Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq.
Abstract
:1. Introduction
2. Results
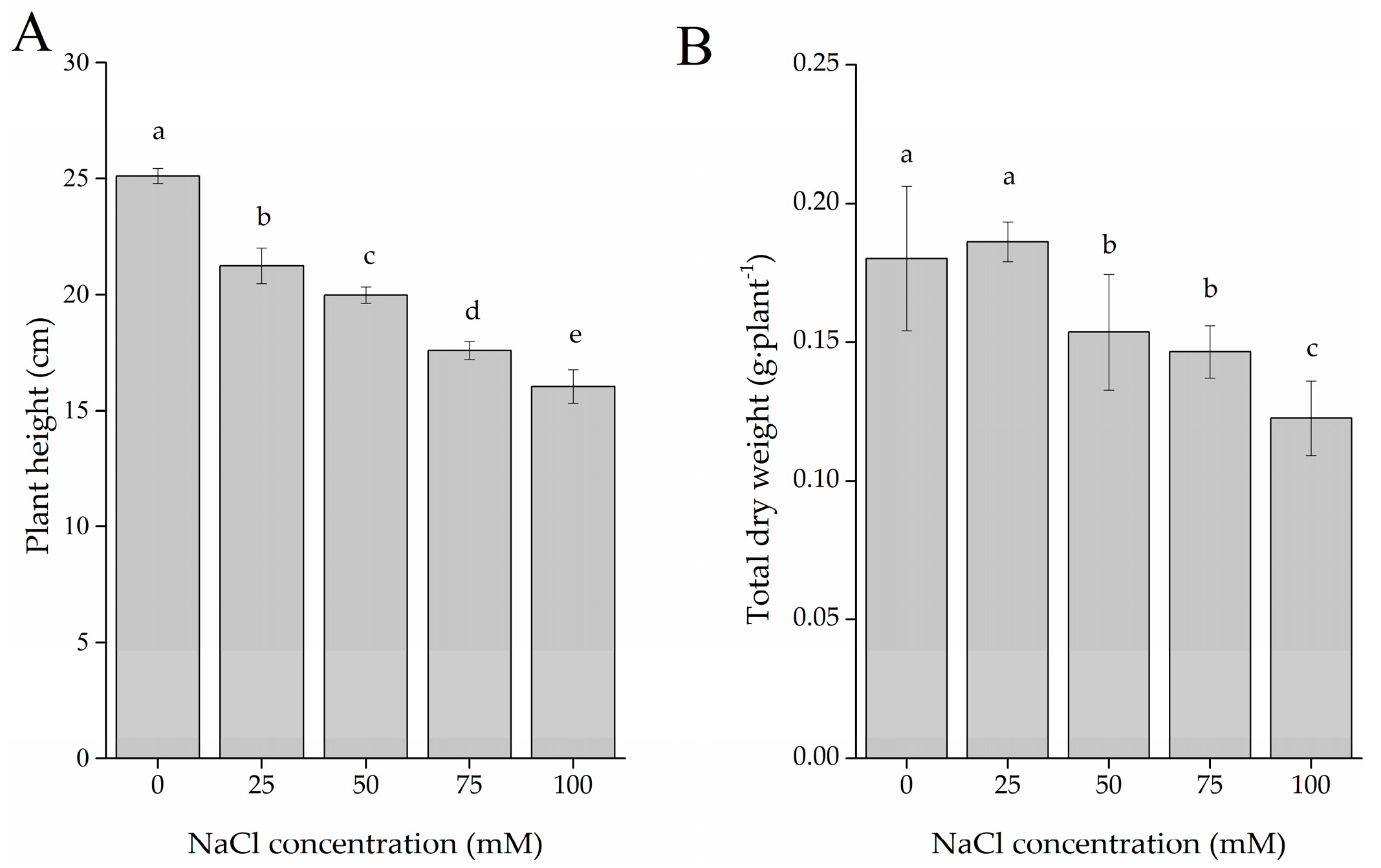
2.1. Plant Growth
2.2. Total Phenolic and Flavonoid Content (TPC and TFC)
2.3. Antioxidant Capacity Evaluation
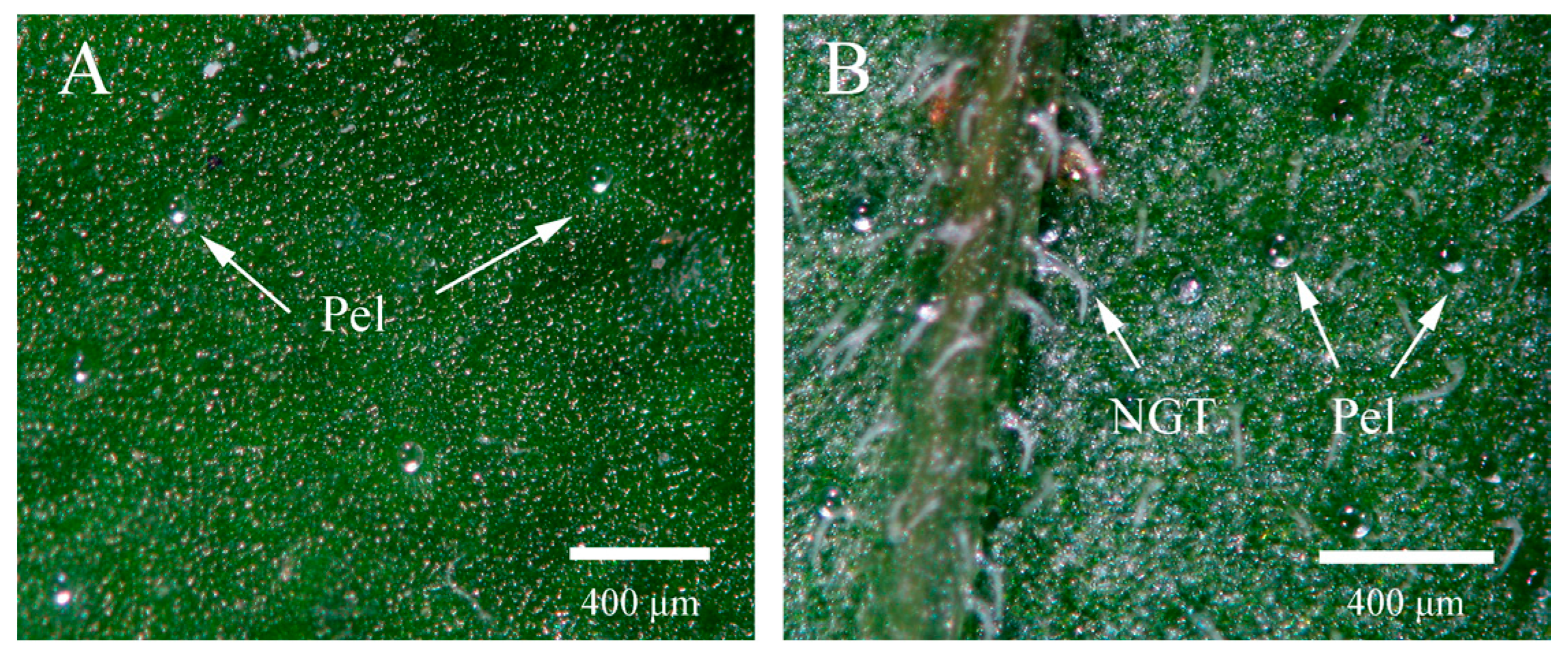
2.4. Glandular Trichome Morphology and Density
2.5. Constituents of Glandular Trichome Volatile Exudates in S. tenuifolia
3. Discussion
4. Materials and Methods
4.1. Plant Material and Salt Treatments
4.2. Chemicals
4.3. Plant Growth Parameters
4.4. Polyphenol Extraction and Analysis
4.4.1. Preparation of Extracts
4.4.2. Determination of Total Phenolic Content (TPC)
4.4.3. Determination of Total Flavonoid Content (TFC)
4.5. Antioxidant Capacity Evaluation
4.5.1. DPPH Radical Scavenging Activity
4.5.2. ABTS·+ Radical Scavenging Activity
4.6. Glandular Trichome Morphology and Density
4.7. Extraction of Glandular Trichome Volatile Exudates
4.8. Gas Chromatography/Mass Spectrometry Analysis of GT Volatile Exudates and Compound Identification
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EO | essential oil |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| NGT | non-glandular trichome |
| GT | glandular trichome |
| Pel | peltate glandular trichome |
| Cap | capitate glandular trichomes |
| SEM | scanning electron microscope |
References
- Chun, M.H.; Kim, E.K.; Yu, S.M.; Oh, M.S.; Moon, K.Y.; Jung, J.H.; Hong, J. GC/MS combined with chemometrics methods for quality control of Schizonepeta tenuifolia Briq: Determination of essential oils. Microchem. J. 2011, 97, 274–281. [Google Scholar] [CrossRef]
- Fung, D.; Lau, C.B.S. Schizonepeta tenuifolia: Chemistry, pharmacology, and clinical applications. J. Clin. Pharmacol. 2002, 42, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Cheng, M.L.; Chen, K.H.; Horng, J.T.; Liu, C.C.; Wang, S.M.; Sakurai, H.; Leu, Y.L.; Wang, S.D.; Ho, H.Y. Antiviral activities of Schizonepeta tenuifolia Briq. against enterovirus 71 in vitro and in vivo. Sci. Rep. 2017, 7, 935. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Kakihara, Y.; Komatsu, K.; Kuraishi, Y. Inhibitory effects of methanol extracts of herbal medicines on substance P-induced itch-scratch response. Biol. Pharm. Bull. 2000, 23, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Huang, G.J.; Tai, H.M.; Huang, M.H. Antioxidant and anti-inflammatory activities of aqueous extracts of Schizonepeta tenuifolia Briq. Food Chem. Toxicol. 2012, 50, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.Q.; Qian, Y.; Yu, S.; Guo, S.C.; Zhang, L.; Ding, A.W.; Wu, Q.N. Anti-inflammatory effect of volatile oil from Schizonepeta tenuifolia on carrageenin-induced pleurisy in rats and its application to study of appropriate harvesting time coupled with multi-attribute comprehensive index method. J. Ethnopharmacol. 2016, 194, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.H.; Park, J.Y.; Shin, S.C.; Kim, J.; Park, I.K. Nematicidal activity of medicinal plant essential oils against the pinewood nematode (Bursaphelenchus xylophilus). Appl. Entomol. Zool. 2007, 42, 397–401. [Google Scholar] [CrossRef]
- Park, I.K.; Kim, L.S.; Choi, I.H.; Lee, Y.S.; Shin, S.C. Fumigant activity of plant essential oils and components from Schizonepeta tenuifolia against Lycoriella ingenua (Diptera: Sciaridae). J. Econ. Entomol. 2006, 99, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Bozovic, M.; Ragno, R. Calamintha nepeta (L.) Savi and its main essential oil constituent pulegone: Biological activities and chemistry. Molecules 2017, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of the People’s Republic of China 1; China Medical Science Press: Beijing, China, 2015; p. 232. (In Chinese) [Google Scholar]
- Sun, J. d-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259. [Google Scholar] [PubMed]
- Miyazawa, M.; Nakanishi, K. Biotransformation of (−)-menthone by human liver microsomes. Biosci. Biotechnol. Biochem. 2006, 70, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, Y.W.; Zhang, L.; Shan, M.Q.; Tang, Y.P.; Ding, A.W. Quantitative comparative analysis of the bio-active and toxic constituents of leaves and spikes of Schizonepeta tenuifolia at different harvesting times. Int. J. Mol. Sci. 2011, 12, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Fahn, A. Secretory Tissues in Vascular Plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef]
- Johnson, S.R.; Lange, I.; Srividya, N.; Lange, B.M. Bioenergetics of monoterpenoid essential oil biosynthesis in nonphotosynthetic glandular trichomes. Plant Physiol. 2017, 175, 681–695. [Google Scholar] [PubMed]
- Gersbach, P.V. The essential oil secretory structures of Prostanthera ovalifolia (Lamiaceae). Ann. Bot. 2002, 89, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tissier, A. Glandular trichomes what comes after expressed sequence tags? Plant J. 2012, 70, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.M.; Han, Y.; Sun, X.; Li, S.H.; Shi, Q.M.; Wang, C.H. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind. Crop. Prod. 2015, 64, 175–181. [Google Scholar]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Aghaei, K.; Komatsu, S. Crop and medicinal plants proteomics in response to salt stress. Front. Plant Sci. 2013, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Kleinwachter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crop. Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Taârit, M.B.; Msaada, K.; Hosni, K.; Marzouk, B. Physiological changes, phenolic content and antioxidant activity of Salvia officinalis L. grown under saline conditions. J. Sci. Food Agric. 2012, 92, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Neffati, M.; Sriti, J.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 2011, 124, 221–225. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.; Orooj, A. Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Trachyspermum ammi [L.] Sprague). J. Arid Environ. 2006, 64, 209–220. [Google Scholar] [CrossRef]
- Oueslati, S.; Karray-Bouraoui, N.; Attia, H.; Rabhi, M.; Ksouri, R.; Lachaal, M. Physiological and antioxidant responses of Mentha pulegium (Pennyroyal) to salt stress. Acta Physiol. Plant 2010, 32, 289–296. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Rahali, F.Z.; Msaada, K.; Ksouri, R.; Marzouk, B. Relation between salt tolerance and biochemical changes in cumin (Cuminum cyminum L.) seeds. J. Food Drug Anal. 2017, 25, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Tananaki, C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ. Exp. Bot. 2012, 79, 37–43. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, K.J.; Kim, B.K.; Jeong, J.W.; Kim, H.J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Dhifi, W.; Limam, F.; Marzouk, B. Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages. Acta Physiol. Plant 2014, 36, 433–445. [Google Scholar] [CrossRef]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Babu, T.S.; Akhtar, T.A.; Lampi, M.A.; Tripuranthakam, S.; Dixon, D.G.; Greenberg, B.M. Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemna gibba: Implication of reactive oxygen species as common signals. Plant Cell Physiol. 2003, 44, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Rius, S.; Emiliani, J.; Pourcel, L.; Feller, A.; Morohashi, K.; Casati, P.; Grotewold, E. Cloning and characterization of a UV-B-inducible maize flavonol synthase. Plant J. 2010, 62, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.F.; Hernandez, J.M.; Grotewold, E. Two cysteines in plant R2R3 MYB domains participate in REDOX-dependent DNA binding. J. Biol. Chem. 2004, 279, 37878–37885. [Google Scholar] [CrossRef] [PubMed]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Marzouk, B. Fatty acids, phenolic changes and antioxidant activity of clary sage (Salvia sclarea L.) rosette leaves grown under saline conditions. Ind. Crop. Prod. 2012, 38, 58–63. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chang, Y.H.; Shao, Y.Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 2006, 98, 529–538. [Google Scholar] [CrossRef]
- Hichem, H.; Mounir, D.; Naceur, E. Differential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop. Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Wong, C.C.; Li, H.B.; Cheng, K.W.; Chen, F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Ascensão, L.; Marques, N.; Pais, M.S. Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceae). Ann. Bot. 1995, 75, 619–626. [Google Scholar] [CrossRef]
- Siebert, D.J. Localization of Salvinorin A and related compounds in glandular trichomes of the psychoactive sage, Salvia divinorum. Ann. Bot. 2004, 93, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Karraybouraoui, N.; Rabhi, M.; Neffati, M.; Baldan, B.; Ranieri, A.; Marzouk, B.; Lachaâl, M.; Smaoui, A. Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of Mentha pulegium. Ind. Crop. Prod. 2009, 30, 338–343. [Google Scholar] [CrossRef]
- Rodrigues, L.; Póvoa, O.; Teixeira, G.; Figueiredo, A.C.; Moldão, M.; Monteiro, A. Trichomes micromorphology and essential oil variation at different developmental stages of cultivated and wild growing Mentha pulegium L. populations from Portugal. Ind. Crop. Prod. 2013, 43, 692–700. [Google Scholar] [CrossRef]
- Farooqi, A.H.A.; Samgwan, N.S.; Sangwan, R.S. Effect of different photoperiodic regimes on growth, flowering and essential oil in Mentha species. Plant Growth Regul. 1999, 29, 181–187. [Google Scholar] [CrossRef]
- Kjær, A.; Grevsen, K.; Jensen, M. Effect of external stress on density and size of glandular trichomes in full-grown Artemisia annua, the source of anti-malarial artemisinin. AoB Plants 2012, 2012, pls018. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, Y.; Taleei, A.; Ranjbar, M. Changes in the expression of key genes involved in the biosynthesis of menthol and menthofuran in Mentha piperita L. under drought stress. Acta Physiol. Plant 2017, 39, 203. [Google Scholar] [CrossRef]
- Mccaskill, D.; Gershenzon, J.; Croteau, R. Morphology and monoterpene biosynthetic capabilities of secretory-cell clusters isolated from glandular trichomes of peppermint (Mentha Piperita L.). Planta 1992, 187, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Serratovalenti, G.; Bisio, A.; Cornara, L.; Ciarallo, G. Structural and histochemical investigation of the glandular trichomes of Salvia aurea L. Leaves, and chemical analysis of the essential oil. Ann. Bot. 1997, 79, 329–336. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; California Agricultural Experiment Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crop. Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Severson, R.F.; Arrendale, R.F.; Chortyk, O.T.; Johnson, A.W.; Jackson, D.M.; Gwynn, G.R.; Chaplin, J.F.; Stephenson, M.G. Quantitation of the major cuticular components from green leaf of different tobacco types. J. Agric. Food Chem. 1984, 32, 566–570. [Google Scholar] [CrossRef]
- Asai, T.; Fujimoto, Y. Cyclic fatty acyl glycosides in the glandular trichome exudate of Silene gallica. Phytochemistry 2010, 71, 1410–1417. [Google Scholar] [CrossRef] [PubMed]



| NaCl Concentration (mM) | TPC (mg GAE/g DW) | TFC (mg Rutin/g DW) | DPPH (% Antioxidant Capacity) | ABTS (μmol TEAC/g DW) |
|---|---|---|---|---|
| 0 | 13.24 ± 1.90 bc | 31.43 ± 2.64 b | 40.17 ± 2.41 c | 54.69 ± 3.26 b |
| 25 | 18.54 ± 0.66 a | 38.99 ± 0.80 a | 62.14 ± 2.67 a | 75.40 ± 5.54 a |
| 50 | 14.56 ± 1.32 b | 36.27 ± 1.01 a | 44.79 ± 2.31 b | 57.44 ± 6.73 b |
| 75 | 12.37 ± 0.51 c | 24.26 ± 1.33 c | 29.56 ± 1.59 d | 44.83 ± 1.61 c |
| 100 | 7.40 ± 0.32 d | 17.18 ± 1.72 d | 18.30 ± 0.70 e | 30.69 ± 2.21 d |
| No. | Compounds | NaCl Concentration (mM) | ||||
|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | ||
| 1 | Heptane,2,2,4,6,6-pentamethyl | 2.44 ± 0.07 d | 7.09 ±0.19 b | 3.99 ± 0.13 c | 7.4 ± 0.18 b | 9.6 ± 0.21 a |
| 2 | 3-Heptanone,5-ethyl-4-methyl | 0.21 ± 0.01 d | 0.79 ± 0.08 a | 0.34 + 0.03 c | 0.53 ± 0.05 b | 0.49 ± 0.04 bc |
| 3 | D-Limonene | 1.54 ± 0.01 a | 1.6 ± 0.01 a | 0.69 ± 0 b | nd | nd |
| 4 | Menthone | 5.48 ± 0.12 b | 11.74 ± 0.30 a | 10.81 ± 0.1 a | 5.67 ± 0.45 b | 3.1 ± 0.09 bc |
| 5 | Anisic acid,tridec-2-ynyl ester | 1.00 ± 0 d | 3.78 ± 0.16 bc | 1.86 ± 0.12 c | 5.38 ± 0.19 b | 13.28 ± 0.33 a |
| 6 | Pulegone | 78.66 ± 5.01 a | 63.73 ± 4.39 b | 63.99 ± 4.98 b | 48.1 ± 3.26 c | 31.03 ± 3.01 d |
| 7 | 2-Cyclohexen-1-ol,2-methyl-5-(1-methylethenyl) | 3.48 ± 0.09 | nd | nd | nd | nd |
| 8 | Ethanone,1-cyclopropyl-2(1-pyrrolidinyl) | nd | 0.8 ± 0.00 b | 0.93 ± 0.01 a | nd | nd |
| 9 | β-Caryophyllene | 2.27 ± 0.11 a | 0.99 ± 0.06 b | 0.99 ± 0.08 b | nd | nd |
| 10 | 1,6-Cyclodecadiene,1-methyl-5-methylene-8-(1-methylethyl) | 3.02 ± 0.14 a | 1.16 ± 0.09 b | 1.28 ± 0.07 b | nd | nd |
| 11 | Glycine,N-(4-butylbenzoyl)-,butyl ester | nd | nd | nd | 0.62 ± 0.02 a | 0.1 ± 0 b |
| 12 | Ethyl propyl ketone | 0.17 ± 0.02 | nd | nd | nd | nd |
| 13 | Sulfurous acid,isobutyl pentyl ester | nd | 0.27 ± 0.01 | nd | nd | nd |
| 14 | Hexanedioic acid,bis(2-ethylhexyl) ester | nd | nd | 2.29 ± 0.14 a | 1.44 ± 0.10 b | 1.76 ± 0.13 b |
| 15 | 2,2′-Methylenebis(6-tert-butyl-4-methyl-phenol) | 0.27 ± 0.04 b | nd | 1.86 ± 0.19 a | 0.22 ± 0.23 b | 1.86 ± 0.20 a |
| 16 | 3-Hexanone,2,2-dimethyl | 0.23 ± 0.01 e | 1.03 ± 0.08 b | 0.5 ± 0.02 d | 0.92 ± 0.07 bc | 2.16 ± 0.15 a |
| 17 | 1,4-Butanediol | 0.09 ± 0.00 d | 0.21 ± 0.01 c | 0.25 ± 0.01 c | 0.39 ± 0.03 b | 0.62 ± 0.07 a |
| 18 | 3-Hexanone,2,5-dimethyl | 0.45 ± 0.03 cd | 0.69 ± 0.08 c | 1.41 ± 0.12 b | 4.03 ± 0.22 a | 4.84 ± 0.22 a |
| 19 | Sulfurous acid,2-ethylhexyl hexyl ester | nd | 5.73 ± 0.71 bc | 7.93 ± 0.87 b | 24.81 ± 2.71 a | 26.70 ± 2.69 a |
| Total | 99.31 | 99.61 | 99.12 | 99.51 | 98.70 | |
| Total identified classes | ||||||
| Alkane | 2.44 ± 0.36 d | 7.09 ± 0.58 b | 3.99 ± 0.41 c | 7.4 ± 0.61 b | 9.6 ± 0.78 a | |
| Ketones | 1.06 ± 0.09 d | 3.31 ± 0.24 c | 3.18 ± 0.21 c | 5.48 ± 0.25 b | 9.64 ± 0.81 a | |
| Monoterpenes | 89.16 ± 3.44 a | 77.07 ± 3.06 b | 75.49 ± 2.71 b | 53.77 ± 1.98 c | 34.13 ± 1.65 d | |
| Esters | 1.00 ± 0.10 e | 9.78 ± 1.51 cd | 12.08 ± 1.78 c | 32.25 ± 2.13 b | 42.85 ± 3.04 a | |
| Sesquiterpene | 5.29 ± 0.56 a | 2.15 ± 0.31 b | 2.27 ± 0.35 b | 0 c | 0 c | |
| Others | 0.36 ± 0.04 d | 0.21 ± 0.02 d | 2.11 ± 0.16 ab | 0.61 ± 0.02 c | 2.48 ± 0.20 a | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. https://doi.org/10.3390/ijms19010252
Zhou Y, Tang N, Huang L, Zhao Y, Tang X, Wang K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. International Journal of Molecular Sciences. 2018; 19(1):252. https://doi.org/10.3390/ijms19010252
Chicago/Turabian StyleZhou, Ying, Nanyu Tang, Lijin Huang, Yongjuan Zhao, Xiaoqing Tang, and Kangcai Wang. 2018. "Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq." International Journal of Molecular Sciences 19, no. 1: 252. https://doi.org/10.3390/ijms19010252