Salvia Species as Sources of Natural Products with Antiprotozoal Activity
Abstract
:1. Introduction
2. Methodology
3. Biological Activity
3.1. Antitrypanosomal Activity
3.1.1. Activity of Crude Extracts and Isolated Constituents from Salvia Species against Trypanosma Brucei
Extracts and Essential Oils
Diterpenoids
Triterpenoids
3.1.2. Activity of Crude Extracts and Isolated Constituents from Salvia Species against Trypanosoma Cruzi
Extracts
Diterpenoids
3.2. Antileishmanial Activity
3.2.1. Extracts and Essential Oils
3.2.2. Diterpenes
3.2.3. Triterpenes
3.2.4. Phenolic Compounds
3.3. Antiplasmodial Activity
3.3.1. Extracts and Essential Oils
3.3.2. Diterpenoids
3.3.3. Triterpenes and Related Terpenoids
3.3.4. Flavonoids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Topçu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B.; Sytsma, K.J. Staminal evolution in the genus Salvia (Lamiaceae): Molecular phylogenetic evidence for multiple origins of the staminal lever. Ann. Bot. 2007, 100, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Goñalons, L. Salvia L. In Flora Ibérica; Morales, R., Quintanar, A., Cabezas, F., Pujadas, A.J., Cirujano, S., Eds.; Real Jardín Botánico, CSIC: Madrid, Sapin, 1974; Volume 12, pp. 1194–1196. [Google Scholar]
- Ślusarczyk, S.; Zimmermann, S.; Kaiser, M.; Matkowski, A.; Hamburger, M.; Adams, M. Antiplasmodial and antitrypanosomal activity of tanshinone-type diterpenoids from Salvia miltiorrhiza. Planta Med. 2011, 77, 1594–1596. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Viljoen, A.M.; Gono-Bwalya, A.B.; van Zyl, R.L.; van Vuuren, S.F.; Lourens, A.C.U.; Başer, K.H.C.; Demirci, B.; Lindsey, K.L.; van Staden, J.; et al. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J. Ethnopharmacol. 2005, 102, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; da Costa, B.D.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; Lago, J.H.G.; et al. The Potential of Secondary Metabolites from Plants as Drugs or Leads Against Protozoan Neglected Diseases—Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef] [PubMed]
- Sairafianpour, M.; Bahreininejad, B.; Witt, M.; Ziegler, H.L.; Jaroszewski, J.W.; Stærk, D. Terpenoids of Salvia hydrangea: Two New, Rearranged 20-Norabietanes and the Effect of Oleanolic Acid on Erythrocyte Membranes. Planta Med. 2003, 69, 846–850. [Google Scholar] [CrossRef] [PubMed]
- WHO Press. Research priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. World Health Organ. Tech. Rep. Ser. 2012, 975, 1–100. [Google Scholar]
- WHO. Health Statistics and Information Systems, Estimates for 2000–2015. Available online: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html (accessed on 1 July 2017).
- WHO. Human African Trypanosomiasis. Available online: http://www.who.int/trypanosomiasis_african/en/ (accessed on 1 December 2017).
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.-P.F.; et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: A pivotal multicentre, randomised, non-inferiority trial. Lancet 2017, 6736, 1–11. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Gascon, J. The importance of the multidisciplinary approach to deal with the new epidemiological scenario of Chagas disease (global health). Acta Trop. 2015, 151, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gascon, J.; Bern, C.; Pinazo, M.J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010, 115, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Info Chagas. Available online: http://www.infochagas.org/en/ (accessed on 13 April 2017).
- Kirmizibekmez, H.; Atay, I.; Kaiser, M.; Yesiladaa, E.; Tasdemir, D. In vitro antiprotozoal activity of extracts of five Turkish Lamiaceae species. Nat. Prod. Commun. 2011, 6, 1697–1700. [Google Scholar]
- Farimani, M.M.; Ebrahimi, S.N.; Salehi, P.; Bahadori, M.B.; Sonboli, A.; Khavasi, H.R.; Zimmermann, S.; Kaiser, M.; Hamburger, M. Antitrypanosomal triterpenoid with an ε-lactone E-ring from Salvia urmiensis. J. Nat. Prod. 2013, 76, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Mokoka, T.A.; Peter, X.K.; Fouche, G.; Moodley, N.; Adams, M.; Hamburger, M.; Kaiser, M.; Brun, R.; Maharaj, V.; Koorbanally, N. Antileishmanial activity of 12-methoxycarnosic acid from Salvia repens Burch. ex Benth. (Lamiaceae). S. Afr. J. Bot. 2014, 90, 93–95. [Google Scholar] [CrossRef]
- Jain, S.; Jacob, M.; Walker, L.; Tekwani, B. Screening North American plant extracts in vitro against Trypanosoma brucei for discovery of new antitrypanosomal drug leads. BMC Complement. Altern. Med. 2016, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, S.A.; Mei, W.; Ali, Z.; Zaki, A.A.; Khan, S.I.; Khan, I.A. Chemical Analysis and Biological Activities of Salvia lavandulifolia Vahl. Essential Oil. Chem. Anal. 2017, 7, 71–78. [Google Scholar]
- Llurba-Montesino, N.; Kaiser, M.; Brun, R.; Schmidt, T.J. Search for Antiprotozoal Activity in Herbal Medicinal Preparations; New Natural Leads against Neglected Tropical Diseases. Molecules 2015, 20, 14118–14138. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Fujiwara, K.; Yamazaki, A.; Sugawara, N.; Yano, R.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K.; Ishiyama, A.; Iwatsuki, M.; et al. Semisynthesis of salviandulin e analogues and their antitrypanosomal activity. Bioorg. Med. Chem. Lett. 2014, 24, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.N.; Zimmermann, S.; Zaugg, J.; Smiesko, M.; Brun, R.; Hamburger, M. Abietane diterpenoids from Salvia sahendica—Antiprotozoal activity and determination of their absolute configurations. Planta Med. 2013, 79, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, Ł.; Kaiser, M.; Wysokińska, H. The production and antiprotozoal activity of abietane diterpenes in Salvia austriaca hairy roots grown in shake flasks and bioreactor. Prep. Biochem. Biotechnol. 2017, 47, 58–66. [Google Scholar] [CrossRef]
- Farimani, M.M.; Bahadori, M.B.; Taheri, S.; Ebrahimi, S.N.; Zimmermann, S.; Brun, R.; Amin, G.; Hamburger, M. Triterpenoids with rare carbon skeletons from Salvia hydrangea: Antiprotozoal activity and absolute configurations. J. Nat. Prod. 2011, 74, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Mokoka, T.A.; Zimmermann, S.; Julianti, T.; Hata, Y.; Moodley, N.; Cal, M.; Adams, M.; Kaiser, M.; Brun, R.; Koorbanally, N.; et al. In vitro screening of traditional South African malaria remedies against Trypanosoma brucei rhodesiense, Trypanosoma cruzi, Leishmania donovani, and Plasmodium falciparum. Planta Med. 2011, 77, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Jimenez-Ortiz, V.; Sartor, T.; Tonn, C.E.; García, E.E.; Nieto, M.; Burgos, M.H.; Sosa, M.A. A novel icetexane diterpene, 5-epi-icetexone from Salvia gilliessi is active against Trypanosoma cruzi. Acta Trop. 2006, 98, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Barrera, P.; Salinas, R.; Vega, I.; Nieto, M.; Tonn, C.; Kemmerling, U.; Mortara, R.A.; Sosa, M.A. Sesquiterpene lactones and the diterpene 5-epi-icetexone affect the intracellular and extracellular stages of Trypanosoma cruzi. Parasitol. Int. 2012, 61, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Strauss, M.; Spina, R.; Cifuente, D.; Tonn, C.; Rivarola, H.W.; Sosa, M.A. The in vivo trypanocidal effect of the diterpene 5-epi-icetexone obtained from Salvia gilliesii. Parasitol. Int. 2016, 65, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.S.; Spina, R.M.; Tonn, C.E.; Sosa, M.A.; Cifuente, D.A. An abietane diterpene from Salvia cuspidata and some new derivatives are active against Trypanosoma cruzi. Bioorg. Med. Chem. Lett. 2015, 25, 5481–5484. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases. Third WHO Report on Neglected Tropical Diseases; Holmes, P., Ed.; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. WHO technical report series: Control of the leishmaniases. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2010; ISBN 9789241209. [Google Scholar]
- Singh, N.; Mishra, B.B.; Bajpai, S.; Singh, R.K.; Tiwari, V.K. Natural product based leads to fight against leishmaniasis. Bioorg. Med. Chem. 2014, 22, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Essid, R.; Zahra, F.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crops Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Nikmehr, B.; Ghaznavi, H.; Rahbar, A.; Sadr, S.; Mehrzadi, S. In vitro anti-leishmanial activity of methanolic extracts of Calendula officinalis flowers, Datura stramonium seeds, and Salvia officinalis leaves. Chin. J. Nat. Med. 2014, 12, 423–427. [Google Scholar] [CrossRef]
- Khan, A.R.; Khan, M.J. In vitro Antileishmanial, Cytotoxic and Antioxidant activities of Salvia bucharica leaves extract and its fractions. Int. J. Basic Appl. Sci. 2003, 13, 74–78. [Google Scholar]
- Et-Touys, A.; Fellah, H.; Sebti, F.; Mniouil, M.; Aneb, M.; Elboury, H.; Talbaoui, A.; Dakka, N.; Sadak, A.; Bakri, Y. In vitro Antileishmanial Activity of Extracts from Endemic Moroccan Medicinal Plant Salvia verbenaca (L.) Briq. Ssp. verbenaca Maire (S. clandestina Batt. non L). Eur. J. Med. Plants 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Tan, N.; Kaloga, M.; Radtke, O.A.; Kiderlen, A.F.; Öksüz, S.; Ulubelen, A.; Kolodziej, H. Abietane diterpenoids and triterpenoic acids from Salvia cilicica and their antileishmanial activities. Phytochemistry 2002, 61, 881–884. [Google Scholar] [CrossRef]
- Búfalo, J.; Cantrell, C.L.; Jacob, M.R.; Schrader, K.K.; Tekwani, B.L.; Kustova, T.S.; Ali, A.; Boaro, C.S.F. Antimicrobial and Antileishmanial Activities of Diterpenoids Isolated from the Roots of Salvia deserta. Planta Med. 2015, 82, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The Potential of Secondary Metabolites from Plants as Drugs or Leads Against Protozoan Neglected Diseases—Part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef] [PubMed]
- Radtke, O.A.; Yeap Foo, L.; Lu, Y.; Kiderlen, A.F.; Kolodziej, H. Evaluation of sage phenolics for their antileishmanial activity and modulatory effects on interleukin-6, interferon and tumour necrosis factor-α-release in RAW 264.7 Cells. Z. Naturforsch. C 2003, 58, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Maubon, D.; Thurot-Guillou, C.; Ravel, C.; Leccia, M.-T.; Pelloux, H. Leishmania killicki imported from Tunisian desert. Emerg. Infect. Dis. 2009, 15, 1864–1865. [Google Scholar] [CrossRef] [PubMed]
- Rotureau, B.; Ravel, C.; Nacher, M.; Couppié, P.; Curtet, I.; Dedet, J.P.; Carme, B. Molecular epidemiology of Leishmania (Viannia) guyanensis in French Guiana. J. Clin. Microbiol. 2006, 44, 468–473. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fact Sheet Malaria. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 1 July 2017).
- Kamatou, G.P.P. Indigenous Salvia Species—An Investigation of Their Pharmacological Activities and Phytochemistry. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2006. [Google Scholar]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, R.; Dehak, K.; Sanon, S.; Mahammed, M.H.; Ouldelhadj, M.D. In Vitro Antimalarial, Antimicrobial and Antioxidants Activities of Salvia chudaei Batt. & Trab. (Lamiaceae) Extracts Roukia. Der Pharma Chem. 2017, 9, 82–89. [Google Scholar]
- Kamatou, G.P.P.; van Zyl, R.L.; Davids, H.; van Heerden, F.R.; Lourens, A.C.U.; Viljoen, A.M. Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. S. Afr. J. Bot. 2008, 74, 238–243. [Google Scholar] [CrossRef]
- Farimani, M.M.; Taheri, S.; Ebrahimi, S.N.; Bahadori, M.B.; Khavasi, H.R.; Zimmermann, S.; Brun, R.; Hamburger, M. Hydrangenone, a new isoprenoid with an unprecedented skeleton from Salvia hydrangea. Org. Lett. 2012, 14, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parasite | Strain | Plant Species | IC50 Extract | Type of Solvent Extract | SI | Ref. |
|---|---|---|---|---|---|---|
| Tbr | STIB 900 | S. tomentosa Mill. (a1) | 3.64 µg/mL | MeOH-extract | 24.73 | [16] |
| Tbr | STIB 900 | S. tomentosa Mill. (a2) | 1.24 µg/mL | n-Hexane-extract | 21.16 | [16] |
| Tbr | STIB 900 | S. tomentosa Mill. (a3) | 2.33 µg/mL | Chloroform-extract | 13.65 | [16] |
| Tbr | STIB 900 | S. tomentosa Mill. (a4) | 10.96 µg/mL | H2O-extract | >8 | [16] |
| Tbr | STIB 900 | S. sclarea L. (b1) | 6.44 µg/mL | MeOH-extract | 13.6 | [16] |
| Tbr | STIB 900 | S. sclarea L. (b2) | 2.4 µg/mL | n-Hexane-extract | 7.63 | [16] |
| Tbr | STIB 900 | S. sclarea L. (b3) | 4.4 µg/mL | Chloroform-extract | 19.07 | [16] |
| Tbr | STIB 900 | S. sclarea L. (b4) | 10.31 µg/mL | H2O-extract | >8.7 | [16] |
| Tbr | STIB 900 | S. dichroantha Stapf. (c1) | 3.58 µg/mL | MeOH-extract | >25 | [16] |
| Tbr | STIB 900 | S. dichroantha Stapf. (c2) | 3.5 µg/mL | n-Hexane-extract | >25.7 | [16] |
| Tbr | STIB 900 | S. dichroantha Stapf. (c3) | 4.4 µg/mL | Chloroform-extract | 19.27 | [16] |
| Tbr | STIB 900 | S. dichroantha Stapf. (c4) | 7.77 µg/mL | H2O-extract | >11.5 | [16] |
| Tbr | STIB 900 | S. hydrangea DC. ex Benth. (d) | 18 µg/mL | n-Hexane-extract | n.d. | [25] |
| Tbr | STIB 900 | S. miltiorrhiza Bunge (e) | 97% inhibition at 0.81 µg/mL | DCM-extract | n.d. | [4] |
| Tbr | STIB 900 | S. repens Burch.ex Benth. (f) | 10.8 µg/mL | DCM-MeOH (1:1) | 3.82 | [26] |
| Tbb | Strain 427 | S. spathacea Greene (g) | 1.13 ± 0.78 µg/mL | Ethanolic extract | n.d. | [19] |
| Tbb | n.d. | S. lavandulifolia Vahl. (h) | No activity at >20 mg/mL | Essential oil | n.d. | [20] |
| Tbr | STIB 900 | S. officinalis L. (i) | 1.86 µg/mL | Ethanolic tincture | 17.3 | [21] |
| Parasite | Form/Strain | Plant Species | IC50 Extract | Isolated Compound | Molecular Formula | Molar Mass (g/mol) | IC50 of the Compound (µM) | SI | Ref |
|---|---|---|---|---|---|---|---|---|---|
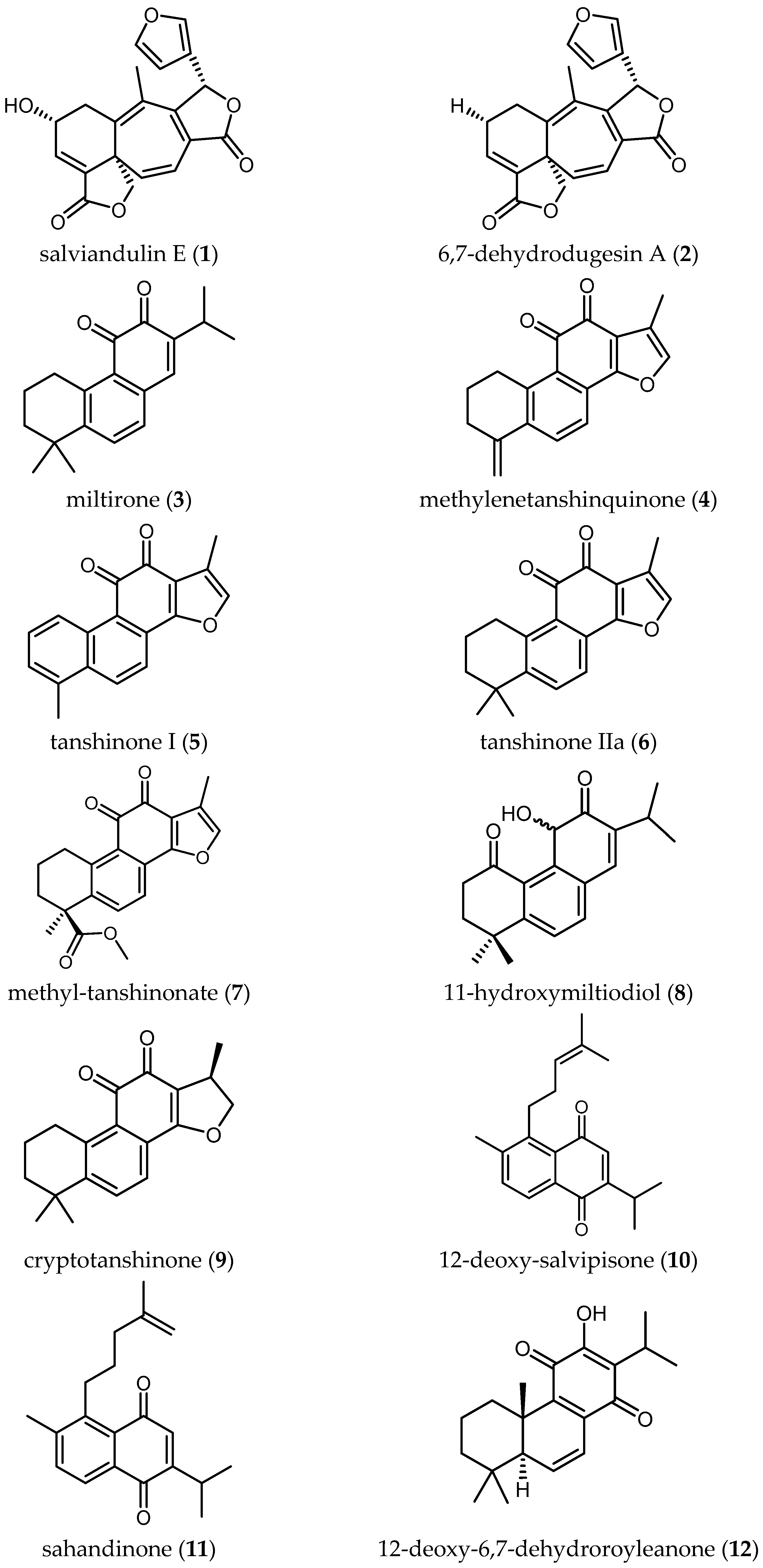
| Tbb | GUT at 3.1 | S. leucantha Cav. | n.d./n.f. | salviandulin E (1) | C20H16O6 | 352.09 | 2.04 µM (0.72 µg/mL) | 1.17 | [22] |
| Tbb | GUT at 3.1 | S. leucantha Cav. | n.d./n.f. | 6,7-dehydrodugesin A (2) | C20H16O5 | 336.09 | >37.19 µM (>12.5 µg/mL) | n.d. | [22] |
| Tbr | Trypomastigotes/ STIB 900 | S. miltiorrhiza Bunge. | 97% inhib. at 0.81 µg/mL | miltirone (3) | C19H22O2 | 282.16 | 0.5 µM | 2.6 | [4] |
| Tbr | Trypomastigotes/ STIB 900 | S. miltiorrhiza Bunge. | 97% inhib. at 0.81 µg/mL | methylenetanshinquinone (4) | C18H14O3 | 278.09 | 0.5 µM | 24.2 | [4] |
| Tbr | Trypomastigotes/ STIB 900 | S. miltiorrhiza Bunge. | 97% inhib. at 0.81 µg/mL | tanshinone I (5) | C18H12O3 | 276.08 | 1.3 µM | 9.5 | [4] |
| Tbr | Trypomastigotes/ STIB 900 | S. miltiorrhiza Bunge. | 97% inhib. at 0.81 µg/mL | tanshinone IIa (6) | C19H18O3 | 294.13 | 1.5 µM | 5 | [4] |
| Tbr | Trypomastigotes/ STIB 900 | S. miltiorrhiza Bunge. | 97% inhib. at 0.81 µg/mL | methyl-tanshinonate (7) | C20H18O5 | 338.12 | 17 µM | 0.4 | [4] |
| Tbr | Trypomastigotes/ STIB 900 | S. miltiorrhiza Bunge. | 97% inhib. at 0.81 µg/mL | 11-hydroxymiltiodiol (8) | C19H22O3 | 298.16 | 3.6 µM | n.d. | [4] |
| Tbr | Trypomastigotes/ STIB 900 | S. miltiorrhiza Bunge. | 97% inhib. at 0.81 µg/mL | cryptotanshinone (9) | C19H20O3 | 296.14 | 26.2 µM | 0.2 | [4] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | 12-deoxy-salvipisone (10) | C20H24O2 | 296.18 | 2.5 µM | 0.2 | [23] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | sahandinone (11) | C20H24O2 | 296.18 | 1.8 µM | 0.2 | [23] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | 12-deoxy-6,7-dehydroroyleanone (12) | C20H26O2 | 298.19 | 32.3 µM | 0.9 | [23] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | 7α-acetoxyroyleanone (13) | C22H30O5 | 374.21 | 2.9 µM | 0.1 | [23] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | Δ 9-ferruginol (14) | C20H28O | 284.21 | 12.8 µM | 1.2 | [23] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | ferruginol (15) | C20H30O | 286.23 | 28.1 µM | 0.5 | [23] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | sahandol (16) | C20H24O2 | 296.18 | 18.4 µM | 0.8 | [23] |
| Tbr | Trypomastigotes/ STIB 900 | S. sahendica Boiss. & Buhse | n.d./n.f. | sahandone (17) | C21H26O3 | 326.19 | 19.5 µM | 0.6 | [23] |
| Tbr | STIB 900 | S.austriaca Jacq. | n.d./n.f. | taxodione (18) | C20H26O3 | 314.19 | 0.05 µM | 38 | [24] |
| Tbr | STIB 900 | S.austriaca Jacq. | n.d./n.f. | 15-deoxy-fuerstione (19) | C20H26O2 | 298.19 | 194.7 µM | 0.69 | [24] |
| Tbr | STIB 900 | S.austriaca Jacq. | n.d./n.f. | 7-(2′-oxohexyl)-taxodione (20) | C26H36O4 | 412.26 | 0.62 µM | 5.0 | [24] |
| Tbr | STIB 900 | S.austriaca Jacq. | n.d./n.f. | taxodone (21) | C20H28O3 | 316.20 | 1.67 µM | 2.4 | [24] |
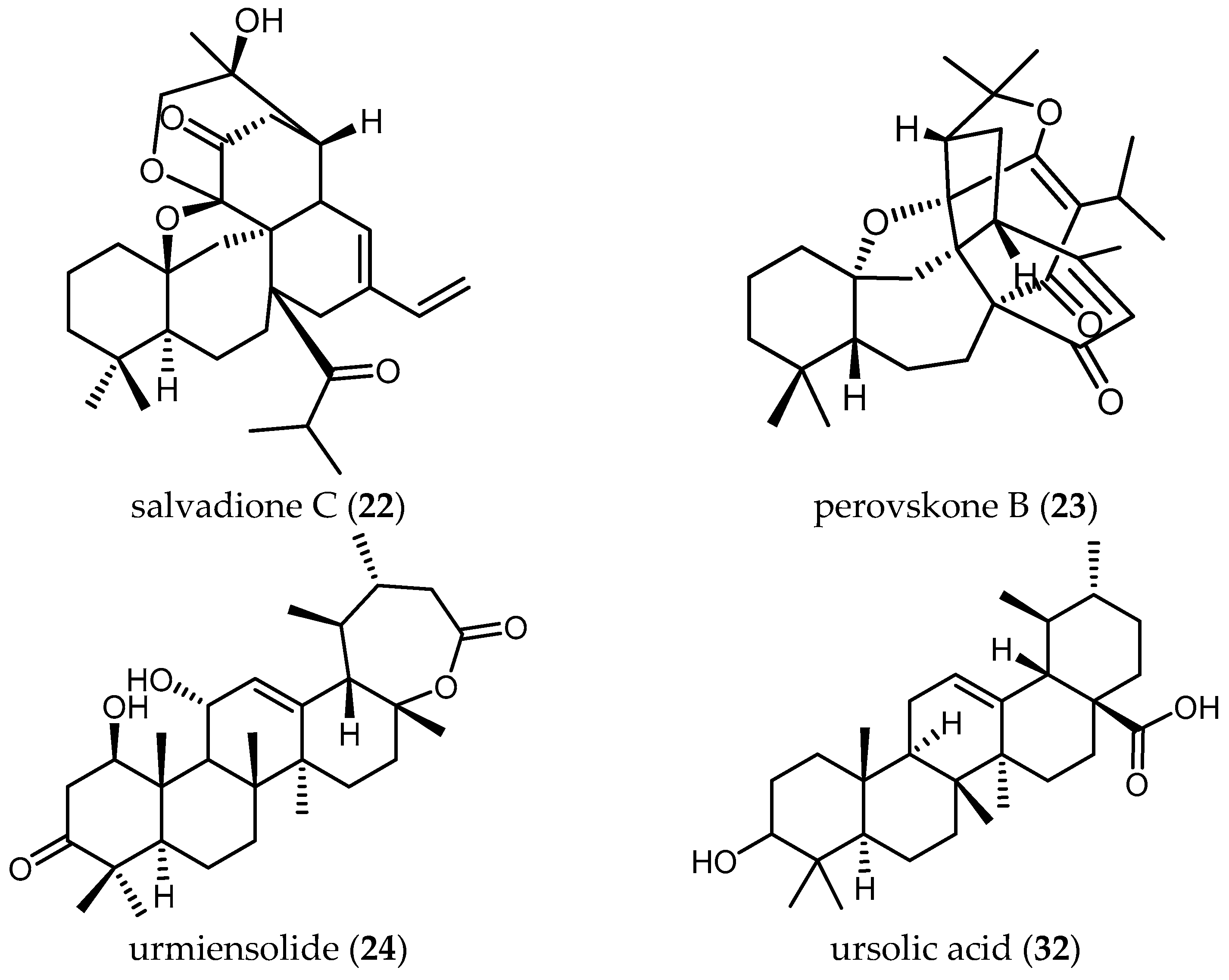
| Tbr | STIB 900 | S. hydrangea DC. Ex Benth. | 18 µg/mL | salvadione C (22) | C30H40O5 | 480.29 | 4.33 µM | 43.2 | [25] |
| Tbr | STIB 900 | S. hydrangea DC. Ex Benth. | 18 µg/mL | perovskone B (23) | C30H40O4 | 464.29 | 15.92 µM | 0.78 | [25] |
| Tbr | STIB 900 | S. urmiensis Bunge. | n.d./n.f. | urmiensolide (24) | C30H46O5 | 486.33 | 5.6 μM | 33 | [17] |
| Parasite | Form/Strain | Plant Species | IC50 Extract | Type of Solvent Extract | SI | Ref. |
|---|---|---|---|---|---|---|
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. tomentosa Mill. (a1) | >90 µg/mL | MeOH-extract | <1 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. tomentosa Mill. (a2) | 28.46 µg/mL | n-Hexane-extract | 0.92 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. tomentosa Mill. (a3) | 35.72 µg/mL | Chloroform-extract | 0.89 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. tomentosa Mill. (a4) | >90 µg/mL | H2O-extract | ≈1 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. sclarea L. (b1) | 56.82 µg/mL | MeOH-extract | 1.54 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. sclarea L. (b2) | 18.17 µg/mL | n-Hexane-extract | 1 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. sclarea L. (b3) | 52.51 µg/mL | Chloroform-extract | 1.59 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. sclarea L. (b4) | >90 µg/mL | H2O-extract | ≈1 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. dichroantha Stapf. (c1) | >90 µg/mL | MeOH-extract | ≈1 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. dichroantha Stapf. (c2) | 41.85 µg/mL | n-Hexane-extract | >2.15 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. dichroantha Stapf. (c3) | 48.99 µg/mL | Chloroform-extract | 1.73 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. dichroantha Stapf. (c4) | >90 µg/mL | H2O-extract | ≈1 | [16] |
| Tc | Trypomastigote/Tulahuen strain C2C4 | S. repens Burch.ex Benth. (f) | 36.2 µg/mL | DCM-MeOH (1:1) | 1.15 | [26] |
| Parasite | Form/Strain | Plant Species | IC50 Extract | Isolated Compound | Molecular Formula | Molar Mass (g/mol) | IC50 of the Compound (µM) | SI | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Tc | Epimastigotes/Tulhauen | S. gilliessi Benth. | n.d. | 5-epi-icetexone (ICTX) (25) | C20H22O5 | 342.15 | 6.5 ± 0.75 µM | n.d. | [27] |
| Tc | Amastigotes/Y Strain | S. gilliessi Benth. | n.d. | 5-epi-icetexone (ICTX) (25) | C20H22O5 | 342.15 | n.d. | n.d. | [28] |
| Tc | Epimastigotes/Dm28c strain | S. cuspidata (Ruiz & Pav. Subsp. gilliesii (Benth.) J.R.I. Wood | n.d. | 12-hydroxy-11,14-diketo-6,8,12-abietatrien-19,20-olide (HABTO) (26) | C17H16O5 | 300.10 | ≈16.6 µM (≈5 µg/mL) | n.d. | [30] |
| Tc | Amastigotes/Tulahuen strain C2C4 containing Lac Z | S. austriaca Jacq. | n.d. | taxodione (18) | C20H26O3 | 314.19 | 7.11 µM | 0.27 | [24] |
| Tc | Amastigotes/Tulahuen strain C2C4 containing Lac Z | S. austriaca Jacq. | n.d. | 15-Deoxy-fuerstione (19) | C20H26O2 | 298.19 | 146.9 µM | 0.91 | [24] |
| Tc | Amastigotes/Tulahuen strain C2C4 containing Lac Z | S. austriaca Jacq. | n.d. | 7-(2′-oxohexyl)-taxodione (20) | C26H36O4 | 412.26 | 7.76 µM | 0.4 | [24] |
| Tc | Amastigotes/Tulahuen strain C2C4 containing Lac Z | S. austriaca Jacq. | n.d. | taxodone (21) | C20H28O3 | 316.20 | 7.63 µM | 0.5 | [24] |
| Parasite | Form/Strain | Plant Species | IC50 Extract | Type of Solvent Extract | SI | Ref. |
|---|---|---|---|---|---|---|
| n.d. | (Popular use antileishmanial remedy) | S. hydrangea DC ex. Benth. (d1) | n.d. | n.d. | n.d. | [8] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. tomentosa Mill. (a1) | 14.92 µg/mL | MeOH-extract | 24.73 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. tomentosa Mill. (a2) | 2.49 µg/mL | n-Hexane-extract | 21.16 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. tomentosa Mill. (a3) | 1.81 µg/mL | Chloroform-extract | 29.47 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. tomentosa Mill. (a4) | >90 µg/mL | H2O-extract | >10 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. sclarea L. (b1) | 12.95 µg/mL | MeOH-extract | 13.6 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. sclarea L. (b2) | 5.25 µg/mL | n-Hexane-extract | 7.63 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. sclarea L. (b3) | 8.31 µg/mL | Chloroform-extract | 19.07 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. sclarea L. (b4) | 47.88 µg/mL | H2O-extract | >9 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. dichroantha Stapf. (c1) | 4.93 µg/mL | MeOH-extract | >25 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. dichroantha Stapf. (c2) | 3.48 µg/mL | n-Hexane-extract | 25.7 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. dichroantha Stapf. (c3) | 2.31 µg/mL | Chloroform-extract | 19.27 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. dichroantha Stapf. (c4) | >90 µg/mL | H2O-extract | >11 | [16] |
| Ld | Axenic amastigotes/ strain MHOM/ET/67/L82 | S. repens Burch. ex Benth. (f) | 5.36 µg/mL | DCM-MeOH (1:1) | 7.74 | [26] |
| Ld | Promastigotes | S. lavandulifolia Vahl. (h) | No activity up to 20 µg/mL | Essential oil | n.d. | [20] |
| Ld | Axenic amastigotes | S. lavandulifolia Vahl. (h) | No activity up to 20 µg/mL | Essential oil | n.d. | [20] |
| Ld | Intracellular amastigotes | S. lavandulifolia Vahl. (h) | No activity up to 20 µg/mL | Essential oil | n.d. | [20] |
| Lm | Promastigote/ LCO3 | S.officinalis L. (i) | 3.40 ± 0.16 µg/mL | Essential oil | 5.92 | [34] |
| Li | Promastigote/ LV20 | S.officinalis L. (i1) | 2.67 ± 0.33 µg/mL | Essential oil | 7.54 | [34] |
| Lm | Promastigotes/ MROH/IR/75/IR | S.officinalis L. (i2) | 184 ± 11.17 µg/mL | MeOH-maceration | n.d. | [35] |
| Lm | Amastogotes | S.officinalis L. (i2) | 58% letally at 184 ± 11.17 µg/mL | MeOH-maceration | n.d. | [35] |
| Lm | Promastigotes | S. bucharica Popov (j) | 72.31 µg/mL | MeOH-extract | n.d. | [36] |
| Lm | Promastigotes | S. bucharica Popov (j1) | 50.51 µg/mL | Chloroform-extract | n.d. | [36] |
| Lm | Promastigotes | S. bucharica Popov (j2) | >100 µg/mL | Acetone-extract | n.d. | [36] |
| Lm | Promastigotes | S. bucharica Popov (j3) | 30.51 µg/mL | H2O extract | n.d. | [36] |
| Lm | Promastigotes/ MHOM/MA/2009/LCER19-09 | S. verbenaca (L.) Briq. ssp verbenaca Maire (k) | 155.43 µg/mL | n-Hexane-extract | n.d. | [37] |
| Lm | Promastigotes/ MHOM/MA/2009/LCER19-09 | S. verbenaca (L.) Briq. ssp verbenaca Maire (k1) | 24.56 µg/mL | DCM-extract | n.d. | [37] |
| Lm | Promastigotes/ MHOM/MA/2009/LCER19-09 | S. verbenaca (L.) Briq. ssp verbenaca Maire (k2) | >1000 µg/mL | MeOH-extract | n.d. | [37] |
| Li | Promastigotes/ MHOM/MA/1998/LVTA | S. verbenaca (L.) Briq. ssp verbenaca Maire (k) | 14.11 µg/mL | n-Hexane-extract | n.d. | [37] |
| Li | Promastigotes/ MHOM/MA/1998/LVTA | S. verbenaca (L.) Briq. ssp verbenaca Maire (k1) | 31.57 µg/mL | DCM-extract | n.d. | [37] |
| Li | Promastigotes/ MHOM/MA/1998/LVTA | S. verbenaca (L.) Briq. ssp verbenaca Maire (k2) | >1000 µg/mL | MeOH-extract | n.d. | [37] |
| Lt | Promastigotes/ MHOM/MA/2010/LCTIOK-4 | S. verbenaca (L.) Briq. ssp verbenaca Maire (k) | 148.23 µg/mL | n-Hexane-extracts | n.d. | [37] |
| Lt | Promastigotes/ MHOM/MA/2010/LCTIOK-4 | S. verbenaca (L.) Briq. ssp verbenaca Maire (k1) | 33.77 µg/mL | DCM-extract | n.d. | [37] |
| Lt | Promastigotes/ MHOM/MA/2010/LCTIOK-4 | S. verbenaca (L.) Briq. ssp verbenaca Maire (k2) | >1000 µg/mL | MeOH-extract | n.d. | [37] |
| Parasite | Form/Strain | Plant Species | IC50 Extract | Isolated Compound | Molecular Formula | Molar Mass (g/mol) | IC50 of the Compound | SI | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ld | Axenic amastigotes/ MHOM/ET/67/L82 | S.repens Burch.ex Benth. | 5.36 µg/mL | 12-methoxycarnosic acid (27) | C21H30O4 | 346.21 | 0.75 μM | 23.06 | [18] |
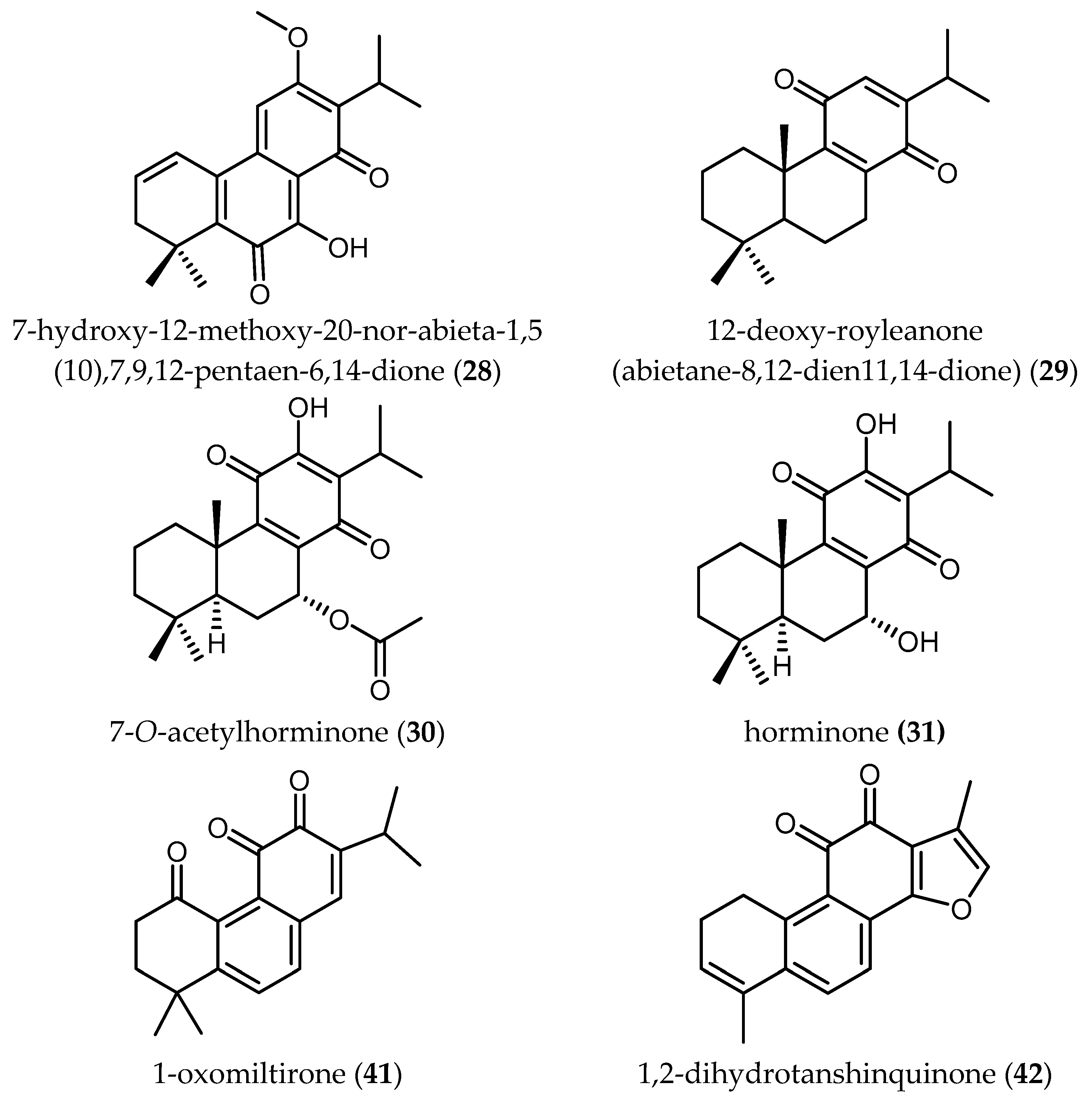
| Ld | Intracellular Amastgitotes /n.d. | S. cilicica Boiss and Kotschy | n.d. | 7-hydroxy-12-methoxy-20-nor-abieta-1,5(10),7,9,12-pentaen-6,14-dione (28) | C20H22O4 | 326.15 | 0.17 μM (170 nM) | >1.76 | [38] |
| Ld | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | 7-hydroxy-12-methoxy-20-nor-abieta-1,5(10),7,9,12-pentaen-6,14-dione (28) | C20 H22 O4 | 326.15 | >0.3 μM (>300 nM) | ≈1 | [38] |
| Lm | Amastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | 7-hydroxy-12-methoxy-20-nor-abieta-1,5(10),7,9,12-pentaen-6,14-dione (28) | C20H22O4 | 326.15 | 0.287 μM (287.4 nM) | 1.04 | [38] |
| Lm | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | 7-hydroxy-12-methoxy-20-nor-abieta-1,5(10),7,9,12-pentaen-6,14-dione (28) | C20H22O4 | 326.15 | ≥0.3 μM (≥300 nM) | ≈1 | [38] |
| Ld | Amastgitotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | abieta-8,12-dien-11,14-dione (12-deoxy-royleanone) (29) | C20H28O2 | 300.21 | 0.121 μM (121 nM) | 1.58 | [38] |
| Ld | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | abieta-8,12-dien-11,14-dione (12-deoxy-royleanone) (29) | C20H28O2 | 300.21 | >0.3 μM (>300 nM) | ≤0.64 | [38] |
| Lm | Amastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | abieta-8,12-dien-11,14-dione (12-deoxy-royleanone) (29) | C20H28O2 | 300.21 | 0.182 μM (182.3 nM) | 1.04 | [38] |
| Lm | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | abieta-8,12-dien-11,14-dione (12-deoxy-royleanone) (29) | C20H28O2 | 300.21 | ≥0.30 μM (≥300 nM) | ≤0.63 | [38] |
| Ld | Promastigotes/n.d. | S. deserta Schang. | n.d. | taxodione (18) | C20H26O3 | 314.42 | 1.46 ± 0.52 μM | 10.34 | [39] |
| Ld | Promastigotes/n.d. | S. deserta Schang. | n.d. | ferruginol (15) | C20H30O | 286.45 | 11.39 ± 1.05 μM | n.d. | [39] |
| Ld | Promastigotes/n.d. | S. deserta Schang. | n.d. | 7-O-acetylhorminone (30) | C22H30O5 | 374.47 | 19.69 ± 0.80 μM | n.d. | [39] |
| Ld | Promastigotes/n.d. | S. deserta Schang. | n.d. | horminone (31) | C20H28O4 | 332.20 | 29.43 ± 3.01 μM | n.d. | [39] |
| Ld | Amastgitotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | ursolic acid (32) | C30H48O3 | 456.36 | 0.0127 μM (12.7 nM) | 2.22 | [38] |
| Ld | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | ursolic acid (32) | C30H48O3 | 456.36 | 0.091 μM (91 nM) | 0.17 | [38] |
| Lm | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | ursolic acid (32) | C30H48O3 | 456.36 | 0.051 μM (51.3 nM) | 0.3 | [38] |
| Lm | Amastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | ursolic acid (32) | C30H48O3 | 456.36 | 0.007 μM (7 nM) | 2.22 | [38] |
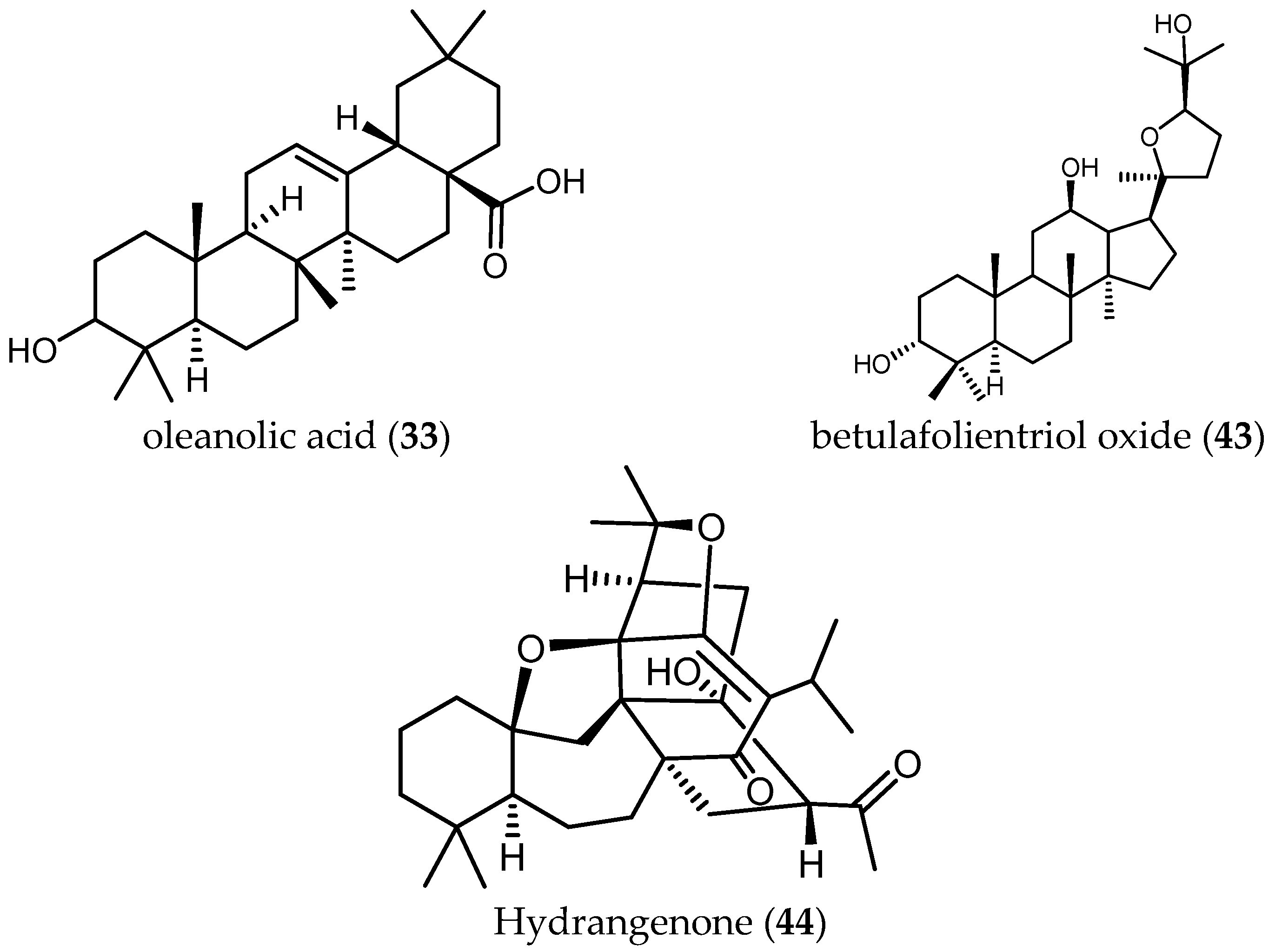
| Ld | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | oleanolic acid (33) | C30H48O3 | 456.36 | 0.091 μM (91 nM) | 1.45 | [38] |
| Ld | Amastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | oleanolic acid (33) | C30H48O3 | 456.36 | 0.063 μM (62.9 nM) | 2.1 | [38] |
| Lm | Promastigotes/n.d. | S. cilicica Boiss and Kotschy | n.d. | oleanolic acid (33) | C30H48O3 | 456.36 | 0.137 μM (137 nM) | 0.97 | [38] |
| Lm | Amastigotes | S. cilicica Boiss and Kotschy | n.d. | oleanolic acid (33) | C30H48O3 | 456.36 | 0.120 μM (119.9 nM) | 1.1 | [38] |
| Lm | Amastigotes/ LV39 strain | S. officinalis L. | n.d. | caffeic acid (34) | C9H8O4 | 180.04 | 0.0044 μM (4.4 nM) | >2200 | [41] |
| Ld | Amastigotes/ LV9 strain | S. officinalis L. | n.d. | caffeic acid (34) | C9H8O4 | 180.04 | 0.0061 μM (6.1 nM) | >500 | [41] |
| Lg | Amastigotes | S. officinalis L. | n.d. | caffeic acid (34) | C9H8O4 | 180.04 | 0.0066 μM (6.6 nM) | >360 | [41] |
| Lk | Amastigotes | S. officinalis L. | n.d. | caffeic acid (34) | C9H8O4 | 180.04 | 0.0039 μM (3.9 nM) | >333 | [41] |
| Lm/Ld/Lk/Lg | Promastigotes | S. officinalis L. | n.d. | caffeic acid (34) | C9H8O4 | 180.04 | >2.8 μM (>2800 nM) | ≈0.78 | [41] |
| Lm | Amastigotes/ LV39 strain | S. officinalis L. | n.d. | rosmarinic acid (35) | C18H16O8 | 360.08 | 0.0592 μM (59.2 nM) | >18 | [41] |
| Ld | Amastigotes/ LV9 strain | S. officinalis L. | n.d. | rosmarinic acid (35) | C18H16O8 | 360.08 | 0.0744 μM (74.4 nM) | >15 | [41] |
| Lg | Amastigotes | S. officinalis L. | n.d. | rosmarinic acid (35) | C18H16O8 | 360.08 | 0.0842 μM (84.2 nM) | >13 | [41] |
| Lk | Amastigotes | S. officinalis L. | n.d. | rosmarinic acid (35) | C18H16O8 | 360.08 | 0.0694 μM (69.4 nM) | >15 | [41] |
| Lm/Ld/ Lk/Lg | Promastigote | S. officinalis L. | n.d. | rosmarinic acid (35) | C18H16O8 | 360.08 | >1.4 μM (>1400 nM) | ≈0.78 | [41] |
| Lm | Amastigotes/ LV39 strain | S. officinalis L. | n.d. | salvianolic acid I (36) | C27H22O12 | 538.11 | 0.1604 μM (160.4 nM) | > 4.35 | [41] |
| Ld | Amastigotes/ LV9 strain | S. officinalis L. | n.d. | salvianolic acid I (36) | C27H22O12 | 538.11 | 0.1758 μM (175.8 nM) | >3.9 | [41] |
| Lg | Amastigotes | S. officinalis L. | n.d. | salvianolic acid I (36) | C27H22O12 | 538.11 | 0.1515 μM (151.5 nM) | >4.62 | [41] |
| Lk | Amastigotes | S. officinalis L. | n.d. | salvianolic acid I (36) | C27H22O12 | 538.11 | 0.1678 μM (167.8 nM) | >4.17 | [41] |
| Lm/Ld/ Lk/Lg | Promastigote | S. officinalis L. | n.d. | salvianolic acid I (36) | C27H22O12 | 538.11 | >0.9 μM (>900 nM) | ≈0.77 | [41] |
| Lm | Amastigotes/strain | S. officinalis L. | n.d. | methyl ester of salvianolic acid I (37) | C28H24O12 | 552.13 | 0.0108 μM (10.8 nM) | >64.81 | [41] |
| Ld | Amastigotes | S. officinalis L. | n.d. | methyl ester of salvianolic acid I (37) | C28H24O12 | 552.13 | 0.0186 μM (18.6 nM) | >37.63 | [41] |
| Lg | Amastigotes | S. officinalis L. | n.d. | methyl ester of salvianolic acid I (37) | C28H24O12 | 552.13 | 0.0152 μM (15.2 nM) | >46 | [41] |
| Lk | Amastigotes | S. officinalis L. | n.d. | methyl ester of salvianolic acid I (37) | C28H24O12 | 552.13 | 0.0136 μM (13.6 nM) | >51.47 | [41] |
| Lm/Ld/ Lk/Lg | Promastigote | S. officinalis L. | n.d. | methyl ester of salvianolic acid I (37) | C28H24O12 | 552.13 | >0.9 μM (>900 nM) | ≈0.77 | [41] |
| Lm | Amastigotes/ LV39 strain | S. officinalis L. | n.d. | salvianolic acid K (38) | C27H24O13 | 556.12 | 0.0183 μM (18.3 nM) | >38.25 | [41] |
| Ld | Amastigotes/ LV9 strain | S. officinalis L. | n.d. | salvianolic acid K (38) | C27H24O13 | 556.12 | 0.0182 μM (18.2 nM) | >38.46 | [41] |
| Lg | Amastigotes | S. officinalis L. | n.d. | salvianolic acid K (38) | C27H24O13 | 556.12 | 0.0133 μM (13.3 nM) | >52.63 | [41] |
| Lk | Amastigotes | S. officinalis L. | n.d. | salvianolic acid K (38) | C27H24O13 | 556.12 | 0.0145 μM (14.5 nM) | >48.27 | [41] |
| Lm/Ld/ Lk/Lg | Promastigote | S. officinalis L. | n.d. | salvianolic acid K (38) | C27H24O13 | 556.12 | >0.9 μM (>900 nM) | ≈0.77 | [41] |
| Lm | Amastigotes/ LV39 strain | S. officinalis L. | n.d. | salvianolic acid L (39) | C36H30O16 | 718.15 | 0.0203 μM (20.3 nM) | >34,48 | [41] |
| Ld | Amastigotes/ LV9 strain | S. officinalis L. | n.d. | salvianolic acid L (39) | C36H30O16 | 718.15 | 0.0154 μM (15.4 nM) | >45.45 | [41] |
| Lg | Amastigotes | S. officinalis L. | n.d. | salvianolic acid L (39) | C36H30O16 | 718.15 | 0.0226 μM (22.6 nM) | >30.97 | [41] |
| Lk | Amastigotes | S. officinalis L. | n.d. | salvianolic acid L (39) | C36H30O16 | 718.15 | 0.013 μM (13.0 nM) | >53.84 | [41] |
| Lm/Ld/ Lk/Lg | Promastigote | S. officinalis L. | n.d. | salvianolic acid L (39) | C36H30O16 | 718.15 | >0.9 μM (>900 nM) | ≈0.77 | [41] |
| Lm | Amastigotes/ LV39 strain | S. officinalis L. | n.d. | sagerinic acid (40) | C36H32O16 | 720.17 | 0.128 μM (128.7 nM) | >4.66 | [41] |
| Ld | Amastigotes/ LV9 strain | S. officinalis L. | n.d. | sagerinic acid (40) | C36H32O16 | 720.17 | 0.122 μM (122.1 nM) | >4.91 | [41] |
| Lg | Amastigotes | S. officinalis L. | n.d. | sagerinic acid (40) | C36H32O16 | 720.17 | 0.142 μM (141.5 nM) | >4.24 | [41] |
| Lk | Amastigotes | S. officinalis L. | n.d. | sagerinic acid (40) | C36H32O16 | 720.17 | 0.155 μM (154.8 nM) | >3.88 | [41] |
| Lm/Ld/ Lk/Lg | Promastigote | S. officinalis L. | n.d. | sagerinic acid (40) | C36H32O16 | 720.17 | >0.7 μM (>700 nM) | ≈0.85 | [41] |
| Parasite | Strain | Plant Species | Type of Solvent Extract | IC50 Solvent Extract | SI | Ref. |
|---|---|---|---|---|---|---|
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. tomentosa Mill. (a1) | MeOH-extract | 9.94 µg/mL | 9.05 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. tomentosa Mill. (a2) | n-Hexane-extract | 3.47 µg/mL | 7.56 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. tomentosa Mill. (a3) | Chloroform-extract | 3.14 µg/mL | 10.12 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. tomentosa Mill. (a4) | H2O-extract | >20 µg/mL | ≈4.5 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. sclarea L. (b1) | MeOH-extract | 6.6 µg/mL | 13.27 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. sclarea L. (b2) | n-Hexane-extract | 3.78 µg/mL | 4.84 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. sclarea L. (b3) | Chloroform-extract | 2.54 µg/mL | 33.03 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. sclarea L. (b4) | H2O-extract | >20 µg/mL | ≈4.5 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. dichroantha Stapf. (c1) | MeOH-extract | 8.85 µg/mL | >10.17 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. dichroantha Stapf. (c2) | n-Hexane-extract | 4.17 µg/mL | >21.58 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. dichroantha Stapf. (c3) | Chloroform-extract | 3.72 µg/mL | 22.79 | [16] |
| Pf | Chloroquine- and Pyrimethamine-resistant K1 strain | S. dichroantha Stapf. (c4) | H2O-extract | >20 µg/mL | ≈4.5 | [16] |
| Pf | K1 strain | S. hydrangea DC ex. Benth. (d) | n-Hexane-extract | 3.2 µg/mL | n.d. | [25] |
| Pf | 3D7 strain | S. hydrangea DC ex. Benth. (d2) | n.d. | <12.5 µg/mL | n.d. | [8] |
| Pf | K1 strain | S. repens Burch. ex Benth. (f) | DCM/MeOH 1:1 | 7.65 µg/mL | 5.4 | [26] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. repens Burch. ex Benth. (f1) | Chloroform/MeOH (1:1) | 8.25 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. repens Burch. ex Benth. (f2) | Essential oil | 1.65 µg/mL | n.d. | [45] |
| Pf | Chloroquine-sensitive strain D10 | S. repens Burch. ex Benth. (f3) | DCM/MeOH 1:1 | 10.8 µg/mL | n.d. | [46] |
| Pf | n.d. | S. repens Burch. ex Benth. (f4) | Methanol extract | 78.9 µg/mL | 1.26 | [6] |
| Pf | n.d. | S. repens Burch. ex Benth. (f5) | Essential oil | 1.68 µg/mL | 5.28 | [6] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. africana–caerulea L. (l) | Essential oil | 4.76 µg/m | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. africana–lutea L. (m) | Chloroform/MeOH (1:1) | 15.86 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. africana–lutea L (m1) | Essential oil | 5.45 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. albicaulis Benth. (n) | Chloroform/MeOH (1:1) | 15.83 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. albicaulis Benth. (n1) | Essential oil | 6.41 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. muirii L. Bol. (o) | Chloroform/MeOH (1:1) | 11.87 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. muirii L. Bol. (o1) | Essential oil | 5.93 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. lanceolata Lam. (p) | Chloroform/MeOH (1:1) | 26.01 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. lanceolata Lam. (p1) | Essential oil | 7.83 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. garipensis E. Mey. (q) | Chloroform/MeOH (1:1) | 13.95 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. dolomitica Codd (r) | Chloroform/MeOH (1:1) | 7.62 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. dolomitica Codd (r1) | Essential oil | 4.81 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. disermas L. (s) | Chloroform/MeOH (1:1) | 24.17 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. chamelaeagnea Berg. (t) | Chloroform/MeOH (1:1) | 8.71 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. chamelaeagnea Berg. (t1) | Essential oil | 8.63 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. aurita L.f. (u) | Chloroform/MeOH (1:1) | 8.92 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. namaensis Schinz (v) | Chloroform/MeOH (1:1) | 25.38 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. runcinata L.f. (v1) | Chloroform/MeOH (1:1) | 16.61 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. runcinata L.f. (v2) | Essential oil | 1.20 µg/mL | n.d. | [45] |
| Pf | n.d. | S. runcinata L.f. (v3) | MeOH extract | 29 µg/mL | >3.44 | [6] |
| Pf | n.d. | S. runcinata L.f. (v4) | Essential oil | 1.23 µg/mL | 2.06 | [6] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. schlechteri Briq. (w) | Chloroform/MeOH (1:1) | 17.51 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. stenophylla Burch. Ex. Benth. (x1) | Chloroform/MeOH (1:1) | 6.5 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. stenophylla Burch. Ex. Benth. (x2) | Essential oil | 4.13 µg/mL | n.d. | [45] |
| Pf | n.d. | S. stenophylla Burch. Ex. Benth. (x3) | MeOH-extract | 17.03 µg/mL | 1.27 | [6] |
| Pf | n.d. | S. stenophylla Burch. Ex. Benth. (x4) | Essential oil | 4.38 µg/mL | 1.53 | [6] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. verbenaca L. (y) | Chloroform/MeOH (1:1) | 23.97 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. radula Benth. (z1) | Chloroform/MeOH (1:1) | 3.91 µg/mL | n.d. | [45] |
| Pf | Chloroquine-resistant Pf FCR-3 strain | S. radula Benth. (z2) | Essential oil | 13.5 µg/mL | n.d. | [45] |
| Pf | Chloroquine resistance Pf K1 strain | S. chudaei Batt. & Trab. (Aa) | n-Hexane-extract | 4.91 ± 2.91 µg/mL | n.d. | [47] |
| Pf | 3D7-chloroquine sensitive strain | S. chudaei Batt. & Trab. (Aa) | n-Hexane extract | 5.69 ± 0.3 µg/mL | n.d. | [47] |
| Pf | Chloroquine resistance Pf K1 strain | S. chudaei Batt. & Trab. (Aa1) | Chloroform-extract | 6.41 ± 0.31 µg/mL | n.d. | [47] |
| Pf | 3D7-chloroquine sensitive strain | S. chudaei Batt. & Trab. (Aa1) | Chloroform-extract | 5.69 ± 2.71 µg/mL | n.d. | [47] |
| Pf | Chloroquine resistance Pf K1 strain | S. chudaei Batt. & Trab. (Aa2) | Ethanolic-extract | 7.99 ± 1.06 µg/mL | n.d. | [47] |
| Pf | 3D7-chloroquine sensitive strain | S. chudaei Batt. & Trab. (Aa2) | Ethanolic-extract | 8.43 ± 0.38 µg/mL | n.d. | [47] |
| Pf | Chloroquine resistance Pf K1 strain | S. chudaei Batt. & Trab. (Aa3) | Essential oil | 2.39 ± 0.24 µg/mL | n.d. | [47] |
| Pf | 3D7-chloroquine sensitive strain | S. chudaei Batt. & Trab. (Aa3) | Essential oil | 2.40 ± 0,77 µg/mL | n.d. | [47] |
| Pf | Chloroquine sensitive D6 strain | S. lavandulifolia Vahl (h) | Essential oil | 47% inhibition at 15.86 µg/mL | n.d. | [20] |
| Parasite | Strain | Plant Species | IC50 Extract | Isolated Compound | Molecular Formula | Molar Mass (g/mol) | IC50 of the Compound | SI | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | miltirone (3) | C19H22O2 | 282.38 | 0.5 µM | 2.6 | [4] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | methylenetanshinquinone (4) | C18H14O3 | 278.09 | 6.3 µM | 1.9 | [4] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | tanshinone I (5) | C18H12O3 | 276.08 | 7.2 µM | 1.7 | [4] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | tanshinone IIa (6) | C19H18O3 | 294.13 | 4 µM | 1.9 | [4] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | methyl-tanshinonate (7) | C20H18O5 | 338.12 | 4.1 µM | 1.7 | [4] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | 11-hydroxymiltiodiol (8) | C19H22O3 | 298.16 | ≥30 µM | n.d. | [4] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | cryptotanshinone (9) | C19H20O3 | 296.14 | 16.1 µM | 0.3 | [4] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | 12-deoxy-salvipisone (10) | C20H24O2 | 296.18 | 8.8 µM | 0.1 | [23] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | sahandinone (11) | C20H24O2 | 296.18 | 5.1 µM | 0.1 | [23] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | 12-deoxy-6,7-dehydroroyleanone (12) | C20H26O2 | 298.19 | 17.8 µM | 0.6 | [23] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | 7α-acetoxyroyleanone (13) | C22H30O5 | 374.21 | 1.3 µM | 0.1 | [23] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | Δ9-ferruginol (14) | C20H28O | 284.21 | 0.9 µM | 18.2 | [23] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | ferruginol (15) | C20H30O | 286.23 | 0.9 µM | 15.6 | [23] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | sahandol (16) | C20H24O2 | 296.18 | 4.7 µM | 3.3 | [23] |
| Pf | K1 strain | S. sahendica Boiss. & Buhse | n.d. | sahandone (17) | C21H26O3 | 326.19 | 17.2 µM | 0.7 | [23] |
| Pf | K1 strain | S. hydrangea DC. ex Benth. | 3.2 µg/mL | salvadione C (22) | C30H40O5 | 480.29 | 1.43 µM | 86.2 | [25] |
| Pf | K1 strain | S. hydrangea DC. ex Benth. | 3.2 µg/mL | perovskone B (23) | C30H40O4 | 464.29 | 18 µM | 69.6 | [25] |
| Pf | 3D7 strain | S. hydrangea DC. ex Benth. | <12.5 µg/mL | oleanolic acid (33) | C30H48O3 | 456.36 | 19.3 µM | n.d. | [8] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | 1-oxomiltirone (41) | C19H20O3 | 296.14 | ≥30 µM | n.d. | [4] |
| Pf | K1 strain | S. miltiorrhiza Bunge. | 34% inhib. at 0.81 µg/mL | 1,2-dihydrotanshinquinone (42) | C18H14O3 | 278.09 | 5.3 µM | 0.8 | [4] |
| Pf | NF54 strain | S. austriaca Jacq. | n.d. | taxodione (18) | C20H26O3 | 314.19 | 1.9 µM | 1.0 | [24] |
| Pf | NF54 strain | S. austriaca Jacq. | n.d. | 15-deoxy-fuerstione (19) | C20H26O2 | 298.19 | >167 µM | n.d. | [24] |
| Pf | NF54 strain | S. austriaca Jacq. | n.d. | 7-(2′-oxohexyl)-taxodion (20) | C26H36O4 | 412.26 | 3.37 µM | 0.9 | [24] |
| Pf | NF54 strain | S. austriaca Jacq. | n.d. | taxodone (21) | C20H28O3 | 316.20 | 3.66 µM | 1.1 | [24] |
| Pf | D6 strain | S. deserta Schang. | n.d. | taxodione (18) | C20H26O3 | 314.19 | 10.49 μM (3.297 mg/L) | >1.4 | [39] |
| Pf | W2 strain | S. deserta Schang. | n.d. | taxodione (18) | C20H26O3 | 314.19 | 9.66 μM (3.036 mg/L) | >1.6 | [39] |
| Pf | D6 strain | S. deserta Schang. | n.d. | ferruginol (15) | C20H30O | 286.23 | 5.64 μM (1.616 mg/L) | 0.8 | [39] |
| Pf | W2 strain | S. deserta Schang. | n.d. | ferruginol (15) | C20H30O | 286.23 | 6.35 μM (1.817 mg/L) | 0.7 | [39] |
| Pf | D6 strain | S. deserta Schang. | n.d. | 7-o-acetylhorminone (30) | C22H30O5 | 374.21 | >12.72 μM (>4.760 mg/L) | 1 | [39] |
| Pf | W2 strain | S. deserta Schang. | n.d. | 7-o-acetylhorminone (30) | C22H30O5 | 374.21 | >12.72 μM (>4.760 mg/L) | 1 | [39] |
| Pf | D6 strain | S. deserta Schang. | n.d. | horminone (31) | C20H28O4 | 332.20 | >14.33 μM (>4.760 mg/L) | 1 | [39] |
| Pf | W2 strain | S. deserta Schang. | n.d. | horminone (31) | C20H28O4 | 332.20 | >14.33 μM (>4.760 mg/L) | 1 | [39] |
| Pf | FCR3 strain | S. radula Benth. | 3.91 µg/mL | betulafolientriol oxide (43) | C30H52O4 | 476.39 | 10.39 μM (4.95 µg/mL) | >20 | [48] |
| Pf | K1 strain | S. hydrangea DC. ex. Benth. | n.d. | hydrangenone (44) | C30H42O5 | 482.30 | 1.4 µM | 6 | [49] |
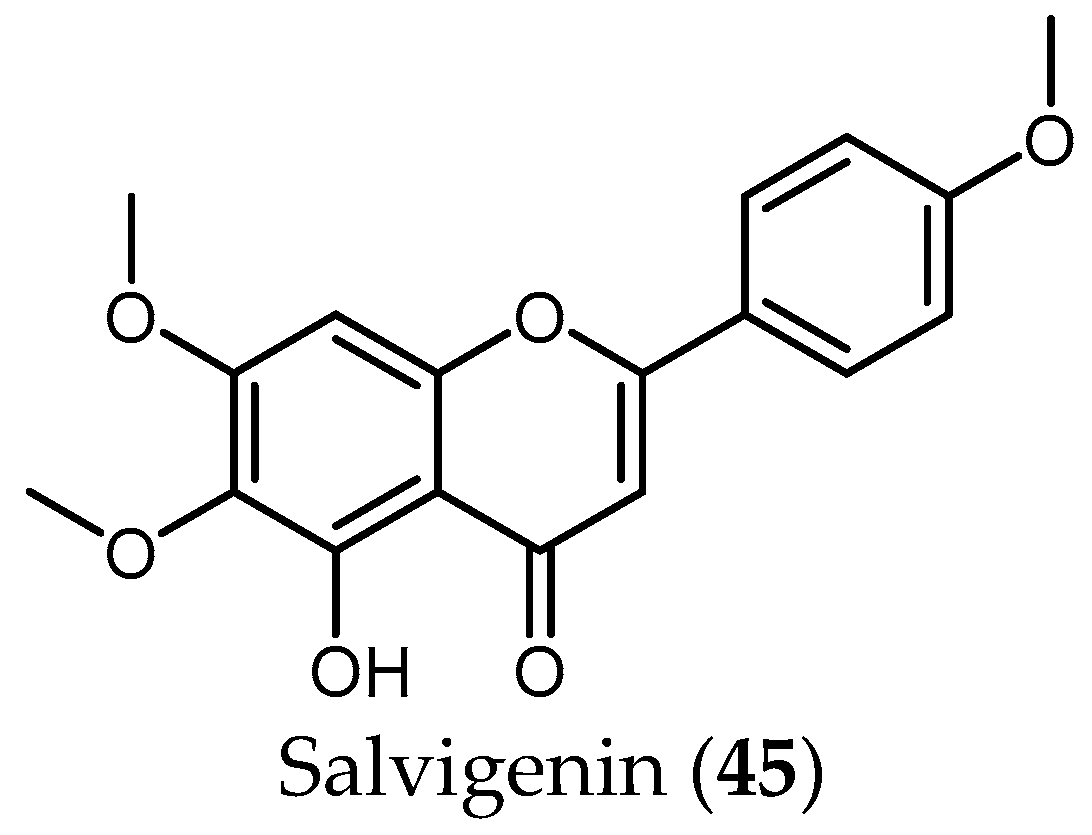
| Pf | FCR3 strain | S. radula Benth. | 3.91 µg/mL | salvigenin (45) | C18H16O6 | 328.09 | 74.98 μM (24.60 µg/mL) | >4 | [48] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llurba-Montesino, N.; Schmidt, T.J. Salvia Species as Sources of Natural Products with Antiprotozoal Activity. Int. J. Mol. Sci. 2018, 19, 264. https://doi.org/10.3390/ijms19010264
Llurba-Montesino N, Schmidt TJ. Salvia Species as Sources of Natural Products with Antiprotozoal Activity. International Journal of Molecular Sciences. 2018; 19(1):264. https://doi.org/10.3390/ijms19010264
Chicago/Turabian StyleLlurba-Montesino, Núria, and Thomas J. Schmidt. 2018. "Salvia Species as Sources of Natural Products with Antiprotozoal Activity" International Journal of Molecular Sciences 19, no. 1: 264. https://doi.org/10.3390/ijms19010264