FM19G11 and Ependymal Progenitor/Stem Cell Combinatory Treatment Enhances Neuronal Preservation and Oligodendrogenesis after Severe Spinal Cord Injury
Abstract
:1. Introduction
2. Results
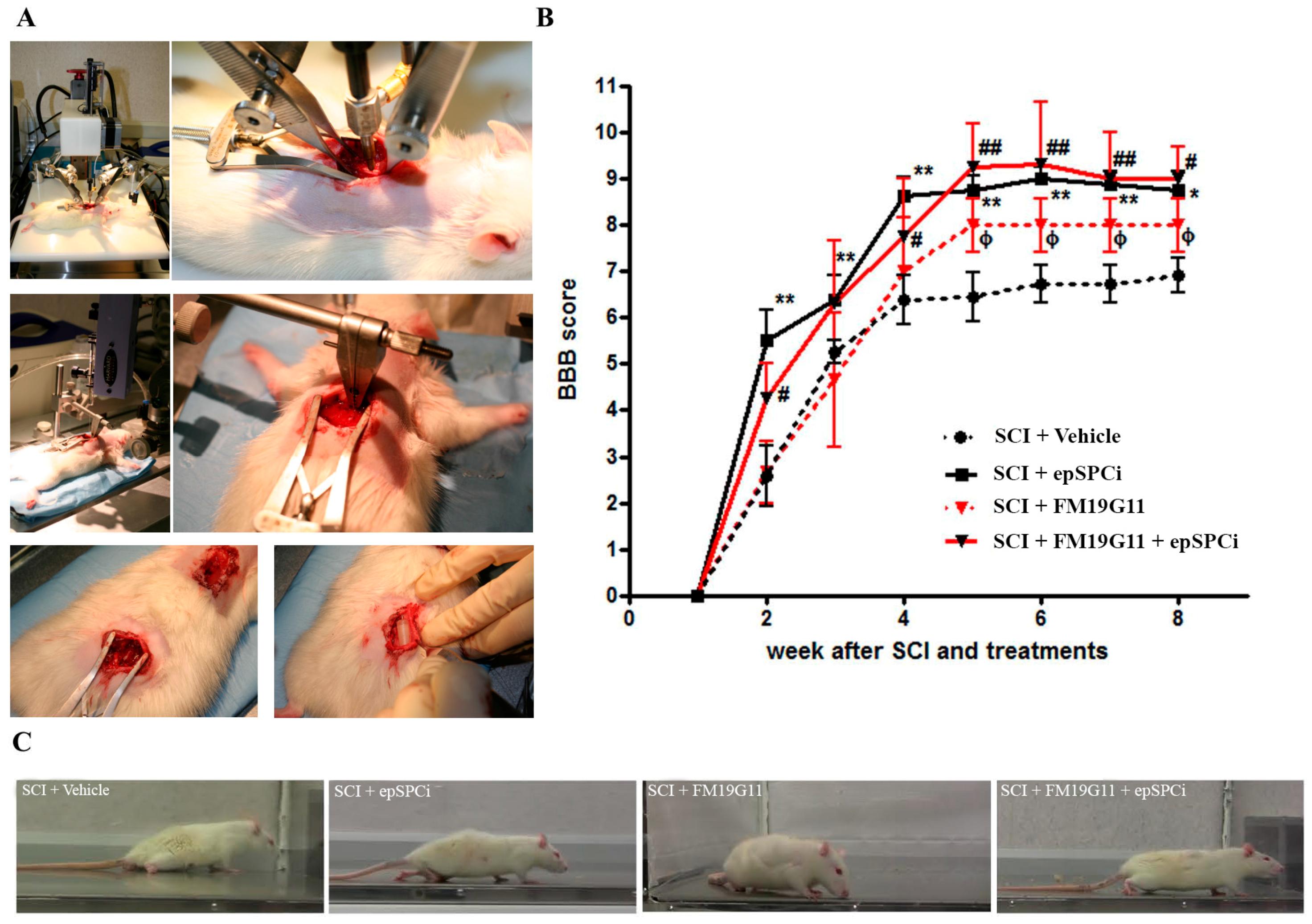
2.1. FM19G11 or epSPCi Improve Locomotion in Severe SCI with No Synergistic Effect
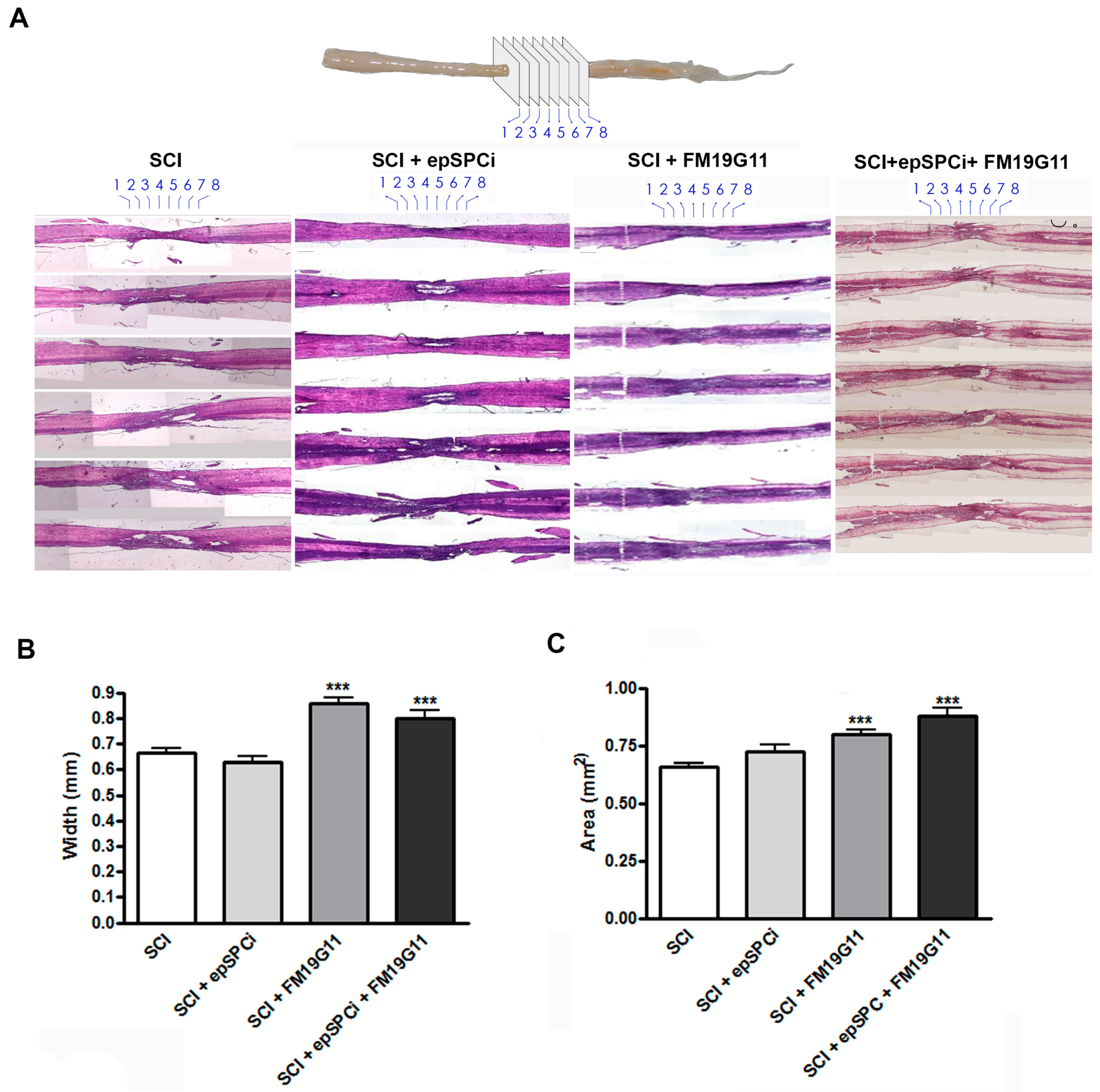
2.2. FM19G11 Intrathecal Administration after SCI Preserves Spinal Cord Tissue
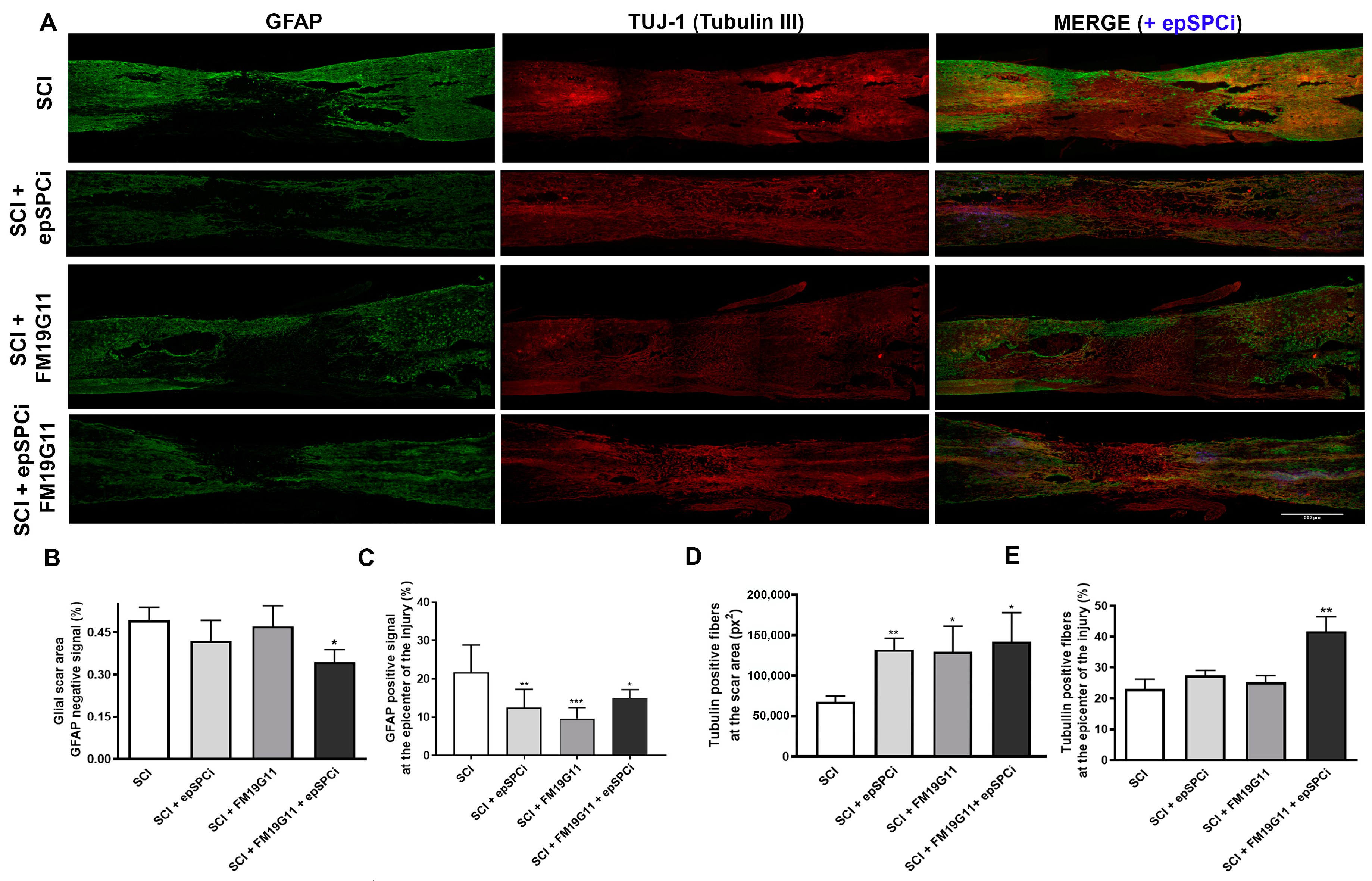
2.3. FM19G11 and epSPCi Combination Treatment Reduces the Scar Extension and Astrogliosis and Increases the Neuronal Fibers at the Injury Area
2.4. FM19G11 and epSPCi Induce the Expression of GAP43 Expression, an Axon Growth Marker, and RIP, a Marker for Myelinated Oligodendrocytes at the Injury
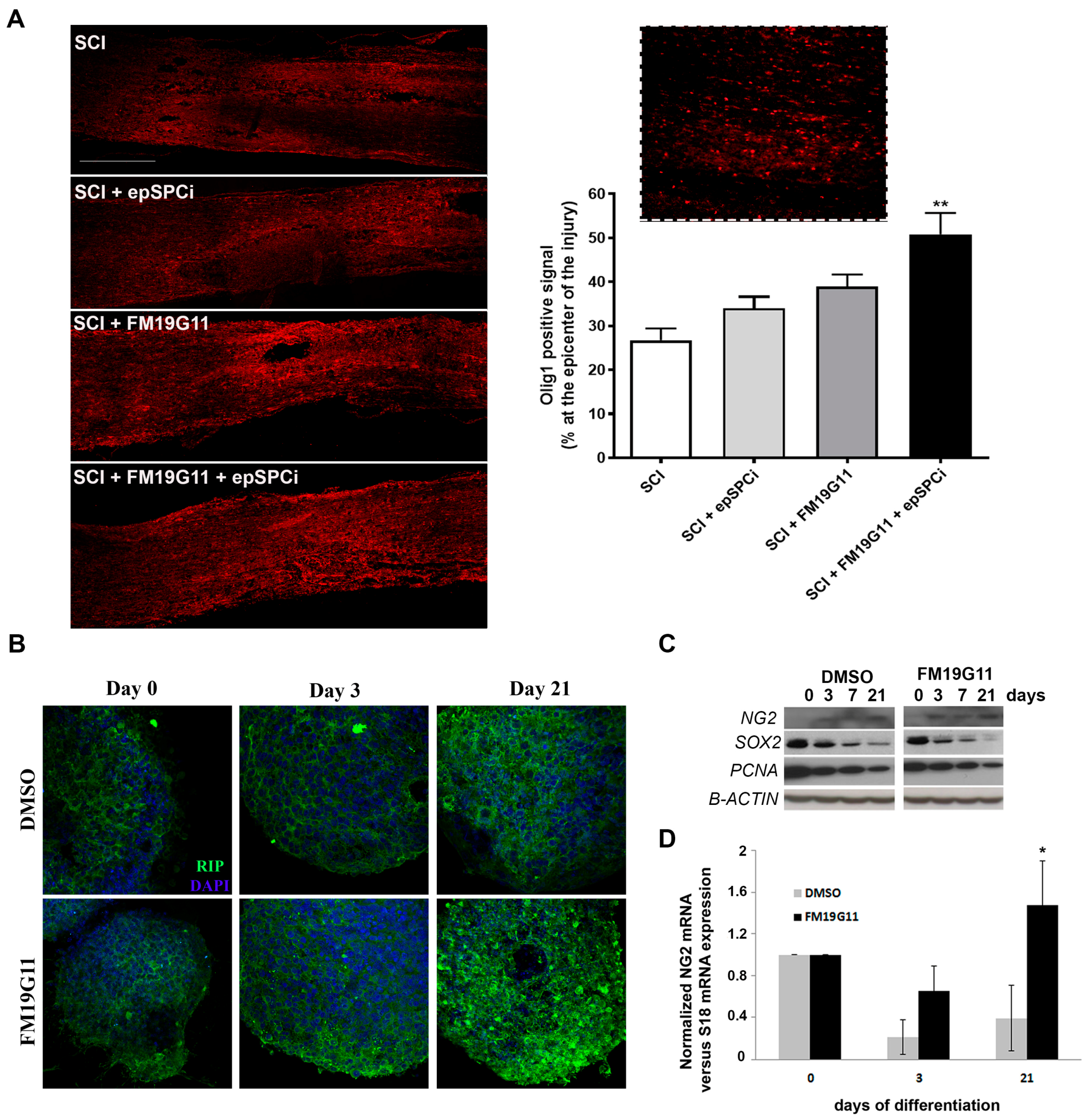
2.5. FM19G11 Favors Oligodendrocyte Replacement
3. Discussion
4. Materials and Methods
4.1. Ethical Statement Regarding the Use of Animals
4.2. EpSPCi Cell Culture
4.3. Spinal Cord Contusion, epSPCi Transplantation, Intrathecal Drug Administration and Functional Locomotor Analysis
4.4. Histology and Quantification of the Tissue Volume at the Injury Area
4.5. Immunocytochemistry and Immunohistochemistry
4.6. Western Blot
4.7. RNA Isolation and Quantitative RT-PCR
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BBB | Basso, Beattie and Bresnahan |
| BSCB | Blood-spinal cord barrier |
| DMSO | Dimethyl sulfoxide |
| epSPC | Ependymal stem/progenitor cells |
| epSPCi | Ependymal stem/progenitor cells of the spinal cord after injury |
| GFAP | Glial fibrillary acidic protein |
| GFP | Green fluorescence protein |
| H&E | Hematoxylin-eosin |
| HIF | Hypoxia inducible factor |
| NPC | Neural precursor cell |
| OPC | Oligodendrocyte precursor cell |
| PFA | Paraformaldehyde |
| PBS | Phosphate buffer solution |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| SC | Spinal cord |
| SCI | Spinal cord injury |
| UCP | Uncoupling protein |
References
- Hulsebosch, C.E. Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol. Educ. 2002, 26, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Lu, P. Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp. Neurol. 2017, 291, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Negro, S.; Lessi, F.; Duregotti, E.; Aretini, P.; La Ferla, M.; Franceschi, S.; Menicagli, M.; Bergamin, E.; Radice, E.; Thelen, M.; et al. CXCL12alpha/SDF-1 from perisynaptic Schwann cells promotes regeneration of injured motor axon terminals. EMBO Mol. Med. 2017, 9, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Li, W.; Zhang, Q.; Li, Y.; Zhang, Z.; Zhu, J.; Chen, B.; Williams, P.R.; Zhang, Y.; et al. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron 2017, 95, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.N.; Tator, C.H. Review of the effect of spinal cord trama on the vessels and blood flow in the spinal cord. J. Neurosurg. 1976, 45, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, M.; Ueno, M.; Itakura, T.; Yamashita, T. Activated microglia inhibit axonal growth through RGMa. PLoS ONE 2011, 6, e25234. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2017, 126, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, D.; Stojkovic, M.; Moreno-Manzano, V.; Jendelova, P.; Sykova, E.; Bhattacharya, S.S.; Erceg, S. Concise review: Reactive astrocytes and stem cells in spinal cord injury: Good guys or bad guys? Stem Cells 2015, 33, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic spinal cord injury-repair and regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.D.; Rosenberg, L.J.; Wrathall, J.R. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp. Neurol. 2001, 168, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Fehlings, M. Concise review: Bridging the gap: Novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl. Med. 2016, 5, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Ronaghi, M.; Erceg, S.; Moreno-Manzano, V.; Stojkovic, M. Challenges of stem cell therapy for spinal cord injury: Human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells 2010, 28, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gazdic, M.; Volarevic, V.; Arsenijevic, A.; Erceg, S.; Moreno-Manzano, V.; Arsenijevic, N.; Stojkovic, M. Stem cells and labeling for spinal cord injury. Int. J. Mol. Sci. 2016, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Requejo-Aguilar, R.; Alastrue-Agudo, A.; Cases-Villar, M.; Lopez-Mocholi, E.; England, R.; Vicent, M.J.; Moreno-Manzano, V. Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials 2017, 113, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Zhu, T.; Yuan, B.; Ni, H.; Jiang, J.; Wang, H.; Liang, W. Anti-inflammatory effects of curcumin in experimental spinal cord injury in rats. Inflamm. Res. 2014, 63, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Ormond, D.R.; Shannon, C.; Oppenheim, J.; Zeman, R.; Das, K.; Murali, R.; Jhanwar-Uniyal, M. Stem cell therapy and curcumin synergistically enhance recovery from spinal cord injury. PLoS ONE 2014, 9, e88916. [Google Scholar] [CrossRef] [PubMed]
- Sahin Kavakli, H.; Koca, C.; Alici, O. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus. Travma Acil Cerrahi Derg. 2011, 17, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Zu, J.N.; Li, J.; Chen, C.; Xi, C.Y.; Yan, J.L. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci. Lett. 2014, 560, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zou, M.; Xiang, X.; Zhu, H.; Chu, W.; Liu, W.; Chen, F.; Lin, J. Curcumin improves neural function after spinal cord injury by the joint inhibition of the intracellular and extracellular components of glial scar. J. Surg. Res. 2015, 195, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Meletis, K.; Barnabe-Heider, F.; Carlen, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villafuertes, R.; Rodriguez-Jimenez, F.J.; Alastrue-Agudo, A.; Stojkovic, M.; Miras-Portugal, M.T.; Moreno-Manzano, V. Purinergic receptors in spinal cord-derived ependymal stem/progenitor cells and their potential role in cell-based therapy for spinal cord injury. Cell Transplant. 2015, 24, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Manzano, V.; Rodriguez-Jimenez, F.J.; Garcia-Rosello, M.; Lainez, S.; Erceg, S.; Calvo, M.T.; Ronaghi, M.; Lloret, M.; Planells-Cases, R.; Sánchez-Puelles, J.M. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells 2009, 27, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Thuret, S.; Moon, L.D.; Gage, F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006, 7, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Manzano, V.; Rodriguez-Jimenez, F.J.; Acena-Bonilla, J.L.; Fustero-Lardies, S.; Erceg, S.; Dopazo, J.; Montaner, D.; Stojkovic, M.; Sánchez-Puelles, J.M. FM19G11, a new hypoxia-inducible factor (HIF) modulator, affects stem cell differentiation status. J. Biol. Chem. 2010, 285, 1333–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Jimenez, F.J.; Moreno-Manzano, V.; Lucas-Dominguez, R.; Sanchez-Puelles, J.M. Hypoxia causes downregulation of mismatch repair system and genomic instability in stem cells. Stem Cells 2008, 26, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jimenez, F.J.; Moreno-Manzano, V.; Mateos-Gregorio, P.; Royo, I.; Erceg, S.; Murguia, J.R.; Sánchez-Puelles, J.M. FM19G11: A new modulator of HIF that links mTOR activation with the DNA damage checkpoint pathways. Cell Cycle 2010, 9, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jimnez, F.J.; Alastrue-Agudo, A.; Erceg, S.; Stojkovic, M.; Moreno-Manzano, V. FM19G11 favors spinal cord injury regeneration and stem cell self-renewal by mitochondrial uncoupling and glucose metabolism induction. Stem Cells 2012, 30, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Miyata, S. Synaptic localization of growth-associated protein 43 in cultured hippocampal neurons during synaptogenesis. Cell Biochem. Funct. 2013, 31, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Sakurai, Y.; Ichinose, T.; Aikawa, Y.; Kotani, M.; Itoh, K. Monoclonal antibody Rip specifically recognizes 2′,3′-cyclic nucleotide 3′-phosphodiesterase in oligodendrocytes. J. Neurosci. Res. 2006, 84, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Barnabe-Heider, F.; Goritz, C.; Sabelstrom, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Hesp, Z.C.; Goldstein, E.Z.; Miranda, C.J.; Kaspar, B.K.; McTigue, D.M. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J. Neurosci. 2015, 35, 1274–1290. [Google Scholar] [CrossRef] [PubMed]
- Sellers, D.L.; Maris, D.O.; Horner, P.J. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J. Neurosci. 2009, 29, 6722–6733. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, R.F.; Harrison, B.M.; McDonald, W.I. Demyelination and remyelination after acute spinal cord compression. Exp. Neurol. 1973, 38, 472–487. [Google Scholar] [CrossRef]
- Harrison, B.M.; McDonald, W.I. Remyelination after transient experimental compression of the spinal cord. Ann. Neurol. 1977, 1, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, Y.; He, Q.; Qiu, M.; Whittemore, S.R.; Cao, Q. Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells 2007, 25, 3204–3214. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.; Martinez-Cerdeno, V. Endogenous proliferation after spinal cord injury in animal models. Stem Cells Int. 2012, 2012, 387513. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, D.; Moreno-Manzano, V.; Lopez-Mocholi, E.; Rodriguez-Jimenez, F.J.; Jendelova, P.; Sykova, E.; Oria, M.; Stojkovic, M.; Erceg, S. Complete rat spinal cord transection as a faithful model of spinal cord injury for translational cell transplantation. Sci. Rep. 2015, 5, 9640. [Google Scholar] [CrossRef] [PubMed]
- Stenudd, M.; Sabelstrom, H.; Frisen, J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015, 72, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Proschel, C.; Zhang, N.; Noble, M.; Mayer-Proschel, M.; Davies, S.J. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J. Biol. 2008, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.; Biziato, D.; Brambilla, E.; Donega, M.; Alfaro-Cervello, C.; Snider, S.; Salani, G.; Pucci, F.; Comi, G.; Garcia-Verdugo, J.M. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain J. Neurol. 2012, 135 Pt 2, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Kopper, T.J.; Zhang, B.; Orr, M.B.; Bailey, W.M. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci. Rep. 2017, 7, 40144. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, M.; Almazan, G.; Tabrizian, M. Oligodendrocyte-protection and remyelination post-spinal cord injuries: A review. Prog. Neurobiol. 2012, 96, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Plemel, J.R.; Keough, M.B.; Duncan, G.J.; Sparling, J.S.; Yong, V.W.; Stys, P.K.; Tetzlaff, W. Remyelination after spinal cord injury: Is it a target for repair? Prog. Neurobiol. 2014, 117, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; He, Q.; Wang, Y.; Cheng, X.; Howard, R.M.; Zhang, Y.; DeVries, W.H.; Shields, C.B.; Magnuson, D.S.; Xu, X.M. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J. Neurosci. 2010, 30, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Plemel, J.R.; Chojnacki, A.; Sparling, J.S.; Liu, J.; Plunet, W.; Duncan, G.J.; Park, S.E.; Weiss, S.; Tetzlaff, W. Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia 2011, 59, 1891–1910. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, C.C.; Li, J.; Guan, X.Y.; Gao, L.; Ma, L.X.; Li, R.X.; Peng, Y.W.; Zhu, G.P. Transplantation of oligodendrocyte precursor cells improves locomotion deficits in rats with spinal cord irradiation injury. PLoS ONE 2013, 8, e57534. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jimenez, F.J.; Alastrue, A.; Stojkovic, M.; Erceg, S.; Moreno-Manzano, V. Connexin 50 modulates Sox2 expression in spinal-cord-derived ependymal stem/progenitor cells. Cell Tissue Res. 2016, 365, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alastrue-Agudo, A.; Rodriguez-Jimenez, F.J.; Mocholi, E.L.; De Giorgio, F.; Erceg, S.; Moreno-Manzano, V. FM19G11 and Ependymal Progenitor/Stem Cell Combinatory Treatment Enhances Neuronal Preservation and Oligodendrogenesis after Severe Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 200. https://doi.org/10.3390/ijms19010200
Alastrue-Agudo A, Rodriguez-Jimenez FJ, Mocholi EL, De Giorgio F, Erceg S, Moreno-Manzano V. FM19G11 and Ependymal Progenitor/Stem Cell Combinatory Treatment Enhances Neuronal Preservation and Oligodendrogenesis after Severe Spinal Cord Injury. International Journal of Molecular Sciences. 2018; 19(1):200. https://doi.org/10.3390/ijms19010200
Chicago/Turabian StyleAlastrue-Agudo, Ana, Francisco Javier Rodriguez-Jimenez, Eric López Mocholi, Francesca De Giorgio, Slaven Erceg, and Victoria Moreno-Manzano. 2018. "FM19G11 and Ependymal Progenitor/Stem Cell Combinatory Treatment Enhances Neuronal Preservation and Oligodendrogenesis after Severe Spinal Cord Injury" International Journal of Molecular Sciences 19, no. 1: 200. https://doi.org/10.3390/ijms19010200




