The Herb–Drug Pharmacokinetic Interaction of 5-Fluorouracil and Its Metabolite 5-Fluoro-5,6-Dihydrouracil with a Traditional Chinese Medicine in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of HPLC–UV Conditions and Sample Preparation
2.2. Validation of Linearity, Recovery, Precision, Accuracy, and Stability
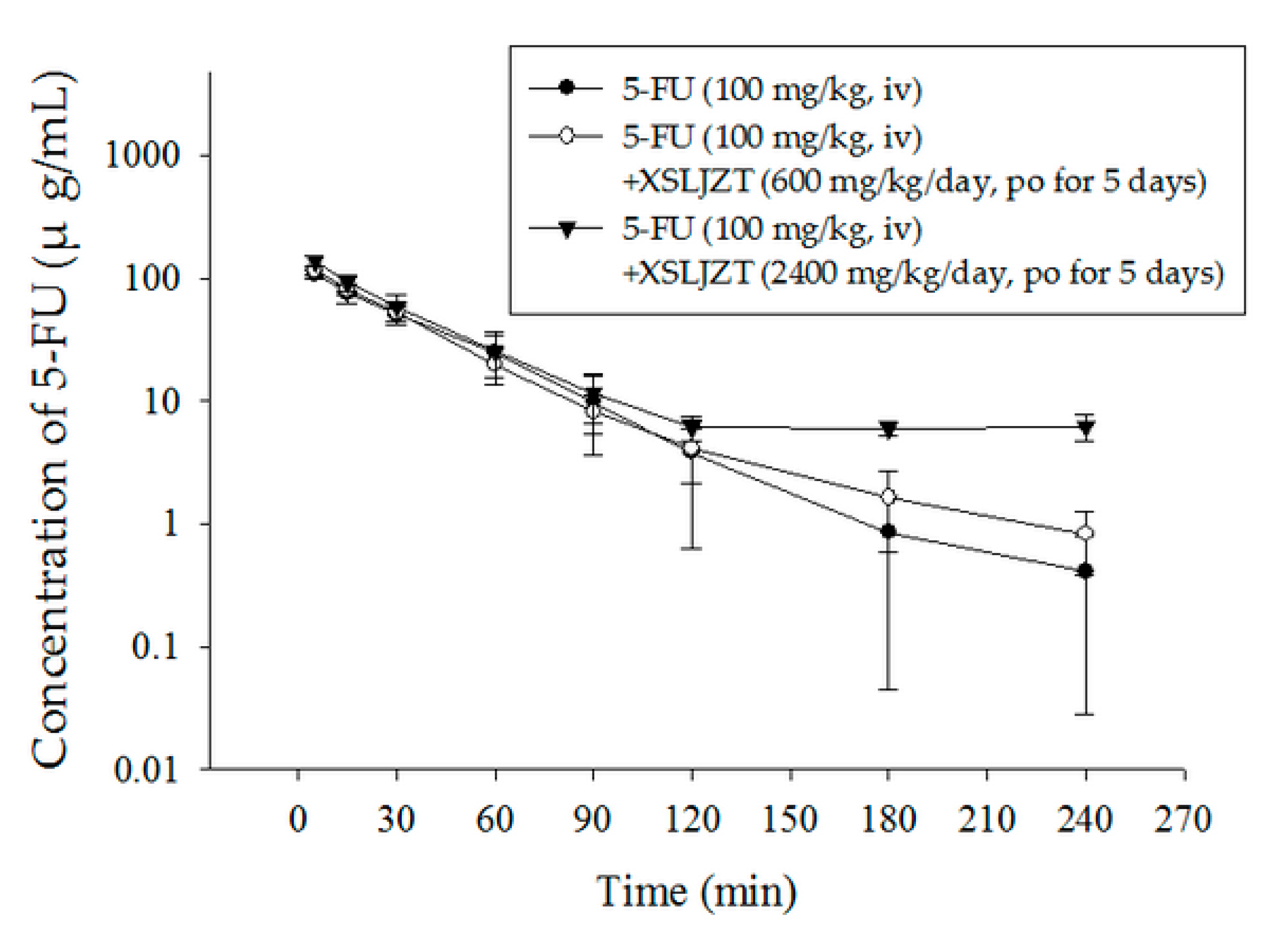
2.3. Herbal–Drug Pharmacokinetic Interaction Study
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instrumentation and HPLC–UV Conditions
3.3. Preparation of 5-FU and 5-FDHU Plasma Extraction
3.4. Method Validation
3.4.1. Calibration Curves
3.4.2. Extraction Recovery
- Set 1
- The stock solutions of 5-FU and 5-FDHU were mixed with 10 µL of amoxicillin (I.S.) solution and diluted to 0.5, 5 and 50 µg/mL in the mobile phase.
- Set 2
- A total of 10 µL of standard solution was added to 50 µL of blank plasma, 10 µL of amoxicillin (I.S.) solution and 130 µL of methanol and prepared as described in the sample preparation section. Pre-extraction samples of 5-FU and 5-FDHU were prepared and used for HPLC–UV analysis. The recovery was calculated as the peak area of Set 2 divided by the peak area of Set 1.
3.4.3. Evaluation of Accuracy and Precision
3.5. Stability Evaluation
- (1)
- Short-term: The samples were stored at room temperature (25 ± 3 °C) for 4 h before analysis.
- (2)
- Post-preparative: The samples were kept at 8 °C for 8 h in an autosampler before analysis.
- (3)
- Freeze and thaw: The samples were stored at −20 °C for 24 h and then thawed at room temperature. The freeze and thaw cycle was repeated three times.
- (4)
- Long-term: The samples were kept at −20 °C for 30 days in darkness before analysis.
3.6. Experimental Animals
3.7. Drug Administration
3.8. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Büchel, B.; Rhyn, P.; Schürch, S.; Bühr, C.; Amstutz, U.; Largiadèr, C.R. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed. Chromatogr. 2013, 27, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Tanaka, N.; Yokoi, K.; Ishikawa, N.; Seya, T.; Horiba, K.; Kanazawa, Y.; Shirakawa, T.; Ohkawa, K.; Kudoh, H.; et al. Prediction of sensitivity to 5-fluorouracil (5-fu) by metabolic and target enzyme activities in colon cancer. Gan to Kagaku Ryoho 2006, 33, 1603–1609. [Google Scholar] [PubMed]
- Levy, E.; Piedbois, P.; Buyse, M.; Pignon, J.; Rougier, P.; Ryan, L.; Hansen, R.; Zee, B.; Weinerman, B.; Pater, J. Toxicity of fluorouracil in patients with advanced colorectal cancer: Effect of administration schedule and prognostic factors. J. Clin. Oncol. 1998, 16, 3537–3541. [Google Scholar] [PubMed]
- Ezzeldin, H.; Diasio, R. Dihydropyrimidine dehydrogenase deficiency, a pharmacogenetic syndrome associated with potentially life-threatening toxicity following 5-fluorouracil administration. Clin. Colorectal Cancer 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Danesi, R.; Bocci, G.; Natale, G.; Del Tacca, M. 5-Fluorouracil catabolism to 5-fluoro-5,6-dihydrouracil is reduced by acute liver impairment in mice. Toxicol. Appl. Pharmacol. 2005, 203, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Harris, B. Clinical pharmacology of 5-fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar]
- Van Kuilenburg, A.B.; Meinsma, R.; Zoetekouw, L.; Van Gennip, A.H. Increased risk of grade IV neutropenia after administration of 5-fluorouracil due to a dihydropyrimidine dehydrogenase deficiency: High prevalence of the IVS14 + 1 g > a mutation. Int. J. Cancer 2002, 101, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vainchtein, L.D.; Rosing, H.; Schellens, J.H.; Beijnen, J.H. A new, validated HPLC-MS/MS method for the simultaneous determination of the anti-cancer agent capecitabine and its metabolites: 5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil and 5-fluorodihydrouracil, in human plasma. Biomed. Chromatogr. 2010, 24, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, A.; Ibrahim, T.; Danesi, R.; Maltoni, M.; Vannozzi, F.; Flamini, E.; Zoli, W.; Amadori, D.; Del Tacca, M. Relationship between plasma concentrations of 5-fluorouracil and 5-fluoro-5,6-dihydrouracil and toxicity of 5-fluorouracil infusions in cancer patients. Ther. Drug Monit. 2002, 24, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.H.; Chang, L.W.; Wang, J.W.; Lin, L.C.; Tsai, T.H. Herb-drug pharmacokinetic interaction of a traditional Chinese medicine Jia-Wei-Xiao-Yao-San with 5-Fluorouracil in the blood and brain of rat using microdialysis. J. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tachjian, A.; Maria, V.; Jahangir, A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 2010, 55, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Fernadez-Ortega, P.; Pud, D.; Ozden, G.; Scott, J.A.; Panteli, V.; Margulies, A.; Browall, M.; Magri, M.; Selvekerova, S.; et al. Use of complementary and alternative medicine in cancer patients: A European survey. Ann. Oncol. 2005, 16, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Factor-Litvak, P.; Cushman, L.F.; Kronenberg, F.; Wade, C.; Kalmuss, D. Use of complementary and alternative medicine among women in New York City: A pilot study. J. Altern. Complement. Med. 2001, 7, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Cassileth, B.R. Integrative oncology: Complementary therapies for pain, anxiety, and mood disturbance. CA Cancer J. Clin. 2005, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sparreboom, A.; Cox, M.C.; Acharya, M.R.; Figg, W.D. Herbal remedies in the United States: Potential adverse interactions with anticancer agents. J. Clin. Oncol. 2004, 22, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Tascilar, M.; de Jong, F.A.; Verweij, J.; Mathijssen, R.H. Complementary and alternative medicine during cancer treatment: Beyond innocence. Oncologist 2006, 11, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.K.; He, S.M.; Liu, L.; Liu, J.P.; Qian, W.M.; Zhou, S.F. Herbal interactions with anticancer drugs: Mechanistic and clinical considerations. Curr. Med. Chem. 2010, 17, 1635–1678. [Google Scholar] [CrossRef]
- Wang, B.R.; Chang, Y.L.; Chen, T.J.; Chiu, J.H.; Wu, J.C.; Wu, M.S.; Chou, C.L.; Chou, Y.C. Coprescription of Chinese herbal medicine and Western medication among female patients with breast cancer in Taiwan: Analysis of national insurance claims. Patient Prefer. Adherence 2014, 8, 671. [Google Scholar] [PubMed]
- Chao, T.H.; Fu, P.K.; Chang, C.H.; Chang, S.N.; Mao, F.C.; Lin, C.H. Prescription patterns of Chinese herbal products for post-surgery colon cancer patients in Taiwan. J. Ethnopharmacol. 2014, 155, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, G. Pharmacological research and clinical application of Xiang Sha Liu Jun Zi Tang. J. Changchun Univ. Tradit. Chin. Med. 2008, 24, 68–69. [Google Scholar]
- Xue, Z.Y. Decoction formulary. In Chinese Herbology; People’s Health Publishing House: Nanking, China, 1998; pp. 326–327. [Google Scholar]
- Peng, X.D.; Huang, Y.X. Effect of Xiang Sha Liu Jun Pills on electrogastrogram and gastrin in patients with functional dyspepsia. Shenzhen J. Integr. Tradit. West. Med. 2001, 11, 20–24. [Google Scholar]
- Xiao, Y.; Liu, Y.Y.; Yu, K.Q.; Ouyang, M.Z.; Luo, R.; Zhao, X.S. Chinese herbal medicine Liu Jun Zi Tang and Xiang Sha Liu Jun Zi Tang for functional dyspepsia: Meta-analysis of randomized controlled trials. J. Evid.-Based Complement. Altern. Med. 2012, 2012, 936459. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Q.; Lu, Z.; Wang, Q.; Wang, M.; Liu, Y.; Fu, S.; Gao, X.; Tang, X. Identification of chemical constituents in traditional Chinese medicine formula using HPLC coupled with linear ion trap-Orbitrap MS from high doses of medicinal materials to equivalent doses of formula: Study on Xiang-Sha-Liu-Jun-Zi-Jia-Jian granules. J. Sep. Sci. 2016, 39, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, J.; Li, Y. Screening for compounds with aromatase inhibiting activities from Atractylodes macrocephala Koidz. Molecules 2011, 16, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Ablise, M.; Leininger-Muller, B.; Dal Wong, C.; Siest, G.; Loppinet, V.; Visvikis, S. Synthesis and in vitro antioxidant activity of glycyrrhetinic acid derivatives tested with the cytochrome P450/NADPH system. Chem. Pharm. Bull. 2004, 52, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zhao, Y.; Chen, P.; Huang, H.; Liu, H.; Jiang, H.; Zhang, R.; Wang, H. Structure-activity relationship and substrate-dependent phenomena in effects of ginsenosides on activities of drug-metabolizing P450 enzymes. PLoS ONE 2008, 3, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.J.; Sloan, K.B. Topical delivery of 5-fluorouracil (5-FU) by 3-alkylcarbonyloxymethyl-5-FU prodrugs. J. Pharm. Sci. 2003, 92, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Yamazaki, H.; Shimada, N.; Nakajima, M.; Yokoi, T. Roles of cytochromes P450 1A2, 2A6, and 2C8 in 5-fluorouracil formation from tegafur, an anticancer prodrug, in human liver microsomes. Drug Metab. Dispos. 2000, 28, 1457–1463. [Google Scholar] [PubMed]
- Hsueh, T.Y.; Ho, J.K.; Lin, L.C.; Chiu, A.W.; Lin, C.H.; Tsai, T.H. Herb–drug interaction of Epimedium extract on the pharmacokinetic of dapoxetine in rats. J. Chromatogr. B 2016, 1014, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.; Frank, A. Simultaneous determination of the inhibitory potency of herbal extracts on the activity of six major cytochrome P450 enzymes using liquid chromatography/mass spectrometry and automated online extraction. Rapid Commun. Mass Spectrom. 2004, 18, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.; Speroni, E.; Broccoli, M.; Cangini, M.; Pasini, P.; Minghetti, A.; Crespi-Perellino, N.; Mirasoli, M.; Cantelli-Forti, G.; Paolini, M. Comparison between Chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin: Antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Sci. 2000, 67, 2997–3006. [Google Scholar] [CrossRef]
- Deb, S.; Chin, M.Y.; Adomat, H.; Guns, E.S.T. Ginsenoside-mediated blockade of 1α, 25-dihydroxyvitamin D 3 inactivation in human liver and intestine in vitro. J. Steroid Biochem. Mol. Biol. 2014, 141, 94–103. [Google Scholar] [CrossRef] [PubMed]
- He, Y.S.; Sun, W.; Wang, C.Z.; Qi, L.W.; Yang, J.; Li, P.; Wen, X.D.; Yuan, C.S. Effects of American ginseng on pharmacokinetics of 5-fluorouracil in rats. Biomed. Chromatogr. 2015, 29, 762–767. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Edeki, T. The inhibitory effects of herbal components on CYP2C9 and CYP3A4 catalytic activities in human liver microsomes. Am. J. Ther. 2004, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kikuchi, K.; Ohishi, T.; Masuike, T. Assay method for uracil, dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil by high-performance liquid chromatography. Gan to Kagaku Ryoho 2004, 31, 381–386. [Google Scholar] [PubMed]
- Ciccolini, J.; Mercier, C.; Blachon, M.F.; Favre, R.; Durand, A.; Lacarelle, B. A simple and rapid high-performance liquid chromatographic (HPLC) method for 5-fluorouracil (5-FU) assay in plasma and possible detection of patients with impaired dihydropyrimidine dehydrogenase (DPD) activity. J. Clin. Pharm. Ther. 2004, 29, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Heggie, G.D.; Sommadossi, J.P.; Cross, D.S.; Huster, W.J.; Diasio, R.B. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987, 47, 2203–2206. [Google Scholar] [PubMed]
- US Food and Drug Administration. Guidance for Industry-Bioanalytical Method Validation; Center for Drug Evaluation and Research (CDER), Department of Health and Human Services, US Food and Drug Administration: Silver Spring, MA, USA, 2001.
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, D.J.; MacIntyre, J.; Catton, G.; Engstrom, P.; Moertel, C. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil-an Eastern Cooperative Oncology Group study. J. Clin. Oncol. 1985, 3, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Jarugula, V.R.; Lam, S.S.; Boudinot, F.D. Nonlinear pharmacokinetics of 5-fluorouracil in rats. J. Pharm. Sci. 1997, 86, 756–758. [Google Scholar] [CrossRef] [PubMed]

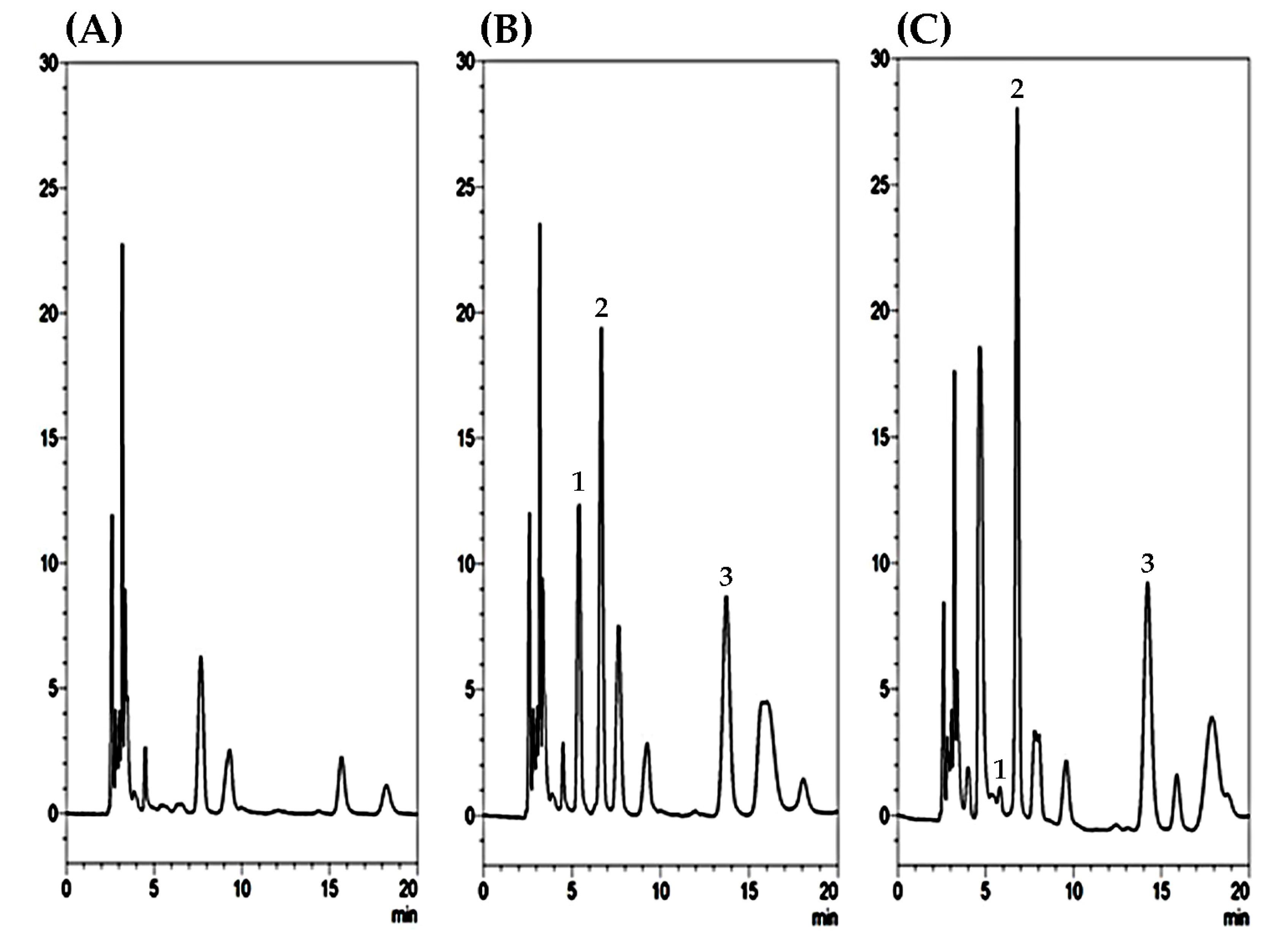

| Compounds | Linear Ranges (µg/mL) | Calibration Curves | r2 | LLOQ (µg/mL) | LOD (µg/mL) |
|---|---|---|---|---|---|
| 5-FU | 0.1–50 | y = 0.126x − 0.015 | 1.0000 | 0.1 | 0.05 |
| 5-FDHU | 0.1–50 | y = 0.066x − 0.001 | 1.0000 | 0.1 | 0.05 |
| Con. (µg/mL) | Spiked in the Mobile Phase (Set 1) | Spiked Before Extraction (Set 2) | Recovery (%) |
|---|---|---|---|
| 5-FU | |||
| 0.5 | 12,884 ± 492 | 13,876 ± 897 | 107.8 ± 0.09 |
| 5 | 149,388 ± 5857 | 161,755 ± 3696 | 108.4 ± 0.07 |
| 50 | 1,487,375 ± 31,269 | 1,562,657 ± 42,890 | 105.1 ± 0.05 |
| 5-FDHU | |||
| 0.5 | 8281 ± 520 | 8699 ± 141 | 105.4 ± 0.09 |
| 5 | 85,864 ± 2239 | 85,206 ± 4730 | 99.29 ± 0.06 |
| 50 | 871,855 ± 13,873 | 887,507 ± 50,640 | 101.7 ± 0.04 |
| Amoxicillin (I.S.) | |||
| 20 | 265,553 ± 4096 | 267,379 ± 10,503 | 100.7 ± 0.05 |
| Nominal Con. (µg/mL) | Intra-Day (n = 6) | Inter-Day (n = 6) | ||||
|---|---|---|---|---|---|---|
| Observed Con. (µg/mL) | Accuracy Bias (%) | Precision RSD (%) | Observed Con. (µg/mL) | Accuracy Bias (%) | Precision RSD (%) | |
| 5-FU | ||||||
| 0.1 | 0.111 ± 0.01 | 10.6 | 6.58 | 0.102 ± 0.01 | 2.02 | 11.5 |
| 0.5 | 0.528 ± 0.04 | 5.69 | 1.03 | 0.487 ± 0.04 | −2.69 | 7.35 |
| 1 | 0.971 ± 0.03 | −2.92 | 2.70 | 0.966 ± 0.03 | −3.45 | 3.32 |
| 5 | 5.018 ± 0.05 | 0.37 | 1.08 | 4.965 ± 0.12 | −0.70 | 2.43 |
| 10 | 10.02 ± 0.12 | 0.21 | 1.16 | 10.04 ± 0.12 | 0.40 | 1.17 |
| 50 | 50.15 ± 0.13 | 0.30 | 0.26 | 49.98 ± 0.02 | −0.04 | 0.05 |
| 5-FDHU | ||||||
| 0.1 | 0.101 ± 0.01 | 0.60 | 8.88 | 0.092 ± 0.01 | −7.98 | 5.70 |
| 0.5 | 0.489 ± 0.04 | −2.23 | 7.43 | 0.503 ± 0.01 | 0.51 | 1.74 |
| 1 | 1.044 ± 0.04 | 4.41 | 3.44 | 1.025 ± 0.04 | 2.45 | 3.61 |
| 5 | 4.766 ± 0.25 | −4.68 | 5.20 | 4.867 ± 0.17 | −2.65 | 3.50 |
| 10 | 10.26 ± 0.31 | 2.61 | 3.03 | 10.11 ± 0.19 | 1.05 | 1.84 |
| 50 | 50.16 ± 0.30 | 0.31 | 0.59 | 49.99 ± 0.04 | −0.02 | 0.09 |
| Analytes/Spiked Concentration (µg/mL) | Short-Term Stability | Autosampler Stability | Freeze-Thaw Stability | Long-Term Stability |
|---|---|---|---|---|
| 5-FU | ||||
| 0.5 | 2.95 ± 0.02 | 4.42 ± 0.04 | −6.10 ± 0.06 | −10.69 ± 0.01 |
| 5 | 1.17± 0.04 | 2.93 ± 0.07 | −2.64 ± 0.07 | −6.74 ± 0.06 |
| 50 | 0.06 ± 0.03 | −0.34 ± 0.05 | −1.41± 0.03 | −2.53 ± 0.08 |
| 5-FDHU | ||||
| 0.5 | 2.53 ± 0.05 | 2.68 ± 0.05 | −5.24 ± 0.05 | −12.16 ± 0.05 |
| 5 | 1.78 ± 0.05 | −2.29 ± 0.02 | −3.85 ± 0.02 | −4.53 ± 0.05 |
| 50 | 0.24 ± 0.11 | −0.23 ± 0.12 | −1.68 ± 0.14 | −1.96 ± 0.03 |
| Parameter | Unit | 5-FU (100 mg/kg, iv) | 5-FU + XSLJZT (600 mg/kg/day, po) | 5-FU + XSLJZT (2400 mg/kg/day, po) |
|---|---|---|---|---|
| AUC | min μg/mL | 4527 ± 974 | 4640 ± 686 | 6343 ± 1272 * |
| C0 | μg/mL | 129.4 ± 14.4 | 140.9 ± 13.6 | 150.9 ± 11.5 |
| t½ | min | 32 ± 12.0 | 50 ± 15.1 * | 50 ± 1.51 * |
| Cl | mL/min/kg | 22.87 ± 4.39 | 21.97 ± 3.43 | 16.32 ± 3.39 * |
| Vss | mL/kg | 770.2 ± 52.1 | 815.4 ± 115 | 1084 ± 188 ** |
| MRT | min | 35 ± 7.06 | 38 ± 6.91 | 67 ± 4.76 ** |
| Parameter | Unit | 5-FU (100 mg/kg, iv) | 5-FU + XSLJZT (600 mg/kg/day, po) | 5-FU + XSLJZT (2400 mg/kg/day, po) |
|---|---|---|---|---|
| AUC | min μg/mL | 321.8 ± 59.7 | 384.0 ± 19.9 | 354.2 ± 45.4 |
| Cmax | μg/mL | 2.904 ± 0.54 | 3.849 ± 0.36 | 3.688 ± 0.34 |
| Tmax | min | 60 | 60 | 45 ± 16.4 |
| t½ | min | 71 ± 9.62 | 68.00 ± 19.9 | 83 ± 32.4 |
| Cl | mL/min/kg | 320.0 ± 61.2 | 261.0 ± 13.4 | 286.8 ± 42.8 |
| MRT | min | 110 ± 18.5 | 102 ± 15.0 | 105 ± 18.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-H.; Cheng, Y.-Y.; Hsieh, C.-H.; Tsai, T.-H. The Herb–Drug Pharmacokinetic Interaction of 5-Fluorouracil and Its Metabolite 5-Fluoro-5,6-Dihydrouracil with a Traditional Chinese Medicine in Rats. Int. J. Mol. Sci. 2018, 19, 25. https://doi.org/10.3390/ijms19010025
Liu J-H, Cheng Y-Y, Hsieh C-H, Tsai T-H. The Herb–Drug Pharmacokinetic Interaction of 5-Fluorouracil and Its Metabolite 5-Fluoro-5,6-Dihydrouracil with a Traditional Chinese Medicine in Rats. International Journal of Molecular Sciences. 2018; 19(1):25. https://doi.org/10.3390/ijms19010025
Chicago/Turabian StyleLiu, Ju-Han, Yung-Yi Cheng, Chen-Hsi Hsieh, and Tung-Hu Tsai. 2018. "The Herb–Drug Pharmacokinetic Interaction of 5-Fluorouracil and Its Metabolite 5-Fluoro-5,6-Dihydrouracil with a Traditional Chinese Medicine in Rats" International Journal of Molecular Sciences 19, no. 1: 25. https://doi.org/10.3390/ijms19010025






