1997, 15, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Scinicariello, F.; Attanasio, R. IgG Fc receptor III homologues in nonhuman primate species: Genetic characterization and ligand interactions. J. Immunol. 2006, 177, 3848–3856. [Google Scholar] [CrossRef] [PubMed]
- Woolhiser, M.R.; Okayama, Y.; Gilfillan, A.M.; Metcalfe, D.D. IgG-dependent activation of human mast cells following up-regulation of FcγRI by IFN-γ. Eur. J. Immunol. 2001, 31, 3298–3307. [Google Scholar] [CrossRef]
- Unkeless, J.C.; Jin, J. Inhibitory receptors, ITIM sequences and phosphatases. Curr. Opin. Immunol. 1997, 9, 338–343. [Google Scholar] [CrossRef]
- Vivier, E.; Daëron, M. Immunoreceptor tyrosine-based inhibition motifs. Immunol. Today 1997, 18, 286–291. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Lachance, G.; Paré, G.; Rollet-Labelle, E.; Naccache, P.H. Signaling through CD16b in human neutrophils involves the Tec family of tyrosine kinases. J. Leukoc. Biol. 2005, 78, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Hořejšı́, V.; Drbal, K.; Cebecauer, M.; Černý, J.; Brdička, T.; Angelisová, P.; Stockinger, H. GPI-microdomains: A role in signalling via immunoreceptors. Immunol. Today 1999, 20, 356–361. [Google Scholar] [CrossRef]
- Cooney, D.S.; Phee, H.; Jacob, A.; Coggeshall, K.M. Signal transduction by human-restricted FcγRIIa involves three distinct cytoplasmic kinase families leading to phagocytosis. J. Immunol. 2001, 167, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Brauweiler, A.; Tamir, I.; Marschner, S.; Helgason, C.D.; Cambier, J.C. Partially distinct molecular mechanisms mediate inhibitory FcγRIIB signaling in resting and activated B cells. J. Immunol. 2001, 167, 204–211. [Google Scholar] [CrossRef] [PubMed]
- De Cordoba, S.R.; Tortajada, A.; Harris, C.L.; Morgan, B.P. Complement dysregulation and disease: From genes and proteins to diagnostics and drugs. Immunobioloy 2012, 217, 1034–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.-X.; Migone, T.-S.; Tseng, M.; Friedmann, M.; Weatherbee, J.A.; Zhou, L.; Yamauchi, A.; Bloom, E.T.; Mietz, J.; John, S. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 1995, 2, 331–339. [Google Scholar] [CrossRef]
- Russell, D.G.; VanderVen, B.C.; Glennie, S.; Mwandumba, H.; Heyderman, R.S. The macrophage marches on its phagosome: Dynamic assays of phagosome function. Nat. Rev. Immunol. 2009, 9, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Keser, T.; Mangino, M.; Bell, J.T.; Erte, I.; Akmačić, I.; Vučković, F.; Baković, M.P.; Gornik, O.; McCarthy, M.I. Glycosylation of immunoglobulin g: Role of genetic and epigenetic influences. PLoS ONE 2013, 8, e82558. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Huffman, J.E.; Adamczyk, B.; Mužinić, A.; Kattla, J.J.; Pučić, M.; Novokmet, M.; Abrahams, J.L.; Hayward, C.; Rudan, I. Association of medication with the human plasma N-glycome. J. Proteom. Res. 2012, 11, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Knežević, A.; Gornik, O.; Polašek, O.; Pučić, M.; Redžić, I.; Novokmet, M.; Rudd, P.M.; Wright, A.F.; Campbell, H.; Rudan, I. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology 2010, 20, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Keser, T.; Vučković, F.; Škaro, V.; Goreta, S.Š.; Pavić, T.; Dumić, J.; Primorac, D.; Lauc, G.; Gornik, O. Estimation of human age using N-glycan profiles from bloodstains. Int. J. Legal Med. 2015, 129, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Vanhooren, V.; Dewaele, S.; Libert, C.; Engelborghs, S.; De Deyn, P.P.; Toussaint, O.; Debacq-Chainiaux, F.; Poulain, M.; Glupczynski, Y.; Franceschi, C.; et al. Serum N-glycan profile shift during human ageing. Exp. Gerontol. 2010, 45, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Catera, M.; Borelli, V.; Malagolini, N.; Chiricolo, M.; Venturi, G.; Reis, C.A.; Osorio, H.; Abruzzo, P.M.; Capri, M.; Monti, D.; et al. Identification of novel plasma glycosylation-associated markers of aging. Oncotarget 2016, 7, 7455–7468. [Google Scholar] [CrossRef] [PubMed]
- Jurić, J.; Peng, H.; Song, M.; Šimunović, J.; Trbojević-Akmačić, I.; Hanić, M.; Zhao, F.; Wang, Y.; Liu, J.; Gao, Q. Immunoglobulin G Glycosylation in Menstrual Cycle. In Proceedings of the 12th Jenner Glycobiology and Medicine Symposium on Translational Glycobiology: From Bench to Bedside, Dubrovnik, Croatia, 6–9 May 2017. [Google Scholar]
- Ercan, A.; Kohrt, W.M.; Cui, J.; Deane, K.D.; Pezer, M.; Yu, E.W.; Hausmann, J.S.; Campbell, H.; Kaiser, U.B.; Rudd, P.M. Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2017, 2, e89703. [Google Scholar] [CrossRef] [PubMed]
- Bondt, A.; Rombouts, Y.; Selman, M.H.J.; Hensbergen, P.J.; Reiding, K.R.; Hazes, J.M.W.; Dolhain, R.J.; Wuhrer, M. Immunoglobulin G (IgG) Fab Glycosylation Analysis Using a New Mass Spectrometric High-throughput Profiling Method Reveals Pregnancy-associated Changes. Mol. Cell. Proteom. 2014, 13, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Spearman, M.; Doering, J.; Lattová, E.; Perreault, H.; Butler, M. The availability of glucose to CHO cells affects the intracellular lipid-linked oligosaccharide distribution, site occupancy and the N-glycosylation profile of a monoclonal antibody. J. Biotechnol. 2014, 170, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jimenez Del Val, I.; Müller, C.; Wagtberg Sen, J.; Rasmussen, S.K.; Kontoravdi, C.; Weilguny, D.; Andersen, M.R. Amino acid and glucose metabolism in fed-batch CHO cell culture affects antibody production and glycosylation. Biotechnol. Bioeng. 2015, 112, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, M.N.; Bakovic, M.P.; Kristic, J.; Novokmet, M.; Huffman, J.E.; Vitart, V.; Hayward, C.; Rudan, I.; Wilson, J.F.; Campbell, H. The association between galactosylation of immunoglobulin G and body mass index. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Kepka, A.; Trbojević-Akmačić, I.; Hui, J.; Hunter, M.; Ugrina, I.; Laws, S.; Wang, W. Higher levels of abdominal body fat are associated with an increase in pro-inflammatory immunoglobulin G N-glycans: Results from the Busselton Healthy Ageing Study. In Proceedings of the 12th Jenner Glycobiology and Medicine Symposium on Translational Glycobiology: From Bench to Bedside, Dubrovnik, Croatia, 6–9 May 2017. [Google Scholar]

| Receptor | Classification | IgG Affinity | Cell Types | Regulation | Produces | Effects | Ref (1st Author) |
|---|---|---|---|---|---|---|---|
| FcγRI (CD64) | Type I Activating | High for IgG1, 3, 4 Low or No affinity for IgG2 | Mast cells, Monocytes, Macrophages, Neutrophils, Dendritic Cells | ↑ IL-10, INF-γ; ↓ IL-4 | IL-6 TNF-α | ↑ B Cell Differentiation, Immunoglobulin Production, Acute-Phase Protein Synthesis; ↑ ADCC, Degranulation, Phagocytosis (depends on expressing cell)—through SRC-family kinase phosphorylation; ↓ Lipo-Protein Lipase, FcγRIIIb on Neutrophils | Akira (1990) [53], Daëron (1997) [54], Lu (2015) [23], Pincetic (2014) [15], Ravetch (2001) [51], Quast (2017) [16], Rogers (2006) [55], Woolhiser (2001) [56] |
| FcγRIIa1/2 (CD32a) | Type I Activating | Low | Mast Cells, Monocytes, Macrophages, Neutrophils, Dendritic Cells, Eosinophils, Basophils, Platelets | ↓ IL-4 | TNF-α | ↑ ADCC, Degranulation, Phagocytosis (depends on expressing cell)—through SRC-family kinase phosphorylation | Daëron (1997) [54], Karsten (2012) [37], Pincetic (2014) [15], Ravetch (2001) [51] |
| FcγRIIb1/2/3 (CD32b) | Type I Inhibitory | Low ↓ with Fuc ↑ with Gal | T Cells, NK Cells, Immature B Cells (only FcR) | ↑ IL-4 | ↓ BCR-Induced Ca2+ Mobilisation, Cell Proliferation, ITAM-Regulated FcRs, Akt; Limits Autoantibody Production (on B Cells) | Karsten (2012) [50], Quast (2017) [16], Subedi (2016) [27], Unkeless (1997) [57], Vivier (1997) [58] | |
| FcγRIIc (CD32c) | Type I Activating | Low ↓ with Fuc | Monocytes, Neutrophils, NK Cells | ↓ IL-4 | TNF-α | ↑ ADCC, Degranulation, Phagocytosis (depends on expressing cell)—through SRC-family kinase phosphorylation | Daëron (1997) [54], Karsten (2012) [37], Pincetic (2014) [15], Ravetch (2001) [51], Subedi (2016) [27] |
| FcγRIIIa (CD16a) | Type I Activating | Medium ↓ with Fuc, Neu5Ac | NK Cells, Macrophages, Monocytes (10%) | ↓ IL-4 | TNF-α | ↑ ADCC | Daëron (1997) [54], Pincetic (2014) [15], Ravetch (2001) [51], Subedi (2016) [27] |
| FcγRIIIb (CD16b) | Type I Activating | Low ↓ with Fuc | T Cells, Neutrophils, Macrophages, Monocytes | ↑ INF-γ ↓ TNF-α | ↑ Ca2+ Mobilisation | Fernandes (2005) [59], Hořejší (1999) [60], Pincetic (2014) [15], Ravetch (2001) [51], Subedi (2016) [27] | |
| FcεRII (CD23) | Type II Inhibitory | ? ↑ with Neu5Ac | B Cells, T Cells, Monocytes, Follicular Dendritic Cells, Macrophages | ↑ Production FcγRIIb—Inhibits further activating FcγR binding | Pincetic (2014) [15], Sondermann (2013) [52] | ||
| DC-SIGN (CD209) | Type II Inhibitory | ? ↑ with Neu5Ac | B Cells, Monocytes, Dendritic Cells, Macrophages | ↑ IL-4, IL-33 | IL-4 | ↑ Production FcγRIIb—Inhibits further activating FcγR binding | Pincetic (2014) [15], Schwab (2013) [4], Sondermann (2013) [52], Quast (2015) [39] |
| Effector Response | Immune Cells | Inflammation | Relation to IgG | Ref (1st Author) |
|---|---|---|---|---|
Cytokines - Molecules with hormone-like function | All | Both | Altered IgG glycosylation may be linked to changes in cytokine expression | Lin (1995) [64] |
Degranulation - Release of antimicrobial cytotoxic agents | Mast Cells, Basophils, Neutrophils, Eosinophils, Cytotoxic T Cells, NK Cells | Pro | ↑ Fcγ-RI binding = ↑ degranulation, thus ↑ localised inflammation | Woolhiser (2001) [56] |
Phagocytosis - Recognising & engulfing large particles or cells opsonised by C3b or IC, or amassed IC | Mast Cells, Basophils, Neutrophils, Eosinophils, Macrophages | Pro | ↑ Fc binding can lead to ↑ localised inflammation | Quast (2014) [6], Russell (2009) [65] |
ADCC - Cell lysis mediated by cytotoxic granules containing perforin & granzymes | NK Cells, Macrophages, Monocytes, Neutrophils, Eosinophils | Pro | ↓ core fucosylated/sialylated IgG = ↑ Fcγ-RIIIa binding = ↑ ADCC Overall, this leads to ↑ localised inflammation/cell apoptosis | Nimmerjahn (2005) [38], Quast (2014) [6] |
Immune Modulation - Upregulation of Fcγ-RIIb, which dominantly inhibits activating FcR | All | Anti | ↑ sialylated IgG = ↑ Fcγ-RIIb binding Overall, this leads to ↑ anti-inflammatory activity | Pincetic (2014) [15], Schwab (2013) [4], Sondermann (2013) [52] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, A.; Adua, E.; Ugrina, I.; Laws, S.; Wang, W. Unravelling Immunoglobulin G Fc N-Glycosylation: A Dynamic Marker Potentiating Predictive, Preventive and Personalised Medicine. Int. J. Mol. Sci. 2018, 19, 390. https://doi.org/10.3390/ijms19020390
Russell A, Adua E, Ugrina I, Laws S, Wang W. Unravelling Immunoglobulin G Fc N-Glycosylation: A Dynamic Marker Potentiating Predictive, Preventive and Personalised Medicine. International Journal of Molecular Sciences. 2018; 19(2):390. https://doi.org/10.3390/ijms19020390
Chicago/Turabian StyleRussell, Alyce, Eric Adua, Ivo Ugrina, Simon Laws, and Wei Wang. 2018. "Unravelling Immunoglobulin G Fc N-Glycosylation: A Dynamic Marker Potentiating Predictive, Preventive and Personalised Medicine" International Journal of Molecular Sciences 19, no. 2: 390. https://doi.org/10.3390/ijms19020390