Effects of Fluoxetine on Hippocampal Neurogenesis and Neuroprotection in the Model of Global Cerebral Ischemia in Rats
Abstract
:1. Introduction
2. Results
2.1. Survival and Neurological Deficit
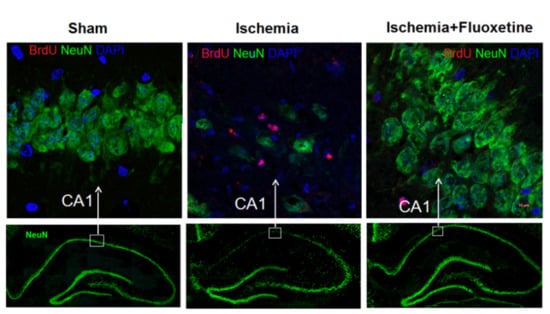
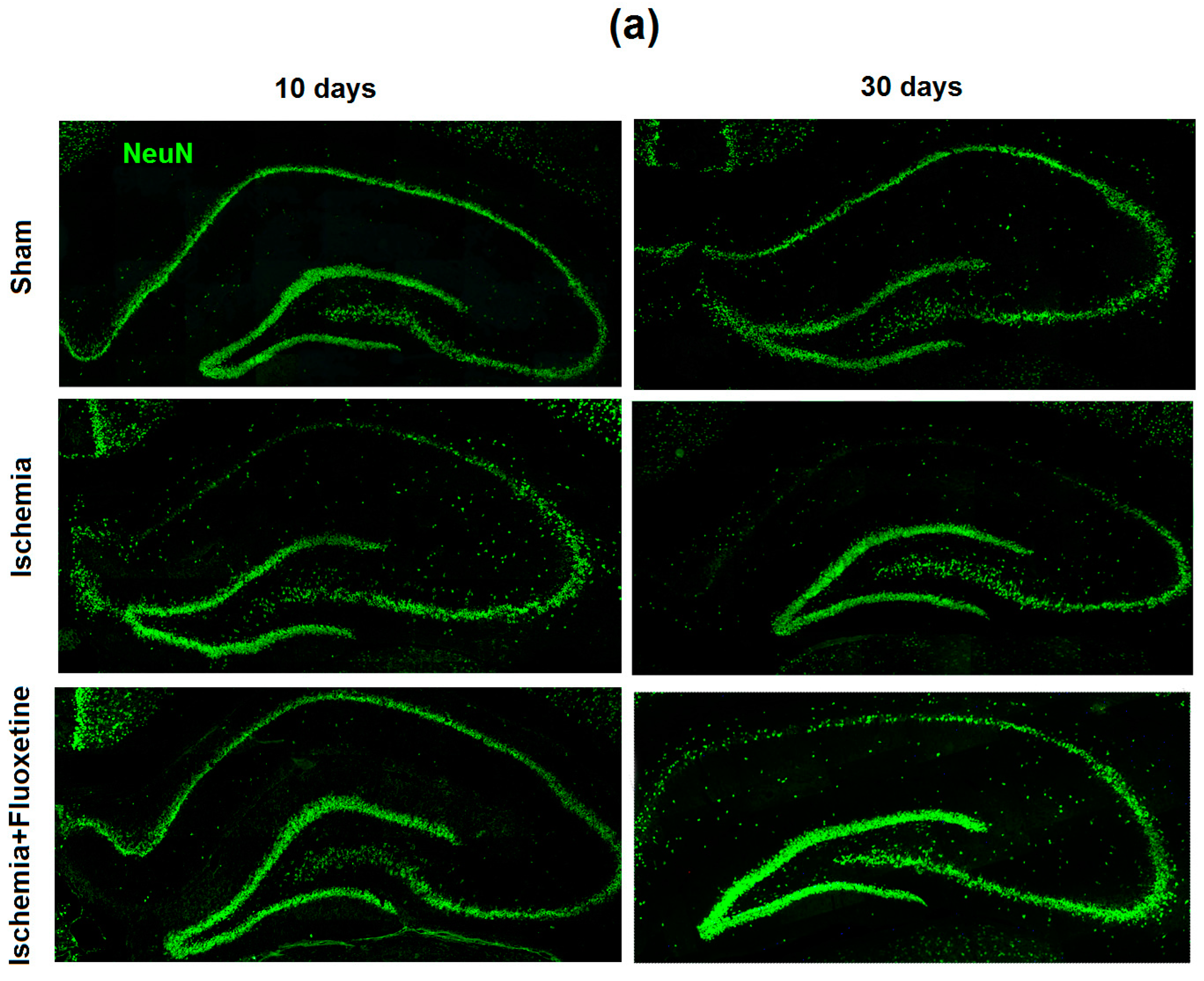
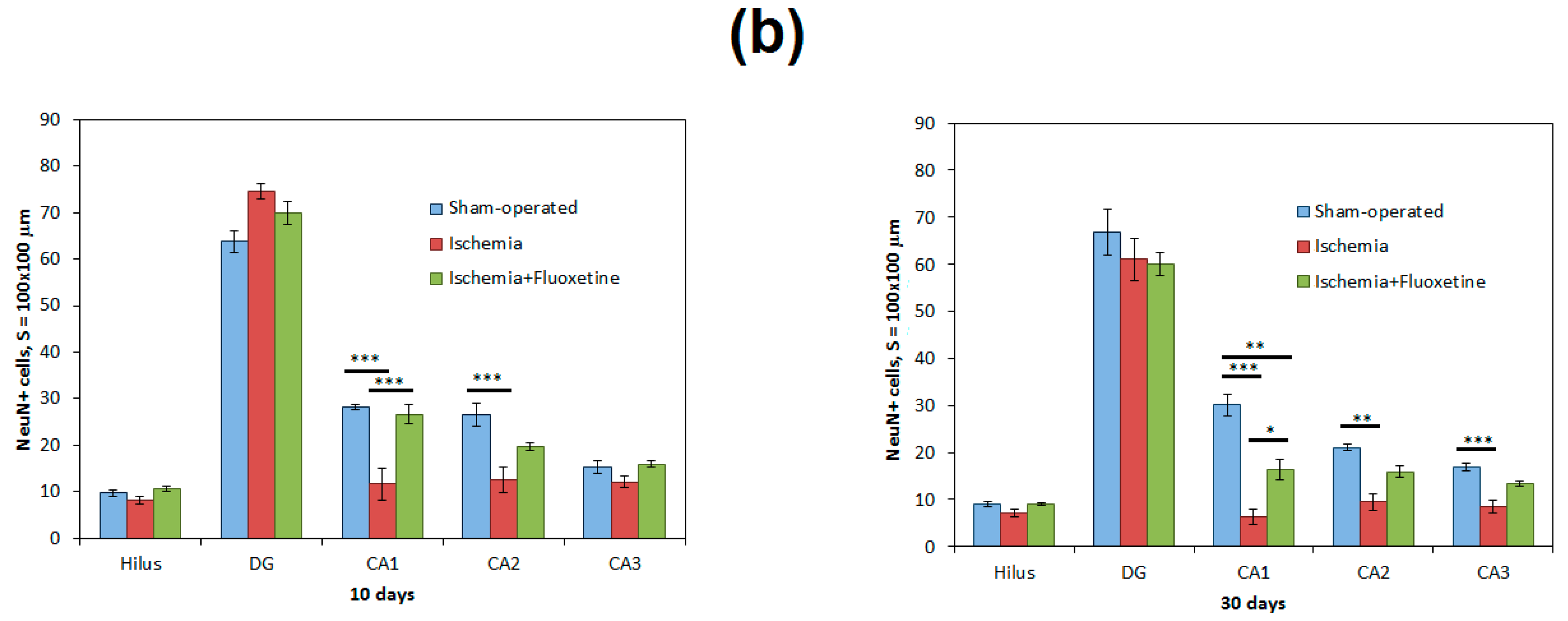
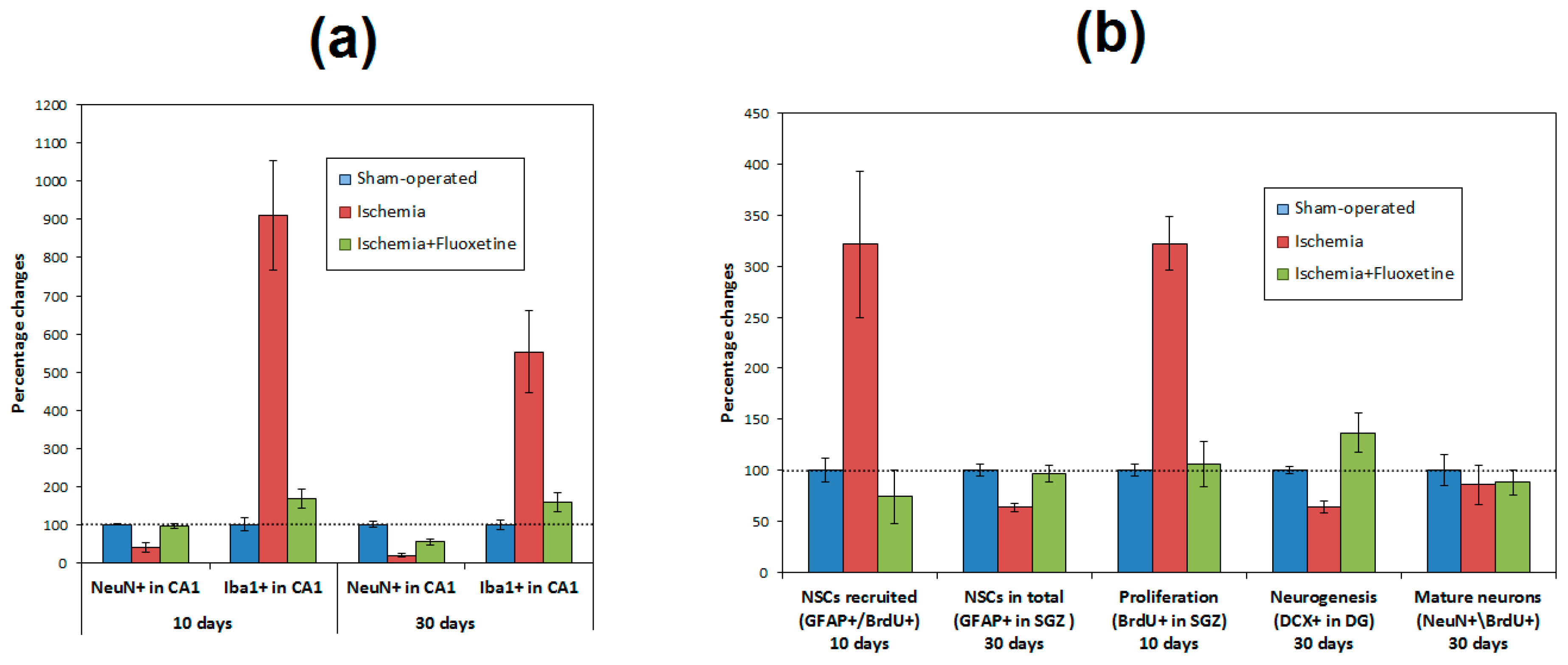
2.2. Fluoxetine Reduces Neuronal Loss in the Hippocampus
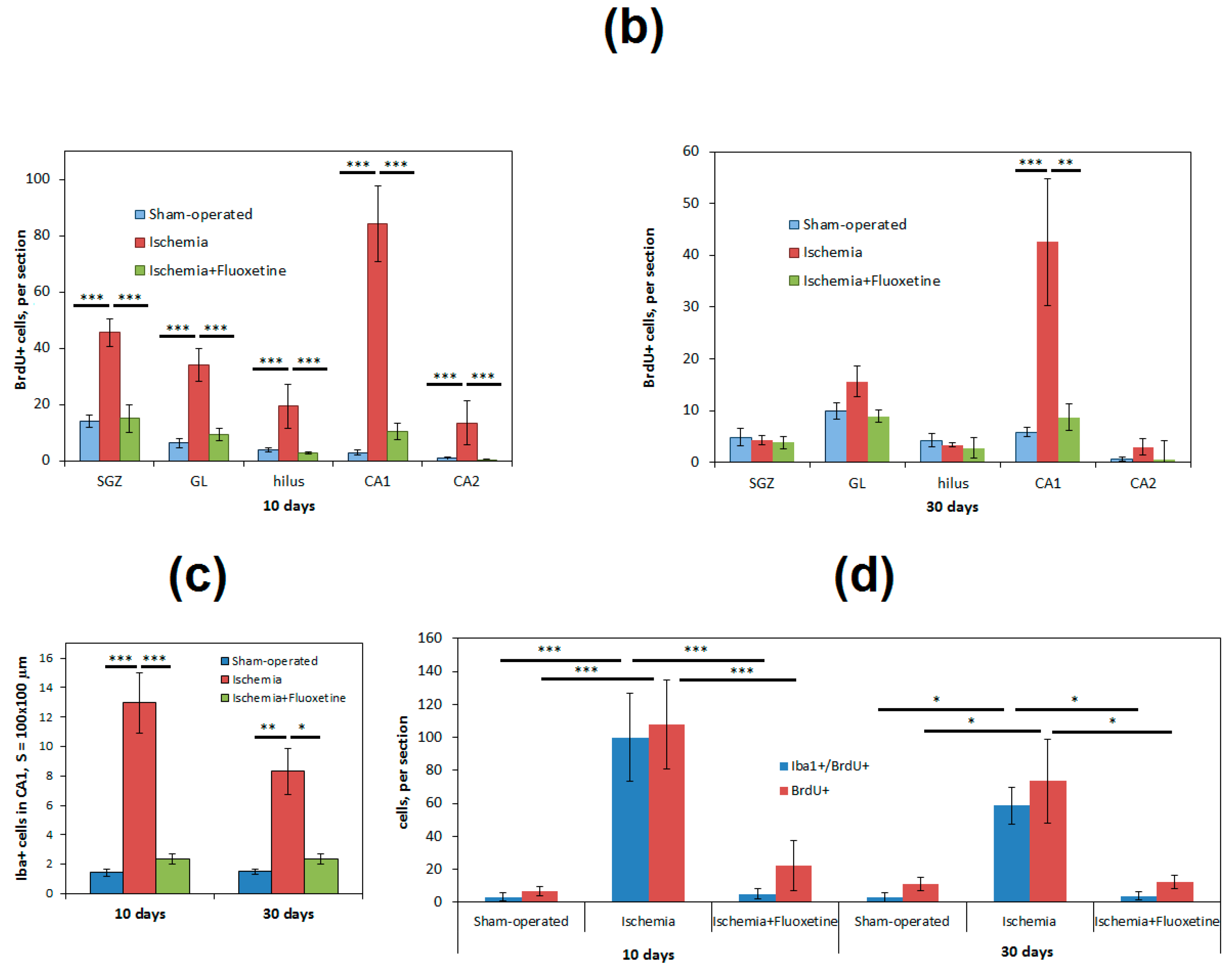
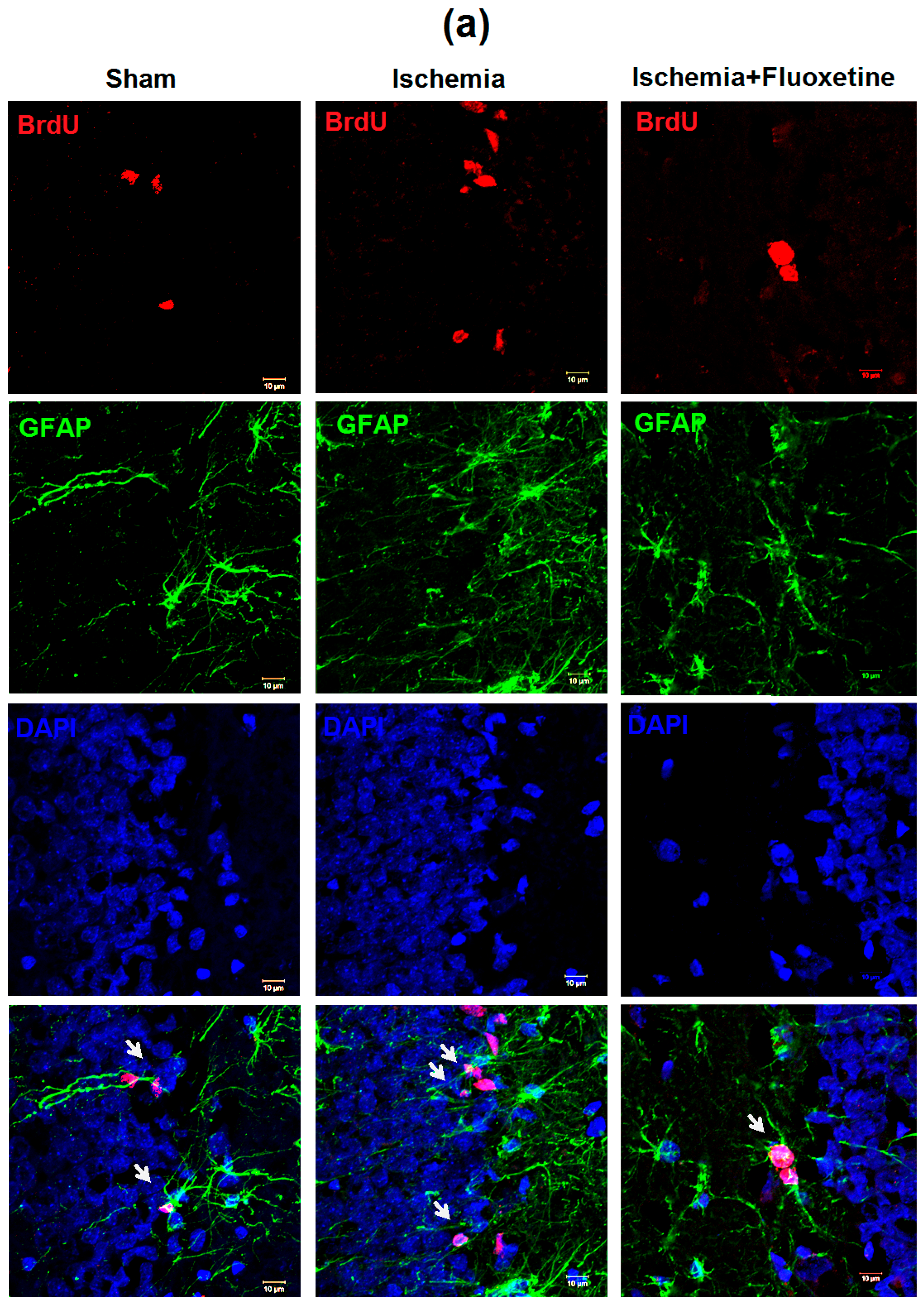
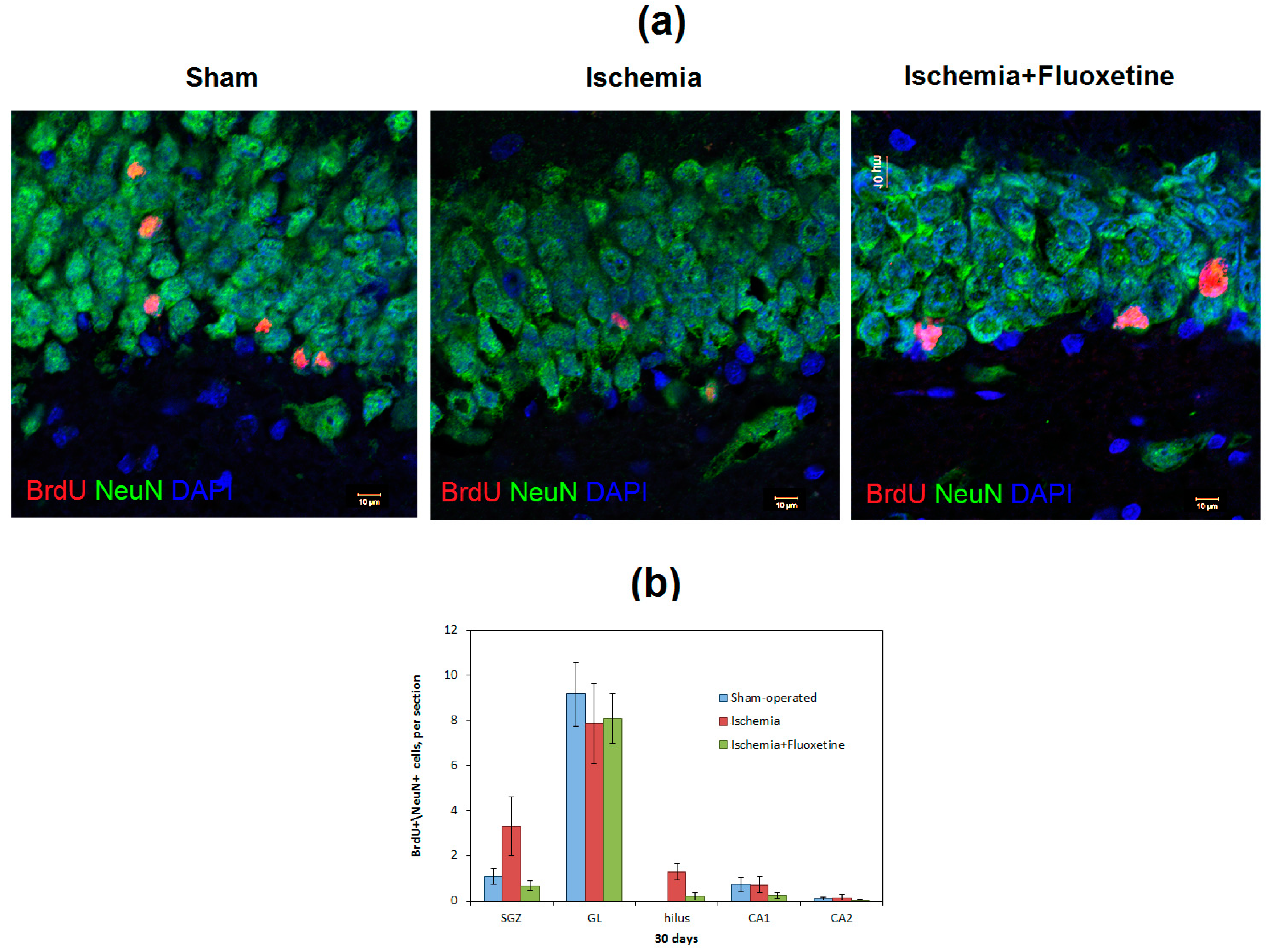
2.3. Fluoxetine Effect on Ischemia-Induced Proliferation in the Hippocampus
2.4. Anti-Inflamatory Effect of Fluoxetine
2.5. Fluoxetine Effect on NSC Recruitment in the DG of the Hippocampus
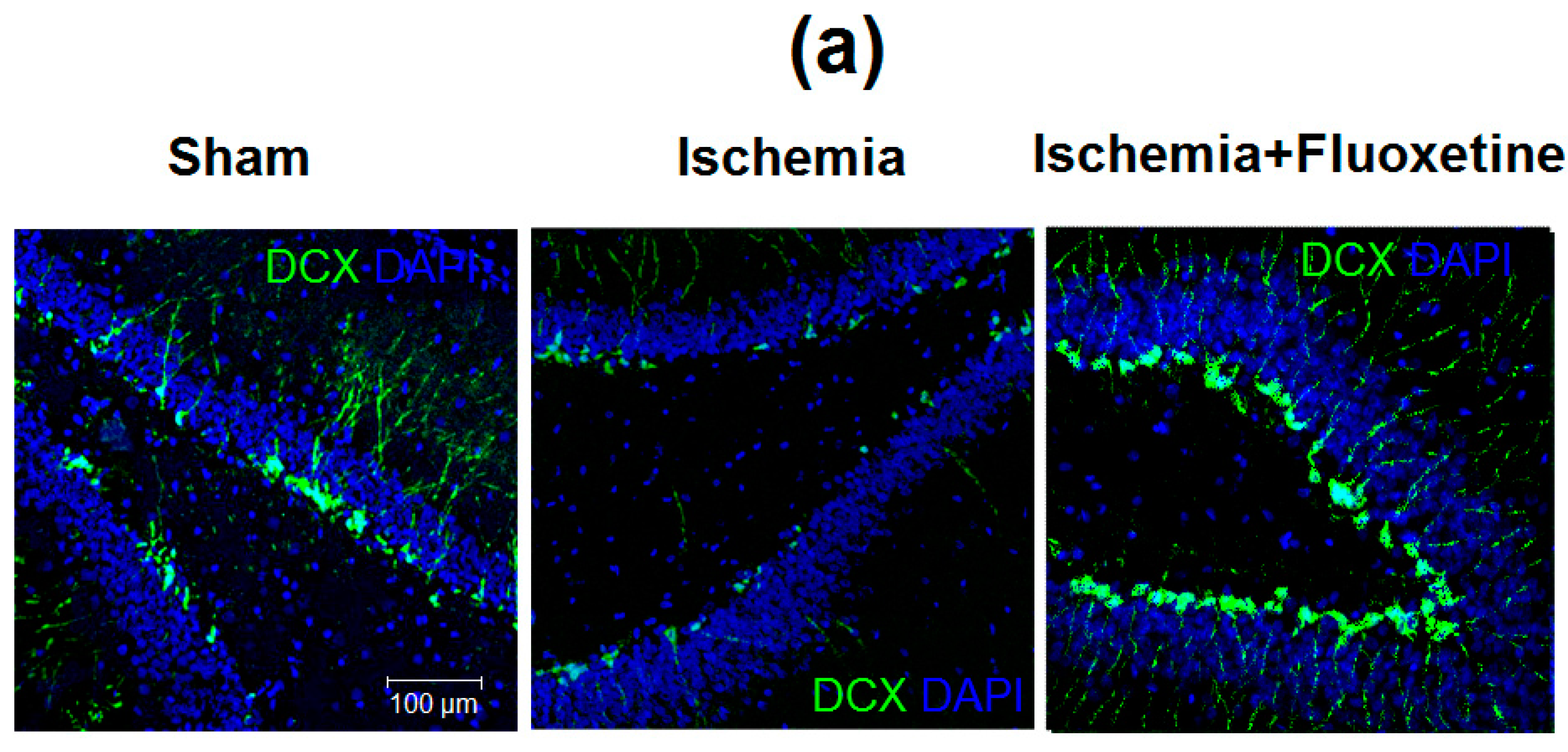
2.6. Fluoxetine Effect on Neurogenesis in the DG of the Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
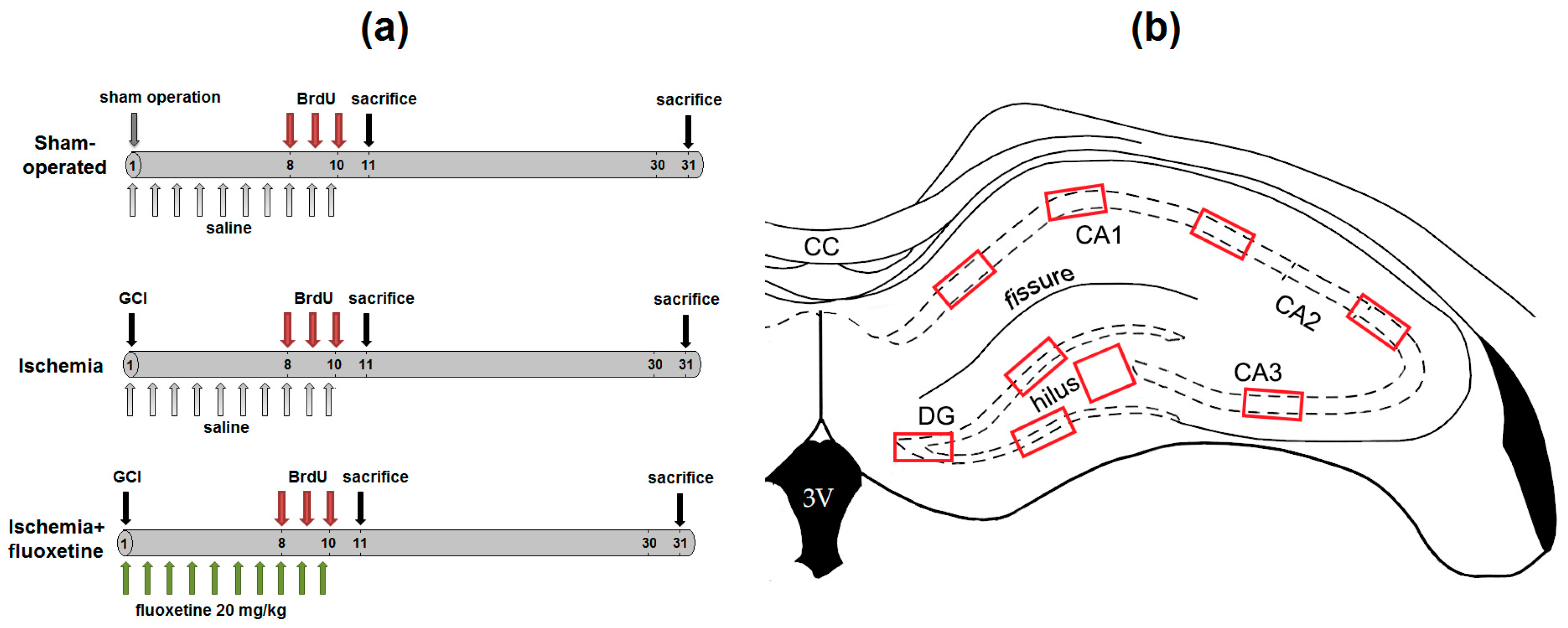
4.2. Experimental Design
4.3. Immunochemistry
4.4. Image Processing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BrdU | 5-Bromo-2′-deoxyuridine |
| CA1 | Cornu Ammonis, area 1 |
| CA2 | Cornu Ammonis, area 2 |
| CA3 | Cornu Ammonis, area 3 |
| CNS | Central nervous system |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DCX | Doublecortin |
| DG | Dentate gyrus of the hippocampus |
| DNA | Desoxynucleic acid |
| GCI | Global cerebral ischemia |
| GFAP | Glial fibrillary acidic protein |
| GL | Granular layer dentate gyrus |
| MCAO | Middle cerebral artery occlusion |
| n | Number of variables |
| NeuN | Neuronal nuclei protein |
| NSC | Neural stem cells |
| p | Significance level |
| PBS | Phosphate buffer |
| r | Pearson’s correlation coefficient |
| ROI | Regions of interest |
| RSCF | Russian Science Foundation |
| SEM | Standard error of mean |
| SGZ | Subgranular zone of dentate gyrus |
| SVZ | Subventricular zone |
References
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [PubMed]
- Encinas, J.M.; Vaahtokari, A.; Enikolopov, G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 2006, 103, 8233–8238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; David, D.J.; Monckton, J.E.; Battaglia, F.; Hen, R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 2008, 28, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Possamai, F.; dos Santos, J.; Walber, T.; Marcon, J.C.; dos Santos, T.S.; Lino de Oliveira, C. Influence of enrichment on behavioral and neurogenic effects of antidepressants in Wistar rats submitted to repeated forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 58, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Klomp, A.; Václavů, L.; Meerhoff, G.F.; Reneman, L.; Lucassen, P.J. Effects of Chronic Fluoxetine Treatment on Neurogenesis and Tryptophan Hydroxylase Expression in Adolescent and Adult Rats. PLoS ONE 2014, 9, e97603. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Cai, H.H.; Wang, B.; Chen, L.; Zhou, Q.G.; Luo, C.X.; Liu, N.; Ding, X.S.; Zhu, D.Y. Chronic Fluoxetine Treatment Improves Ischemia-Induced Spatial Cognitive Deficits Through Increasing Hippocampal Neurogenesis After Stroke. J. Neurosci. Res. 2009, 87, 112–122. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.K.; Irvine, C.; Mead, G.E.; Sena, E.S.; Currie, G.L.; Egan, K.E.; Macleod, M.R.; Howells, D.W. Efficacy of antidepressants in animal models of ischemic stroke: A systematic review and meta-analysis. Stroke 2014, 45, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, X.; Liu, T.; Zhao, M.; Zhao, S.; Xiao, T.; Jolkkonen, J.; Zhao, C. Fluoxetine enhanced neurogenesis is not translated to functional outcome in stroke rats. Neurosci. Lett. 2015, 603, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Khodanovich, M.Yu.; Kisel, A.A.; Chernyshova, G.A.; Smol’yakova, V.I.; Savchenko, R.R.; Plotnikov, M.B. Effect of Fluoxetine on Neurogenesis in Hippocampal Dentate Gyrus after Global Transient Cerebral Ischemia in Rats. Bull. Exp. Biol. Med. 2016, 161, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Zhang, Z.J.; Wang, S.H.; Sui, Y.X.; Sun, Y. Notch1 signaling, hippocampal neurogenesis and behavioral responses to chronic unpredicted mild stress in adult ischemic rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Cayre, M.; Canoll, P.; Goldman, J.E. Cell migration in the normal and pathological postnatal mammalian brain. Prog. Neurobiol. 2009, 88, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Colocho, P.; Lanciego, J.L.; Del Rio, J.; Frechilla, D. Ischemia induces cell proliferation and neurogenesis in the gerbil hippocampus in response to neuronal death. Neurosci. Res. 2008, 61, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.J.; Preston, E.; Wojtowicz, J.M. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp. Brain Res. 2001, 136, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Solway, K.; Messing, R.O.; Sharp, F.R. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998, 18, 7768–7778. [Google Scholar] [PubMed]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef]
- Yagita, Y.; Kitagawa, K.; Ohtsuki, T.; Takasawa, K.-I.; Miyata, T.; Okano, H.; Hori, M.; Matsumoto, M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke 2001, 32, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Wiltrout, C.; Lang, B.; Yan, Y.; Dempsey, R.J.; Vemuganti, R. Repairing brain after stroke: A review on post-ischemic neurogenesis. Neurochem. Int. 2007, 50, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Heldmann, U.; Lindvall, O.; Kokaia, Z. Stroke-induced neurogenesis in aged brain. Stroke 2005, 36, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Kokaia, Z.; Thored, P.; Arvidsson, A.; Lindval, O. Regulation of Stroke-Induced Neurogenesis in Adult Brain—Recent Scientific Progress. Cereb. Cortex 2006, 16, i162–i167. [Google Scholar] [CrossRef] [PubMed]
- Thored, P.; Arvidsson, A.; Cacci, E.; Ahlenius, H.; Kallur, T.; Darsalia, V.; Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 2006, 24, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gu, W.; Brännström, T.; Rosqvist, R.; Wester, P. Cortical Neurogenesis in Adult Rats After Transient Middle Cerebral Artery Occlusion. Stroke 2001, 32, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Hayashi, T.; Zhang, W.R.; Sato, K.; Manabe, Y.; Abe, K. Induction of highly polysialylated neural cell adhesion molecule (PSA-NCAM) in postischemic gerbil hippocampus mainly dissociated with neural stem cell proliferation. Brain Res. 2001, 902, 288–293. [Google Scholar] [CrossRef]
- Lim, C.M.; Kim, S.W.; Park, J.Y.; Kim, C.; Yoon, S.H.; Lee, J.K. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J. Neurosci. Res. 2009, 87, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, H.E.; Kang, S.R.; Choi, H.Y.; Ryu, J.H.; Yune, T.Y. Fluoxetine inhibits transient global ischemia-induced hippocampal neuronal death and memory impairment by preventing blood-brain barrier disruption. Neuropharmacology 2014, 79, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.K.; Kang, M.S.; Lee, H.Y.; Seo, M.S.; Kim, S.G.; Kim, C.D.; Lee, W.S. Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J. Physiol. Pharmacol. 2009, 13, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Li, H.; Yoo, K.Y.; Lee, B.H.; Hwang, I.K.; Won, M.H. Effects of fluoxetine in ischemic cells and expressions in BDNF and some antioxidants in the gerbil hippocampal CA1 region induced by transient ischemia. Exp. Neurol. 2007, 204, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, N.; Nakayama, S.; Tanaka, M. Fluoxetine has neuroprotective effects after cardiac arrest and cardiopulmonary resuscitation in mouse. Resuscitation 2012, 83, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Chernysheva, G.A.; Smol’yakova, V.I.; Osipenko, A.N.; Plotnikov, M.B. Evaluation of survival and neurological deficit in rats in the new model of global transient cerebral ischemia. Bull. Exp. Biol. Med. 2014, 158, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Atochin, D.N.; Chernysheva, G.A.; Aliev, O.I.; Smolyakova, V.I.; Osipenko, A.N.; Logvinov, S.V.; Zhdankina, A.A.; Plotnikova, T.M.; Plotnikov, M.B. An improved three-vessel occlusion model of global cerebral ischemia in rats. Brain Res. Bull. 2017, 132, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Jolkkonen, J.; Puurunen, K.; Rantakömi, S.; Sirviö, J.; Haapalinna, A.; Sivenius, J. Effects-of fluoxetine on sensorimotor and spatial learning deficits following focal cerebral ischemia in rats. Restor. Neurol. Neurosci. 2000, 17, 211–216. [Google Scholar] [PubMed]
- Windle, V.; Corbett, D. Fluoxetine and recovery of motor function after focal ischemia in rats. Brain Res. 2005, 1044, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Danton, G.H.; Dietrich, W.D. Inflammatory Mechanisms after Ischemia and Stroke. J. Neuropathol. Exp. Neurol. 2003, 62, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Caiaffo, V.; Oliveira, B.D.; de Sá, F.B.; Evêncio Neto, J. Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine. Pharmacol. Res. Perspect. 2016, 4, e00231. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-Coupled Astrocytic Differentiation and Age-Related Depletion of Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef] [PubMed]
- McGraw, C.P. Experimental cerebral infarction effects on pentobarbital in Mongolian gerbils. Arch. Neurol. 1977, 34, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007; ISBN 9780125476126. [Google Scholar]



| Group | Total Number | Survival (%) | Neurological Scores | ||
|---|---|---|---|---|---|
| 10 Days | 30 Days | 10 Days | 30 Days | ||
| Sham-operated | 10 | 100 | 100 | 0 ## | 0 ## |
| Ischemia | 27 | 40.7 | 37.0 | 5.0 (2–9) ** | 4 (1–5) ** |
| Ischemia + Fluoxetine | 18 | 61.1 | 61.1 | 3 (1–8) * | 1 (3–0) # |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodanovich, M.; Kisel, A.; Kudabaeva, M.; Chernysheva, G.; Smolyakova, V.; Krutenkova, E.; Wasserlauf, I.; Plotnikov, M.; Yarnykh, V. Effects of Fluoxetine on Hippocampal Neurogenesis and Neuroprotection in the Model of Global Cerebral Ischemia in Rats. Int. J. Mol. Sci. 2018, 19, 162. https://doi.org/10.3390/ijms19010162
Khodanovich M, Kisel A, Kudabaeva M, Chernysheva G, Smolyakova V, Krutenkova E, Wasserlauf I, Plotnikov M, Yarnykh V. Effects of Fluoxetine on Hippocampal Neurogenesis and Neuroprotection in the Model of Global Cerebral Ischemia in Rats. International Journal of Molecular Sciences. 2018; 19(1):162. https://doi.org/10.3390/ijms19010162
Chicago/Turabian StyleKhodanovich, Marina, Alena Kisel, Marina Kudabaeva, Galina Chernysheva, Vera Smolyakova, Elena Krutenkova, Irina Wasserlauf, Mark Plotnikov, and Vasily Yarnykh. 2018. "Effects of Fluoxetine on Hippocampal Neurogenesis and Neuroprotection in the Model of Global Cerebral Ischemia in Rats" International Journal of Molecular Sciences 19, no. 1: 162. https://doi.org/10.3390/ijms19010162