Comparative Genomics of the Balsaminaceae Sister Genera Hydrocera triflora and Impatiens pinfanensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. The I. pinfanensis and H. triflora Chloroplast Genome Structure and Gene Content
2.2. Codon Usage
2.3. SSR Analysis Results
2.4. Selection Pressure Analysis of Evolution
2.5. IR Expansion and Contraction
2.6. Phylogenetic Analysis
3. Materials and Methods
3.1. Plant Materials and DNA Extraction
3.2. Chloroplast Genome Sequence Assembly and Annotation
3.3. Genome Comparison and Structure Analyses
3.4. Substitution Rate Analysis—Synonymous (Ks) and Non-Synonymous (Ka)
3.5. Phylogenetic Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IR | Inverted repeat |
| LSC | Large single copy |
| SSC | Small single copy |
| SSR | Simple sequence repeats |
| RSCU | Relative synonymous codon usage |
References
- Grey-Wilson, C. Impatiens of Africa; Morphology, Pollination and Pollinators, Ecology, Phytogeography, Hybridization, Keys and a Systematics of All African Species with a Note on Collecting and Cultivation; AA Balkema: Rotterdam, The Netherlands, 1980. [Google Scholar]
- Janssens, S.B.; Smets, E.F.; Vrijdaghs, A. Floral development of Hydrocera and Impatiens reveals evolutionary trends in the most early diverged lineages of the Balsaminaceae. Ann. Bot. 2012, 109, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Rahelivololona, M.E. New taxa of Impatiens (Balsaminaceae) from Madagascar iii. Adansonia 2004, 26, 37–52. [Google Scholar]
- Janssens, S.; Geuten, K.; Yuan, Y.-M.; Song, Y.; Küpfer, P.; Smets, E. Phylogenetics of Impatiens and Hydrocera (Balsaminaceae) using chloroplast atpb-rbcl spacer sequences. Syst. Bot. 2006, 31, 171–180. [Google Scholar] [CrossRef]
- Janssens, S.B.; Knox, E.B.; Huysmans, S.; Smets, E.F.; Merckx, V.S. Rapid radiation of Impatiens (Balsaminaceae) during pliocene and pleistocene: Result of a global climate change. Mol. Phylogenet. Evol. 2009, 52, 806–824. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.B.; Viaene, T.; Huysmans, S.; Smets, E.F.; Geuten, K.P. Selection on length mutations after frameshift can explain the origin and retention of the AP3/DEF-like paralogues in Impatiens. J. Mol. Evol. 2008, 66, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-M.; Song, Y.; Geuten, K.; Rahelivololona, E.; Wohlhauser, S.; Fischer, E.; Smets, E.; Küpfer, P. Phylogeny and biogeography of Balsaminaceae inferred from its sequences. Taxon 2004, 53, 391. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, Y.-M.; Küpfer, P. Chromosomal evolution in Balsaminaceae, with cytological observations on 45 species from Southeast Asia. Caryologia 2003, 56, 463–481. [Google Scholar] [CrossRef]
- Tan, Y.-H.; Liu, Y.-N.; Jiang, H.; Zhu, X.-X.; Zhang, W.; Yu, S.-X. Impatiens pandurata (Balsaminaceae), a new species from Yunnan, China. Bot. Stud. 2015, 56, 29. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, Y.-N.; Gogoi, R.; Zhang, L.-J.; Yu, S.-X. Impatiens tianlinensis (Balsaminaceae), a new species from Guangxi, China. Phytotaxa 2015, 227, 253–260. [Google Scholar] [CrossRef]
- Raju, R.; Dhanraj, F.I.; Arumugam, M.; Pandurangan, A. Impatiens matthewiana, a new scapigerous balsam from Western Ghats, India. Phytotaxa 2015, 227, 268–274. [Google Scholar] [CrossRef]
- Guo, H.; Wei, L.; Hao, J.-C.; Du, Y.-F.; Zhang, L.-J.; Yu, S.-X. Impatiens occultans (Balsaminaceae), a newly recorded species from Xizang, China, and its phylogenetic position. Phytotaxa 2016, 275, 62–68. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kim, B.-Y.; Park, H.-S.; Phourin, C.; Kim, Y.-D. Impatiens bokorensis (Balsaminaceae), a new species from Cambodia. PhytoKeys 2017, 77, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, S.-S.; Maung, K.W.; Tan, Y.-H. Two new species of Impatiens (Balsaminaceae) from Putao, Kachin state, northern Myanmar. Phytotaxa 2017, 321, 103–113. [Google Scholar] [CrossRef]
- Hooker, J.D. Les Espèces du Genre “Impatiens” dans l’herbier du Museum de Paris. Nov. Arch. Mus. Nat. Hist. Paris Ser. 1908, 10, 233–272. [Google Scholar]
- Bhaskar, V. Taxonomic Monograph on ‘Impatiens’ (‘Balsaminaceae’) of Western Ghats, South India: The Key Genus for Endemism; Centre for Plant Taxonomic Studies: Bengaluru, India, 2012. [Google Scholar]
- Baskar, N.; Devi, B.P.; Jayakar, B. Anticancer studies on ethanol extract of Impatiens balsamina. Int. J. Res. Ayurveda Pharm. 2012, 3, 631–633. [Google Scholar]
- Cimmino, A.; Mathieu, V.; Evidente, M.; Ferderin, M.; Banuls, L.M.Y.; Masi, M.; De Carvalho, A.; Kiss, R.; Evidente, A. Glanduliferins A and B, two new glucosylated steroids from Impatiens glandulifera, with in vitro growth inhibitory activity in human cancer cells. Fitoterapia 2016, 109, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Zidorn, C.; Biernasiuk, A.; Komsta, Ł.; Granica, S. Polyphenols from Impatiens (Balsaminaceae) and their antioxidant and antimicrobial activities. Ind. Crops Prod. 2016, 86, 262–272. [Google Scholar] [CrossRef]
- Sugiura, M. The chloroplast genome. In 10 Years Plant Molecular Biology; Springer: Berlin, Germany, 1992; pp. 149–168. [Google Scholar]
- Chumley, T.W.; Palmer, J.D.; Mower, J.P.; Fourcade, H.M.; Calie, P.J.; Boore, J.L.; Jansen, R.K. The complete chloroplast genome sequence of Pelargonium × hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 2006, 23, 2175–2190. [Google Scholar] [CrossRef] [PubMed]
- Tangphatsornruang, S.; Sangsrakru, D.; Chanprasert, J.; Uthaipaisanwong, P.; Yoocha, T.; Jomchai, N.; Tragoonrung, S. The chloroplast genome sequence of mungbean (Vigna radiata) determined by high-throughput pyrosequencing: Structural organization and phylogenetic relationships. DNA Res. 2009, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wicke, S.; Schneeweiss, G.M.; Müller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K.; Cai, Z.; Raubeson, L.A.; Daniell, H.; Leebens-Mack, J.; Müller, K.F.; Guisinger-Bellian, M.; Haberle, R.C.; Hansen, A.K.; Chumley, T.W. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA 2007, 104, 19369–19374. [Google Scholar] [CrossRef] [PubMed]
- Parks, M.; Cronn, R.; Liston, A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Soltis, P.S.; Bell, C.D.; Burleigh, J.G.; Soltis, D.E. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc. Natl. Acad. Sci. USA 2010, 107, 4623–4628. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Guo, W.; Gupta, S.; Fan, W.; Mower, J.P. Evolutionary dynamics of the plastid inverted repeat: The effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016, 209, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Maliga, P. Engineering the plastid genome of higher plants. Curr. Opin. Plant Biol. 2002, 5, 164–172. [Google Scholar] [CrossRef]
- Shaw, J.; Shafer, H.L.; Leonard, O.R.; Kovach, M.J.; Schorr, M.; Morris, A.B. Chloroplast DNA sequence utility for the lowest phylogenetic and phylogeographic inferences in angiosperms: The tortoise and the hare iv. Am. J. Bot. 2014, 101, 1987–2004. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Gao, L.M.; Soltis, D.E.; Soltis, P.S.; Yang, J.B.; Fang, L.; Yang, S.X.; Li, D.Z. Insights into the historical assembly of East Asian subtropical evergreen broadleaved forests revealed by the temporal history of the tea family. New Phytol. 2017, 215, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Allantospermum, A.; Apodanthaceae, A.; Boraginales, B.; Buxaceae, C.; Centrolepidaceae, C.; Cynomoriaceae, D.; Dilleniales, D.; Dipterocarpaceae, E.; Forchhammeria, F.; Gesneriaceae, H. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Lan, Y.; Cheng, L.; Huang, W.; Cao, Q.; Zhou, Z.; Luo, A.; Hu, G. The complete chloroplast genome sequence of Actinidia kolomikta from north China. Conserv. Genet. Resour. 2017, 1–3. [Google Scholar] [CrossRef]
- Wang, W.-C.; Chen, S.-Y.; Zhang, X.-Z. Chloroplast genome evolution in Actinidiaceae: Clpp loss, heterogenous divergence and phylogenomic practice. PLoS ONE 2016, 11, e0162324. [Google Scholar] [CrossRef] [PubMed]
- Logacheva, M.D.; Schelkunov, M.I.; Shtratnikova, V.Y.; Matveeva, M.V.; Penin, A.A. Comparative analysis of plastid genomes of non-photosynthetic Ericaceae and their photosynthetic relatives. Sci. Rep. 2016, 6, 30042. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, D.; Senalik, D.; Ames, M.; Zhu, H.; Steffan, S.A.; Harbut, R.; Polashock, J.; Vorsa, N.; Gillespie, E.; Kron, K. Complete plastid genome sequence of Vaccinium macrocarpon: Structure, gene content, and rearrangements revealed by next generation sequencing. Tree Genet. Genomes 2013, 9, 489–498. [Google Scholar] [CrossRef]
- Fu, J.; Liu, H.; Hu, J.; Liang, Y.; Liang, J.; Wuyun, T.; Tan, X. Five complete chloroplast genome sequences from Diospyros: Genome organization and comparative analysis. PLoS ONE 2016, 11, e0159566. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, H.-W.; Kim, Y.-K.; Cheon, S.-H.; Kim, K.-J. The first complete plastome sequence from the family Sapotaceae, Pouteria campechiana (kunth) baehni. Mitochondr. DNA Part B 2016, 1, 734–736. [Google Scholar] [CrossRef]
- Ku, C.; Hu, J.-M.; Kuo, C.-H. Complete plastid genome sequence of the basal Asterid Ardisia polysticta miq. and comparative analyses of Asterid plastid genomes. PLoS ONE 2013, 8, e62548. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Liu, T.-J.; Yan, H.-F.; Ge, X.-J.; Hao, G. The complete chloroplast genome of a rare candelabra primrose Primula stenodonta (Primulaceae). Conserv. Genet. Resour. 2017, 9, 123–125. [Google Scholar] [CrossRef]
- Wang, L.-L.; Zhang, Y.; Yang, Y.-C.; Du, X.-M.; Ren, X.-L.; Liu, W.-Z. The complete chloroplast genome of Sinojackia xylocarpa (Ericales: Styracaceae), an endangered plant species endemic to China. Conserv. Genet. Resour. 2017. [Google Scholar] [CrossRef]
- Yao, X.; Tang, P.; Li, Z.; Li, D.; Liu, Y.; Huang, H. The first complete chloroplast genome sequences in Actinidiaceae: Genome structure and comparative analysis. PLoS ONE 2015, 10, e0129347. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Suzuki, H.; Kusumegi, T.; Hirose, T.; Yukawa, Y.; Sugiura, M. Translation of psbC mRNAs starts from the downstream GUG, not the upstream AUG, and requires the extended shine–dalgarno sequence in tobacco chloroplasts. Plant Cell Physiol. 2007, 48, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Härtel, B.; Brennicke, A. RNA editing in plants and its evolution. Annu. Rev. Genet. 2013, 47, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qi, B.; Ding, L.; Tang, X. Based on RSCU and QRSCU research codon bias of F/10 and G/11 xylanase. J. Food Sci. Biotechnol. 2010, 29, 755–764. [Google Scholar]
- Zuo, L.-H.; Shang, A.-Q.; Zhang, S.; Yu, X.-Y.; Ren, Y.-C.; Yang, M.-S.; Wang, J.-M. The first complete chloroplast genome sequences of Ulmus species by de novo sequencing: Genome comparative and taxonomic position analysis. PLoS ONE 2017, 12, e0171264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, X.; Cui, Y.; Sun, W.; Li, Y.; Wang, Y.; Song, J.; Yao, H. Molecular structure and phylogenetic analyses of complete chloroplast genomes of two Aristolochia medicinal species. Int. J. Mol. Sci. 2017, 18, 1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, H.; Wang, J.; Lei, W.; Gao, J.; Qiu, X.; Wang, J. The complete chloroplast genome sequences of the medicinal plant Forsythia suspensa (Oleaceae). Int. J. Mol. Sci. 2017, 18, 2288. [Google Scholar] [CrossRef] [PubMed]
- Gichira, A.W.; Li, Z.; Saina, J.K.; Long, Z.; Hu, G.; Gituru, R.W.; Wang, Q.; Chen, J. The complete chloroplast genome sequence of an endemic monotypic genus Hagenia (Rosaceae): Structural comparative analysis, gene content and microsatellite detection. PeerJ 2017, 5, e2846. [Google Scholar] [CrossRef] [PubMed]
- Makałowski, W.; Boguski, M.S. Evolutionary parameters of the transcribed mammalian genome: An analysis of 2,820 orthologous rodent and human sequences. Proc. Natl. Acad. Sci. USA 1998, 95, 9407–9412. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Cheon, K.-S.; Yoo, K.-O.; Lee, H.-O.; Cho, K.-S.; Suh, J.-T.; Kim, S.-J.; Nam, J.-H.; Sohn, H.-B.; Kim, Y.-H. Complete chloroplast genome sequences and comparative analysis of Chenopodium quinoa and C. album. Front. Plant Sci. 2017, 8, 1696. [Google Scholar] [CrossRef] [PubMed]
- Saina, J.K.; Gichira, A.W.; Li, Z.-Z.; Hu, G.-W.; Wang, Q.-F.; Liao, K. The complete chloroplast genome sequence of Dodonaea viscosa: Comparative and phylogenetic analyses. Genetica 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Raubeson, L.A.; Peery, R.; Chumley, T.W.; Dziubek, C.; Fourcade, H.M.; Boore, J.L.; Jansen, R.K. Comparative chloroplast genomics: Analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genom. 2007, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-J.; Cheng, C.-L.; Chang, C.-C.; Wu, C.-L.; Su, T.-M.; Chaw, S.-M. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 2008, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Huotari, T.; Korpelainen, H. Complete chloroplast genome sequence of Elodea canadensis and comparative analyses with other monocot plastid genomes. Gene 2012, 508, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Chung, M.G.; Park, S. The complete chloroplast genome sequences of three Veroniceae species (Plantaginaceae): Comparative analysis and highly divergent regions. Front. Plant Sci. 2016, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J. DNA protocols for plants. In Molecular Techniques in Taxonomy; Springer: Berlin, Germany, 1991; pp. 283–293. [Google Scholar]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Schattner, P.; Brooks, A.N.; Lowe, T.M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005, 33, W686–W689. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). TAG Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, F.; Wang, L.; Huang, S.; Yu, J. Nonsynonymous substitution rate (Ka) is a relatively consistent parameter for defining fast-evolving and slow-evolving protein-coding genes. Biol. Direct 2011, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. Jmodeltest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
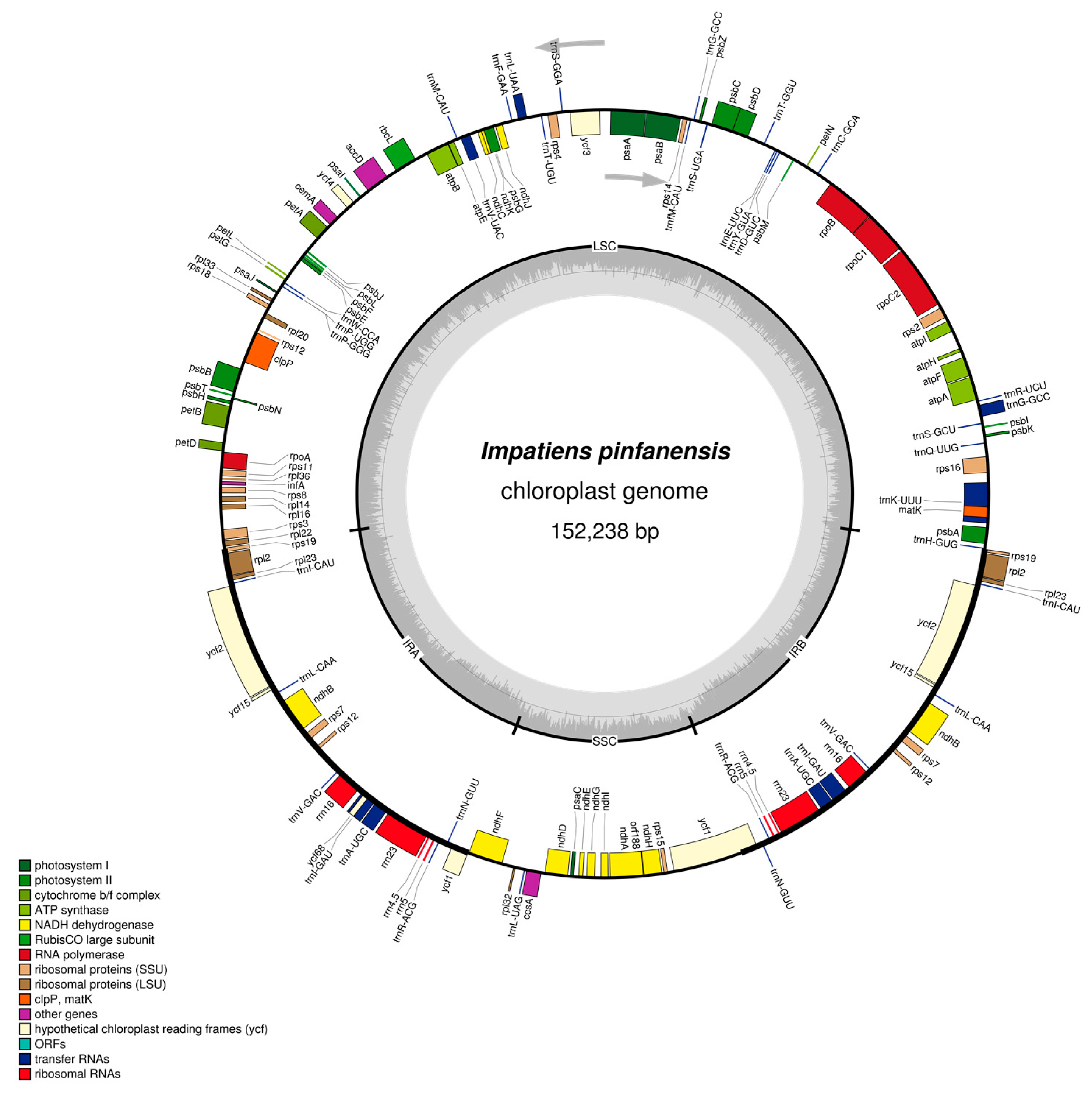
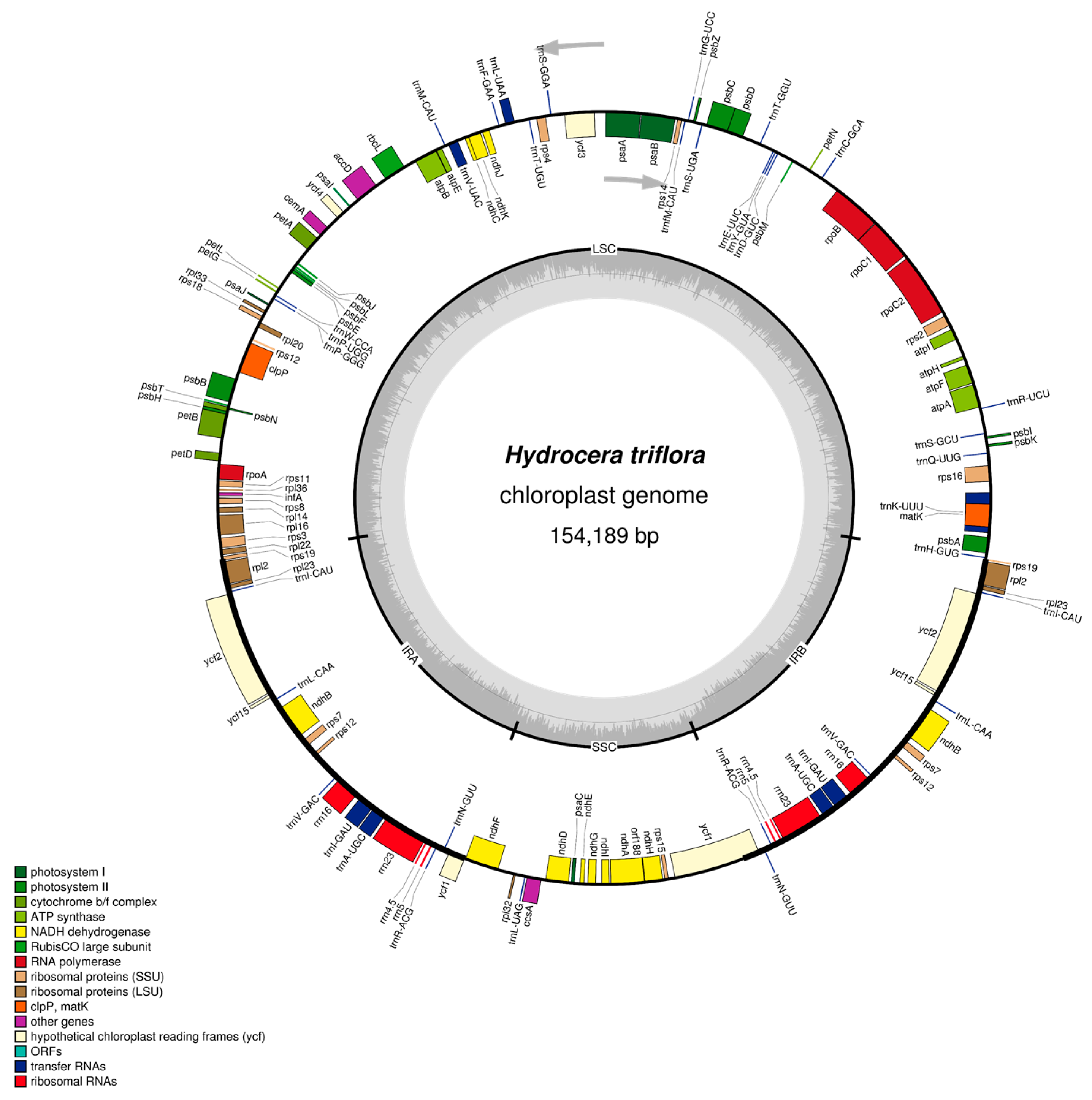



| Species | Impatiens pinfanensis | Hydrocera triflora |
|---|---|---|
| Total Genome length (bp) | 154,189 | 152,238 |
| Overall G/C content (%) | 36.8 | 36.9 |
| Large single copy region | 83,117 | 84,865 |
| GC content (%) | 34.5 | 34.7 |
| Short single copy region | 25,755 | 25,622 |
| GC content (%) | 29.3 | 29.9 |
| Inverted repeat region | 17,611 | 18,082 |
| GC content (%) | 43.1 | 43.1 |
| Protein-Coding Genes | 80 | 80 |
| tRNAs | 31 | 31 |
| rRNAs | 4 | 4 |
| Genes with introns | 17 | 17 |
| Genes duplicated by IR | 18 | 18 |
| Group of Genes | Gene Name |
|---|---|
| rRNA genes | rrn16(×2), rrn23(×2), rrn4.5(×2), rrn5(×2), |
| tRNA genes | trnA-UGC * (×2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-GCC *, trnG-UCC, trnH-GUG, trnI-CAU(×2), trnI-GAU * (×2), trnK-UUU *, trnL-CAA(×2), trnL-UAA *, trnL-UAG, trnfM-CAU, trnM-CAU, trnN-GUU(×2), trnP-GGG trnP-UGG, trnQ-UUG, trnR-ACG(×2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(×2), trnV-UAC *, trnW-CCA, trnY-GUA |
| Ribosomal small subunit | rps2, rps3, rps4, rps7(×2), rps8, rps11, rps12_5’end, rps12_3’end * (×2), rps14, rps15, rps16 *, rps18, rps19 |
| Ribosomal large subunit | rpl2 * (×2), rpl14, rpl16, rpl20, rpl22, rpl23(×2), rpl32, rpl33, rpl36 |
| DNA-dependent RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 |
| Large subunit of rubisco | rbcL |
| Photosystem I | psaA, psaB, psaC, psaI, psaJ, ycf3 ** |
| Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ |
| NADH dehydrogenase | ndhA *, ndhB * (×2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK |
| Cytochrome b/f complex | petA, petB *, petD, petG, petL, petN |
| ATP synthase | atpA, atpB, atpE, atpF *, atpH, atpI |
| Maturase | matK |
| Subunit of acetyl-CoA carboxylase | accD |
| Envelope membrane protein | cemA |
| Protease | clpP ** |
| Translational initiation factor | infA |
| c-type cytochrome synthesis | ccsA |
| Conserved open reading frames (ycf) | ycf1, ycf2(×2), ycf4, ycf15(×2) |
| Amino Acid | Codon | Number | RSCU | Amino Acid | Codon | Number | RSCU | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I. pinfanensis | H. triflora | I. pinfanensis | H. triflora | I. pinfanensis | H. triflora | I. pinfanensis | H. triflora | ||||
| Phe | UUU | 913 | 908 | 1.40 | 1.38 | Ser | UCU | 482 | 482 | 1.69 | 1.67 |
| UUC | 387 | 406 | 0.60 | 0.62 | UCC | 252 | 264 | 0.88 | 0.92 | ||
| Leu | UUA | 854 | 842 | 2.11 | 2.07 | UCA | 360 | 324 | 1.26 | 1.12 | |
| UUG | 468 | 486 | 1.16 | 1.20 | UCG | 142 | 181 | 0.50 | 0.63 | ||
| CUU | 517 | 503 | 1.28 | 1.24 | Pro | CCU | 376 | 371 | 1.59 | 1.58 | |
| CUC | 160 | 162 | 0.40 | 0.40 | CCC | 175 | 167 | 0.74 | 0.71 | ||
| CUA | 310 | 315 | 0.77 | 0.78 | CCA | 294 | 290 | 1.24 | 1.23 | ||
| CUG | 121 | 128 | 0.30 | 0.32 | CCG | 103 | 112 | 0.43 | 0.48 | ||
| Ile | AUU | 1035 | 1020 | 1.54 | 1.52 | Thr | ACU | 493 | 500 | 1.70 | 1.74 |
| AUC | 359 | 376 | 0.53 | 0.56 | ACC | 198 | 180 | 0.68 | 0.63 | ||
| AUA | 624 | 611 | 0.93 | 0.91 | ACA | 358 | 368 | 1.24 | 1.28 | ||
| Met | AUG | 547 | 548 | 1.00 | 1.00 | ACG | 108 | 104 | 0.37 | 0.36 | |
| Val | GUU | 482 | 469 | 1.55 | 1.52 | Ala | GCU | 580 | 593 | 1.86 | 1.85 |
| GUC | 134 | 135 | 0.43 | 0.44 | GCC | 183 | 191 | 0.59 | 0.60 | ||
| GUA | 457 | 457 | 1.47 | 1.48 | GCA | 346 | 353 | 1.11 | 1.10 | ||
| GUG | 167 | 174 | 0.54 | 0.56 | GCG | 141 | 143 | 0.45 | 0.45 | ||
| Tyr | UAU | 704 | 697 | 1.64 | 1.65 | Cys | UGU | 191 | 196 | 1.53 | 1.51 |
| UAC | 155 | 146 | 0.36 | 0.35 | UGC | 58 | 63 | 0.47 | 0.49 | ||
| TER | UAA | 41 | 44 | 1.50 | 1.63 | TER | UGA | 18 | 18 | 0.66 | 0.67 |
| UAG | 23 | 19 | 0.84 | 0.70 | Trp | UGG | 412 | 412 | 1.00 | 1.00 | |
| His | CAU | 405 | 421 | 1.54 | 1.57 | Arg | AGA | 406 | 407 | 1.81 | 1.77 |
| CAC | 121 | 114 | 0.46 | 0.43 | AGG | 134 | 143 | 0.60 | 0.62 | ||
| Gln | CAA | 627 | 626 | 1.54 | 1.53 | Arg | CGU | 302 | 299 | 1.35 | 1.30 |
| CAG | 186 | 192 | 0.46 | 0.47 | CGC | 88 | 95 | 0.39 | 0.41 | ||
| Asn | AAU | 885 | 868 | 1.59 | 1.57 | CGA | 317 | 333 | 1.41 | 1.45 | |
| AAC | 231 | 238 | 0.41 | 0.43 | CGG | 98 | 103 | 0.44 | 0.45 | ||
| Lys | AAA | 976 | 978 | 1.55 | 1.54 | Ser | AGU | 363 | 72 | 1.27 | 1.29 |
| AAG | 284 | 289 | 0.45 | 0.46 | AGC | 110 | 108 | 0.39 | 0.37 | ||
| Asp | GAU | 720 | 737 | 1.64 | 1.64 | Gly | GGU | 525 | 525 | 1.33 | 1.35 |
| GAC | 159 | 160 | 0.36 | 0.36 | GGC | 160 | 165 | 0.40 | 0.42 | ||
| Glu | GAA | 914 | 929 | 1.55 | 1.55 | GGA | 639 | 625 | 1.62 | 1.61 | |
| GAG | 264 | 272 | 0.45 | 0.45 | GGG | 258 | 238 | 0.65 | 0.61 | ||
| SSR Type | Repeat Unit | Amount | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Impatiens pinfanensis | Hydrocera triflora | Actinidia kolomikta | Ardisia polysticta | Diospyros lotus | Barringtonia fusicarpa | Pouteria campechiana | Primula persimilis | ||
| Mono | A/T | 176 | 139 | 117 | 153 | 146 | 154 | 161 | 134 |
| C/G | 4 | 2 | 4 | 4 | 4 | 8 | 1 | 4 | |
| Di | AT/AT | 8 | 9 | 8 | 5 | 3 | 13 | 11 | 6 |
| Tri | AAG/CTT | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| AAT/ATT | 3 | 3 | 2 | 1 | 1 | 2 | 4 | 0 | |
| AGC/CTG | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Tetra | AAAG/CTTT | 1 | 0 | 3 | 2 | 1 | 3 | 1 | 1 |
| AAAT/ATTT | 2 | 3 | 3 | 3 | 4 | 3 | 6 | 2 | |
| AATG/ATTC | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| AATT/AATT | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| AGAT/ATCT | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAGT/ACTT | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| AACT/AGTT | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| AATC/ATTG | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | |
| AAAC/GTTT | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| AAGG/CCTT | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Penta | AATAC/ATTGT | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| AAAAT/ATTTT | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| AAATT/AATTT | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| AATGT/ACATT | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| AATAT/ATATT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Hexa | AATCCC/ATTGGG | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| AGATAT/ATATCT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| AAGATG/ATCTTC | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Total | 197 | 159 | 143 | 171 | 161 | 187 | 188 | 150 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-Z.; Saina, J.K.; Gichira, A.W.; Kyalo, C.M.; Wang, Q.-F.; Chen, J.-M. Comparative Genomics of the Balsaminaceae Sister Genera Hydrocera triflora and Impatiens pinfanensis. Int. J. Mol. Sci. 2018, 19, 319. https://doi.org/10.3390/ijms19010319
Li Z-Z, Saina JK, Gichira AW, Kyalo CM, Wang Q-F, Chen J-M. Comparative Genomics of the Balsaminaceae Sister Genera Hydrocera triflora and Impatiens pinfanensis. International Journal of Molecular Sciences. 2018; 19(1):319. https://doi.org/10.3390/ijms19010319
Chicago/Turabian StyleLi, Zhi-Zhong, Josphat K. Saina, Andrew W. Gichira, Cornelius M. Kyalo, Qing-Feng Wang, and Jin-Ming Chen. 2018. "Comparative Genomics of the Balsaminaceae Sister Genera Hydrocera triflora and Impatiens pinfanensis" International Journal of Molecular Sciences 19, no. 1: 319. https://doi.org/10.3390/ijms19010319






