Telomere Biology and Thoracic Aortic Aneurysm
Abstract
:1. Introduction
2. Telomeres: Structure, Functions and Maintenance
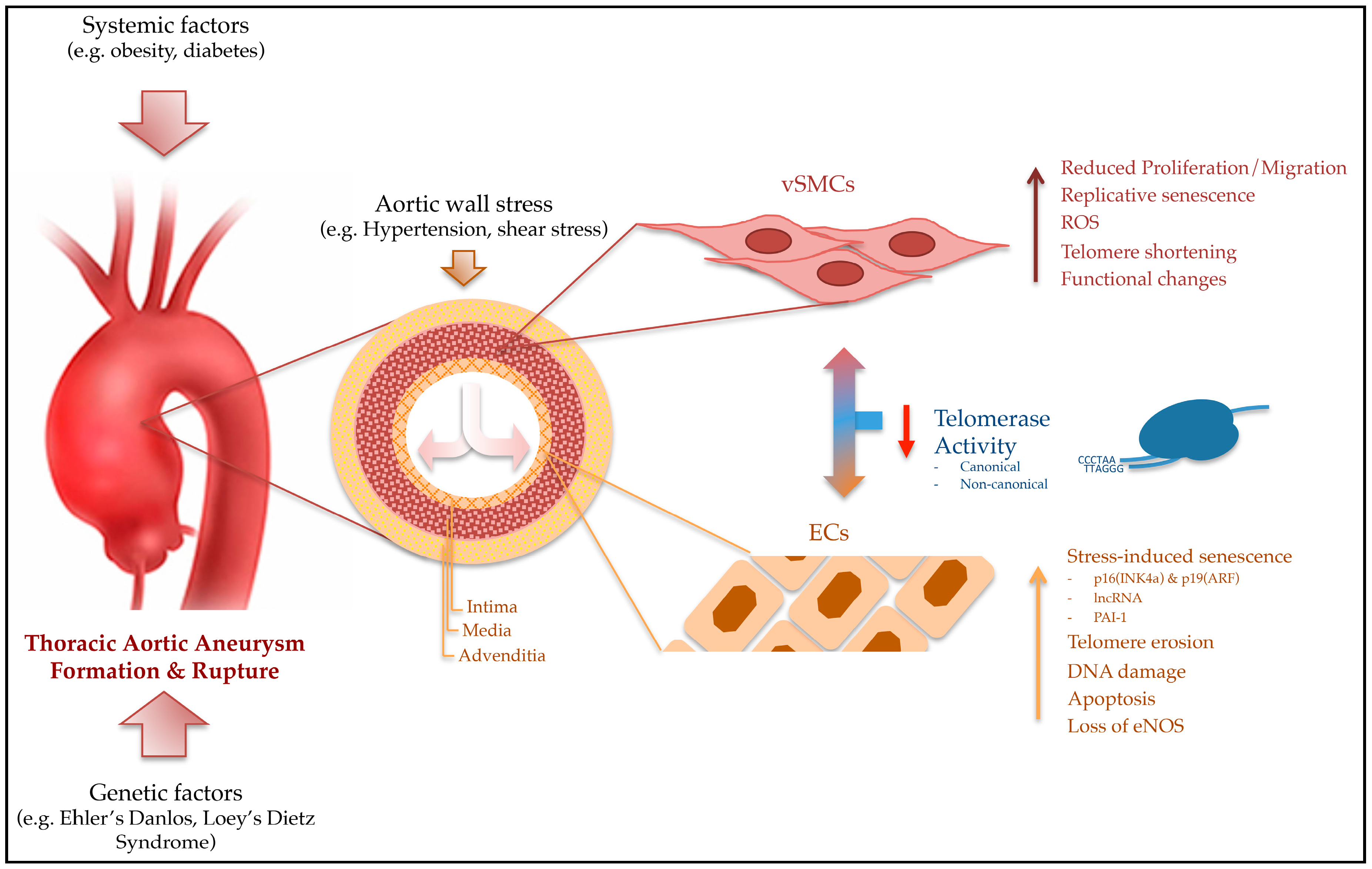
3. Telomeres and Pathobiology of Aortic Aneurysms
4. Regulation of Telomeres and Senescence in Cells of the Aortic Wall
5. Factors Affecting Telomeres and the Risk for TAA Formation
5.1. Oxidative Stress
5.2. Nutrition
5.3. Hypertension
5.4. Diabetes Mellitus
6. Telomeres as Markers in Leukocytes
7. Mechanism to Preserve Telomere Length
8. Conclusions
Conflicts of Interest
Funding
Abbreviations
| AAA | Abdominal aortic aneurysm |
| ALT | Alternative lengthening of telomeres |
| BAV | Bicuspid aortic valve |
| CKD | Chronic kidney disease |
| CVD | Cardiovascular disease |
| DOX | Doxorubicin |
| ds/ss | Double-stranded/single-stranded |
| DSB | Double strand break |
| EC | Endothelial cell |
| eNOS | Endothelial nitrate oxide synthase |
| FB | Fibroblast |
| LXR | Liver X receptor |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NO | Nitride oxide |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PARP | poly-ADP ribose polymerase |
| POT1 | Protection of telomeres 1 |
| RAP1 | Repressor activator protein-1 |
| ROS | Reactive oxygen species |
| RTL | Relative telomere length |
| SIPS | Stress-induced senescence program |
| (v)SMC | (vascular)smooth muscle cell |
| TA | Telomerase activity |
| TAA | Thoracic aortic aneurysm |
| TAV | Tricuspid aortic valve |
| TBP | Telomere binding protein |
| (h)TERC/(h)TR | (human)telomerase RNA-component |
| (h)TERT | (human)telomerase reverse transcriptase |
| TIN2 | TRF1-interacting protein 2 |
| TL | Telomere length |
| TPP1 | Tripeptidyl peptidase 1 |
| TRF | Telomere repeat-binding factor |
| TZAP | Telomeric zinc finger-associated protein |
| WBC | White blood cell |
References
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Telomeres. Trends Biochem. Sci. 1991, 16, 378–381. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M.; Avilion, A.A.; LeFeuvre, C.E.; Stewart, N.G.; Greider, C.W.; Harley, C.B.; Bacchetti, S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992, 11, 1921–1929. [Google Scholar] [PubMed]
- Karlseder, J.; Smogorzewska, A.; de Lange, T. Senescence induced by altered telomere state, not telomere loss. Science 2002, 295, 2446–2449. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Blasco, M.A. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer 2011, 11, 161–176. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Protection of mammalian telomeres. Oncogene 2002, 21, 532–540. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Morin, G.B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989, 59, 521–529. [Google Scholar] [CrossRef]
- Fleisig, H.B.; Hukezalie, K.R.; Thompson, C.A.; Au-Yeung, T.T.; Ludlow, A.T.; Zhao, C.R.; Wong, J.M. Telomerase reverse transcriptase expression protects transformed human cells against DNA-damaging agents, and increases tolerance to chromosomal instability. Oncogene 2016, 35, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Nagamatsu, G.; Saito, S.; Takubo, K.; Horimoto, K.; Suda, T. Telomerase reverse transcriptase has an extratelomeric function in somatic cell reprogramming. J. Biol. Chem. 2014, 289, 15776–15787. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Chen, G.; Zhou, J.; Xu, G.; Wang, S.; Wu, P.; Zhu, T.; Zhang, A.; Yang, W.; Xu, Q.; Lu, Y.; Ma, D. Inhibition of telomerase enhances apoptosis induced by sodium butyrate via mitochondrial pathway. Apoptosis 2006, 11, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Forsdahl, S.H.; Singh, K.; Solberg, S.; Jacobsen, B.K. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromso Study, 1994–2001. Circulation 2009, 119, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- El-Hamamsy, I.; Yacoub, M.H. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 2009, 6, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Booher, A.M.; Eagle, K.A.; Bossone, E. Acute aortic syndromes. Herz 2011, 36, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.G.; Ives, S.J.; Lesniewski, L.A.; Cawthon, R.M.; Andtbacka, R.H.; Noyes, R.D.; Richardson, R.S.; Donato, A.J. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H251–H258. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Rietzschel, E.R.; De Buyzere, M.L.; Van Criekinge, W.; Bekaert, S. Telomere length and cardiovascular aging: The means to the ends? Ageing Res. Rev. 2011, 10, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, Y.; Chen, C.; Peng, J.; Ding, H.; Wang, D.W. Short leukocyte telomere length is associated with aortic dissection. Intern. Med. 2011, 50, 2871–2875. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Merkin, S.S.; Cawthon, R.; Blackburn, E.H.; Adler, N.E.; Pletcher, M.J.; Seeman, T.E. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging 2008, 1, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G.; Diaz, V.A. Telomere length as a risk marker for cardiovascular disease: The next big thing? Expert Rev. Mol. Diagn. 2010, 10, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Huzen, J.; de Boer, R.A.; van Veldhuisen, D.J.; van Gilst, W.H.; van der Harst, P. The emerging role of telomere biology in cardiovascular disease. Front. Biosci. 2010, 15, 35–45. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kronmal, R.A.; Kimura, M.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Hardikar, S.; Aviv, A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Ince, H.; Nienaber, C.A. Etiology, pathogenesis and management of thoracic aortic aneurysm. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A.; Farkas, E.A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Levy, D. Telomeres, atherosclerosis, and the hemothelium: The longer view. Annu. Rev. Med. 2012, 63, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat. Res. 2012, 730, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Origin of concatemeric T7 DNA. Nat. New Biol. 1972, 239, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Olovnikov, A.M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Samassekou, O.; Gadji, M.; Drouin, R.; Yan, J. Sizing the ends: Normal length of human telomeres. Ann. Anat. 2010, 192, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Farrell, A.S.; Lakamp, A.S.; Ouellette, M.M. Characterization of the DNA binding specificity of Shelterin complexes. Nucleic Acids Res. 2011, 39, 9206–9223. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Fuste, J.M.; Simavorian, T.; Bartocci, C.; Tsai, J.; Karlseder, J.; Denchi, E.L. TZAP: A telomere-associated protein involved in telomere length control. Science 2017, 355, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Nera, B.; Huang, H.S.; Lai, T.; Xu, L. Elevated levels of TRF2 induce telomeric ultrafine anaphase bridges and rapid telomere deletions. Nat. Commun. 2015, 6, 10132. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, N.; Tahimic, C.G.; Otsuki, A.; Burma, S.; Fukuhara, A.; Sato, K.; Shiota, G.; Oshimura, M.; Chen, D.J.; Kurimasa, A. Ku70/80 modulates ATM and ATR signaling pathways in response to DNA double strand breaks. J. Biol. Chem. 2007, 282, 10138–10145. [Google Scholar] [CrossRef] [PubMed]
- Oeseburg, H.; de Boer, R.A.; van Gilst, W.H.; van der Harst, P. Telomere biology in healthy aging and disease. Pflügers Arch. Eur. J. Physiol. 2010, 459, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Dantzer, F.; Ame, J.C.; de Murcia, G. Poly(ADP-ribose): Novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, F.; Ame, J.C.; Schreiber, V.; Nakamura, J.; Menissier-de Murcia, J.; de Murcia, G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006, 409, 493–510. [Google Scholar] [PubMed]
- Saliques, S.; Zeller, M.; Lorin, J.; Lorgis, L.; Teyssier, J.R.; Cottin, Y.; Rochette, L.; Vergely, C. Telomere length and cardiovascular disease. Arch. Cardiovasc. Dis. 2010, 103, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Counter, C.M.; Botelho, F.M.; Wang, P.; Harley, C.B.; Bacchetti, S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J. Virol. 1994, 68, 3410–3414. [Google Scholar] [PubMed]
- Prowse, K.R.; Greider, C.W. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl. Acad. Sci. USA 1995, 92, 4818–4822. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.E.; Shay, J.W. Telomere biology in aging and cancer. J. Am. Geriatr. Soc. 2005, 53, S292–S294. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W. Meeting report: The role of telomeres and telomerase in cancer. Cancer Res. 2005, 65, 3513–3517. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Mammalian telomeres and telomerase: Why they matter for cancer and aging. Eur. J. Cell Biol. 2003, 82, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gilson, E.; Segal-Bendirdjian, E. The telomere story or the triumph of an open-minded research. Biochimie 2010, 92, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Geserick, C.; Blasco, M.A. Novel roles for telomerase in aging. Mech. Ageing Dev. 2006, 127, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tergaonkar, V. Noncanonical functions of telomerase: Implications in telomerase-targeted cancer therapies. Cancer Res. 2014, 74, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Neumann, A.A.; Yeager, T.R.; Reddel, R.R. Alternative lengthening of telomeres in mammalian cells. Oncogene 2002, 21, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Bohrson, C.; Pike, A.M.; Wheelan, S.J.; Greider, C.W. ATM Kinase Is Required for Telomere Elongation in Mouse and Human Cells. Cell Rep. 2015, 13, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Colonna-Romano, G.; Lio, D.; Candore, G.; Caruso, C. TLR4 polymorphisms and ageing: Implications for the pathophysiology of age-related diseases. J. Clin. Immunol. 2009, 29, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chuturgoon, A.A.; Naidoo, D.P. Telomeres and atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.H.; Pawlina, W. Cardiovascular system. In Histology: A Text and Atlas, 5th ed.; Taylor, C., Scogna, K.H., Ajello, J.P., Eds.; Lippincott Williams and Wilkins: Baltimore, MA, USA, 2006; pp. 364–394. [Google Scholar]
- Darland, D.C.; D’Amore, P.A. Cell-cell interactions in vascular development. Curr. Top. Dev. Biol. 2001, 52, 107–149. [Google Scholar] [PubMed]
- Lin, C.H.; Lilly, B. Notch signaling governs phenotypic modulation of smooth muscle cells. Vasc. Pharmacol. 2014, 63, 88–96. [Google Scholar] [CrossRef] [PubMed]
- High, F.A.; Lu, M.M.; Pear, W.S.; Loomes, K.M.; Kaestner, K.H.; Epstein, J.A. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. USA 2008, 105, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Khan, M.Y.; Skurnick, J.; Kimura, M.; Aviv, H.; Aviv, A. Telomere attrition of the human abdominal aorta: Relationships with age and atherosclerosis. Atherosclerosis 2000, 152, 391–398. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Katsargyris, A.; Klonaris, C.; Avgerinos, E.D.; Fragou-Plemenou, M.; Kouraklis, G.; Liapis, C.D. Telomerase expression on aortic wall endothelial cells is attenuated in abdominal aortic aneurysms compared to healthy nonaneurysmal aortas. J. Vasc. Surg. 2011, 54, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Blunder, S.; Messner, B.; Aschacher, T.; Zeller, I.; Turkcan, A.; Wiedemann, D.; Andreas, M.; Bluschke, G.; Laufer, G.; Schachner, T.; et al. Characteristics of TAV- and BAV-associated thoracic aortic aneurysms—Smooth muscle cell biology, expression profiling, and histological analyses. Atherosclerosis 2012, 220, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Drose, S.; Buchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Bianchessi, V.; Badi, I.; Bertolotti, M.; Nigro, P.; D’Alessandra, Y.; Capogrossi, M.C.; Zanobini, M.; Pompilio, G.; Raucci, A.; Lauri, A. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J. Mol. Cell. Cardiol. 2015, 81, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Boe, A.E.; Eren, M.; Murphy, S.B.; Kamide, C.E.; Ichimura, A.; Terry, D.; McAnally, D.; Smith, L.H.; Miyata, T.; Vaughan, D.E. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nomega-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 2013, 128, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J. Mechanistic links between aging and lung fibrosis. Biogerontology 2013, 14, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, H.; Mu, F.T.; Ebisui, O.; Funder, J.W.; Liu, J.P. Telomerase activation causes vascular smooth muscle cell proliferation in genetic hypertension. FASEB J. 2002, 16, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Pili, R.; Guo, Y.; Chang, J.; Nakanishi, H.; Martin, G.R.; Passaniti, A. Altered angiogenesis underlying age-dependent changes in tumor growth. J. Natl. Cancer Inst. 1994, 86, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Rivard, A.; Fabre, J.E.; Silver, M.; Chen, D.; Murohara, T.; Kearney, M.; Magner, M.; Asahara, T.; Isner, J.M. Age-dependent impairment of angiogenesis. Circulation 1999, 99, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Klagsbrun, M.; D’Amore, P.A. Regulators of angiogenesis. Annu. Rev. Physiol. 1991, 53, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Harley, C.B. Telomere length and replicative aging in human vascular tissues. Proc. Natl. Acad. Sci. USA 1995, 92, 11190–11194. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.; Qureshi, M.; Verma, R.; Lacy, P.S.; Williams, B. Telomere attrition and accumulation of senescent cells in cultured human endothelial cells. Cell Prolif. 2004, 37, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Tateno, K.; Kunieda, T.; Komuro, I. Vascular cell senescence and vascular aging. J. Mol. Cell. Cardiol. 2004, 36, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.; Boyer, M.; Solan, A.; Dahl, S.L.; Pedrotty, D.; Banik, S.S.; McKee, J.A.; Klinger, R.Y.; Counter, C.M.; Niklason, L.E. Blood vessels engineered from human cells. Lancet 2005, 365, 2122–2124. [Google Scholar] [CrossRef]
- Minamino, T.; Kourembanas, S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ. Res. 2001, 89, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, E.; Cherry, A.M.; Bangs, C.D.; Oei, Y.; Bodnar, A.; Bronstein, A.; Chiu, C.P.; Herron, G.S. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 1999, 274, 26141–26148. [Google Scholar] [CrossRef] [PubMed]
- Trivier, E.; Kurz, D.J.; Hong, Y.; Huang, H.L.; Erusalimsky, J.D. Differential regulation of telomerase in endothelial cells by fibroblast growth factor-2 and vascular endothelial growth factor-a: Association with replicative life span. Ann. N. Y. Acad. Sci. 2004, 1019, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed]
- McCord, R.A.; Michishita, E.; Hong, T.; Berber, E.; Boxer, L.D.; Kusumoto, R.; Guan, S.; Shi, X.; Gozani, O.; Burlingame, A.L.; et al. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging 2009, 1, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.T.; Cesare, A.J.; Fitzpatrick, J.A.; Lazzerini-Denchi, E.; Karlseder, J. A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat. Struct. Mol. Biol. 2012, 19, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Spallarossa, P.; Altieri, P.; Aloi, C.; Garibaldi, S.; Barisione, C.; Ghigliotti, G.; Fugazza, G.; Barsotti, A.; Brunelli, C. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H2169–H2181. [Google Scholar] [CrossRef] [PubMed]
- Lechel, A.; Satyanarayana, A.; Ju, Z.; Plentz, R.R.; Schaetzlein, S.; Rudolph, C.; Wilkens, L.; Wiemann, S.U.; Saretzki, G.; Malek, N.P.; et al. The cellular level of telomere dysfunction determines induction of senescence or apoptosis in vivo. EMBO Rep. 2005, 6, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Nomiyama, T.; Nakamachi, T.; Heywood, E.B.; Stone, J.F.; Berger, J.P.; Law, R.E.; Bruemmer, D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ. Res. 2006, 98, e50–e59. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Mitsialis, S.A.; Kourembanas, S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol. Cell. Biol. 2001, 21, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kurz, D.J.; Decary, S.; Hong, Y.; Trivier, E.; Akhmedov, A.; Erusalimsky, J.D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004, 117, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Hoffmann, J.; Zeiher, A.M.; Dimmeler, S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: A novel vasculoprotective function of statins. Circulation 2004, 110, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Von Zglinicki, T.; Burkle, A.; Kirkwood, T.B. Stress, DNA damage and ageing—An integrative approach. Exp. Gerontol. 2001, 36, 1049–1062. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; FitzGerald, G.A. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation 2003, 108, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Mactaggart, J.; Knispel, R.; Worth, J.; Zhu, Z.; Li, Y.; Sun, Y.; Baxter, B.T.; Johanning, J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009, 202, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Acilan, C.; Potter, D.M.; Saunders, W.S. DNA repair pathways involved in anaphase bridge formation. Genes Chromosomes Cancer 2007, 46, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.H.; Meyer, J.N.; Skorvaga, M.; Annab, L.A.; Van Houten, B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 2004, 3, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Chiodi, I.; Mondello, C. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front. Oncol. 2012, 2, 133. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; De Caterina, R.; Willerson, J.T.; Geng, Y.J. Biologic function and clinical potential of telomerase and associated proteins in cardiovascular tissue repair and regeneration. Eur. Heart J. 2011, 32, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Cafueri, G.; Parodi, F.; Pistorio, A.; Bertolotto, M.; Ventura, F.; Gambini, C.; Bianco, P.; Dallegri, F.; Pistoia, V.; Pezzolo, A.; et al. Endothelial and smooth muscle cells from abdominal aortic aneurysm have increased oxidative stress and telomere attrition. PLoS ONE 2012, 7, e35312. [Google Scholar] [CrossRef] [PubMed]
- Rhee, D.B.; Ghosh, A.; Lu, J.; Bohr, V.A.; Liu, Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair 2011, 10, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Beyer, A.M.; Freed, J.K.; Durand, M.J.; Riedel, M.; Ait-Aissa, K.; Green, P.; Hockenberry, J.C.; Morgan, R.G.; Donato, A.J.; Peleg, R.; et al. Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation. Circ. Res. 2016, 118, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chamseddine, A.H.; Carrell, S.; Miller, F.J., Jr. Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014, 2, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Buchner, N.; Ale-Agha, N.; Jakob, S.; Sydlik, U.; Kunze, K.; Unfried, K.; Altschmied, J.; Haendeler, J. Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp. Gerontol. 2013, 48, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Martin-Gronert, M.S.; Chen, J.H.; Cripps, R.L.; Ozanne, S.E. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 2008, 22, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Silventoinen, K.; Saijonmaa, O.; Kontula, K.; Devereux, R.B.; de Faire, U.; Os, I.; Dahlof, B. Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: The LIFE study. J. Hum. Hypertens. 2011, 25, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Demissie, S.; Levy, D.; Benjamin, E.J.; Cupples, L.A.; Gardner, J.P.; Herbert, A.; Kimura, M.; Larson, M.G.; Meigs, J.B.; Keaney, J.F.; et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 2006, 5, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rivero, G.; Ruiz-Torres, M.P.; Rivas-Elena, J.V.; Jerkic, M.; Diez-Marques, M.L.; Lopez-Novoa, J.M.; Blasco, M.A.; Rodriguez-Puyol, D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation 2006, 114, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Hingorani, A.; Ascher, E. Evidence for telomerase activation in VSMCs exposed to hyperglycemic and hyperhomocysteinemic conditions. Angiology 2009, 60, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Matsui-Hirai, H.; Hayashi, T.; Yamamoto, S.; Ina, K.; Maeda, M.; Kotani, H.; Iguchi, A.; Ignarro, L.J.; Hattori, Y. Dose-dependent modulatory effects of insulin on glucose-induced endothelial senescence in vitro and in vivo: A relationship between telomeres and nitric oxide. J. Pharmacol. Exp. Ther. 2011, 337, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Hastie, N.D.; Dempster, M.; Dunlop, M.G.; Thompson, A.M.; Green, D.K.; Allshire, R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990, 346, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Pisano, C.; Candore, G.; Maresi, E.; Codispoti, M.; Ruvolo, G. Focus on the unique mechanisms involved in thoracic aortic aneurysm formation in bicuspid aortic valve versus tricuspid aortic valve patients: Clinical implications of a pilot study. Eur. J. Cardiothorac. Surg. 2013, 43, e180–e186. [Google Scholar] [CrossRef] [PubMed]
- Huusko, T.J.; Santaniemi, M.; Kakko, S.; Taskinen, P.; Ukkola, O.; Kesaniemi, Y.A.; Savolainen, M.J.; Salonurmi, T. Long telomeres in blood leukocytes are associated with a high risk of ascending aortic aneurysm. PLoS ONE 2012, 7, e50828. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Herbert, K.E.; Mistry, Y.; Stevens, S.E.; Patel, H.R.; Hastings, R.A.; Thompson, M.M.; Williams, B. Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur. Heart J. 2008, 29, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Atturu, G.; Brouilette, S.; Samani, N.J.; London, N.J.; Sayers, R.D.; Bown, M.J. Short leukocyte telomere length is associated with abdominal aortic aneurysm (AAA). Eur. J. Vasc. Endovasc. Surg. 2010, 39, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Samani, N.J.; Boultby, R.; Butler, R.; Thompson, J.R.; Goodall, A.H. Telomere shortening in atherosclerosis. Lancet 2001, 358, 472–473. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Pisano, C.; Martorana, A.; Triolo, O.F.; Lio, D.; Candore, G.; Ruvolo, G. Are the leukocyte telomere length attrition and telomerase activity alteration potential predictor biomarkers for sporadic TAA in aged individuals? Age 2014, 36, 9700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balistreri, C.R.; Pisano, C.; Merlo, D.; Fattouch, K.; Caruso, M.; Incalcaterra, E.; Colonna-Romano, G.; Candore, G. Is the mean blood leukocyte telomere length a predictor for sporadic thoracic aortic aneurysm? Data from a preliminary study. Rejuvenation Res. 2012, 15, 170–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschenberger, J.; Kollerits, B.; Ritchie, J.; Lane, B.; Kalra, P.A.; Ritz, E.; Kronenberg, F. Association of relative telomere length with progression of chronic kidney disease in two cohorts: Effect modification by smoking and diabetes. Sci. Rep. 2015, 5, 11887. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Seals, D.R.; Pierce, G.L. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech. Ageing Dev. 2010, 131, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Belcher, M.; van der Harst, P. Healthy aging and disease: Role for telomere biology? Clin. Sci. 2011, 120, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Tarkanyi, I.; Aradi, J. Pharmacological intervention strategies for affecting telomerase activity: Future prospects to treat cancer and degenerative disease. Biochimie 2008, 90, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh-Far, R.; Lin, J.; Epel, E.S.; Harris, W.S.; Blackburn, E.H.; Whooley, M.A. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010, 303, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Riordan, S.M.; Heruth, D.P.; Grigoryev, D.N.; Zhang, L.Q.; Ye, S.Q. A critical role of nicotinamide phosphoribosyltransferase in human telomerase reverse transcriptase induction by resveratrol in aortic smooth muscle cells. Oncotarget 2015, 6, 10812–10824. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chi, H.Y.; Yu, Z.H.; Chen, J.L. [Effects of Chinese herbal medicine Shoushen Granule on telomere length and telomerase activity of peripheral white blood cells and vascular cells in rats with atherosclerosis]. Zhong Xi Yi Jie He Xue Bao 2012, 10, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Solito, R.; Cetti, E.; Corti, F.; Giachetti, A.; Carra, S.; Beltrame, M.; Cotelli, F.; Ziche, M. Abeta peptides accelerate the senescence of endothelial cells in vitro and in vivo, impairing angiogenesis. FASEB J. 2010, 24, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Donnini, S.; Terzuoli, E.; Ziche, M.; Morbidelli, L. Sulfhydryl angiotensin-converting enzyme inhibitor promotes endothelial cell survival through nitric-oxide synthase, fibroblast growth factor-2, and telomerase cross-talk. J. Pharmacol. Exp. Ther. 2010, 332, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Spyridopoulos, I.; Haendeler, J.; Urbich, C.; Brummendorf, T.H.; Oh, H.; Schneider, M.D.; Zeiher, A.M.; Dimmeler, S. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation 2004, 110, 3136–3142. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Minami, Y.; Takahashi, Y.; Tabuchi, T.; Itoh, T.; Nakamura, M. Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clin. Sci. 2009, 116, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Saliques, S.; Teyssier, J.R.; Vergely, C.; Lorgis, L.; Lorin, J.; Farnier, M.; Donzel, A.; Sicard, P.; Berchoud, J.; Lagrost, A.C.; et al. Circulating leukocyte telomere length and oxidative stress: A new target for statin therapy. Atherosclerosis 2011, 219, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Gensch, C.; Poss, J.; Haendeler, J.; Bohm, M.; Laufs, U. Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis 2011, 216, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kotani, H.; Yamaguchi, T.; Taguchi, K.; Iida, M.; Ina, K.; Maeda, M.; Kuzuya, M.; Hattori, Y.; Ignarro, L.J. Endothelial cellular senescence is inhibited by liver X receptor activation with an additional mechanism for its atheroprotection in diabetes. Proc. Natl. Acad. Sci. USA 2014, 111, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aschacher, T.; Salameh, O.; Enzmann, F.; Messner, B.; Bergmann, M. Telomere Biology and Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2018, 19, 3. https://doi.org/10.3390/ijms19010003
Aschacher T, Salameh O, Enzmann F, Messner B, Bergmann M. Telomere Biology and Thoracic Aortic Aneurysm. International Journal of Molecular Sciences. 2018; 19(1):3. https://doi.org/10.3390/ijms19010003
Chicago/Turabian StyleAschacher, Thomas, Olivia Salameh, Florian Enzmann, Barbara Messner, and Michael Bergmann. 2018. "Telomere Biology and Thoracic Aortic Aneurysm" International Journal of Molecular Sciences 19, no. 1: 3. https://doi.org/10.3390/ijms19010003




