Characterization of Different Types of Excitability in Large Somatosensory Neurons and Its Plastic Changes in Pathological Pain States
Abstract
:1. Introduction
2. Results
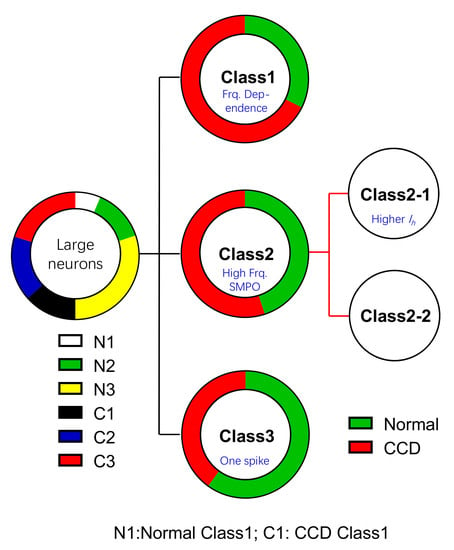
2.1. Functional Classification of Large DRG Neurons under Physiological States
2.2. Ionic Mechanisms Underlying Different Excitability Type of Large DRG Neurons
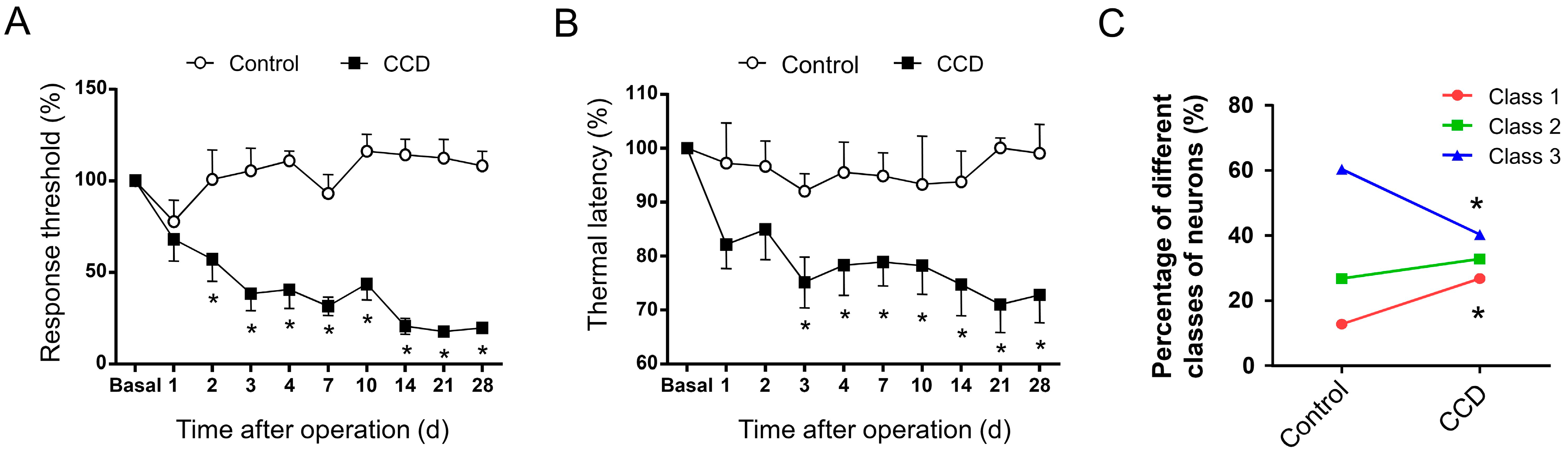
2.3. Development of Mechanical Hypersensitivity and Thermal Hyperalgesia in Rats Subjected to Chronic Compression of Lumbar DRG
2.4. Chronic Compression of Lumbar DRGs Induces Transition of Excitability Type in Large DRG Neurons
3. Discussion
4. Methods
4.1. Animals
4.2. Chronic Compression of Lumbar DRGs (CCD)
4.3. Behavioral Tests
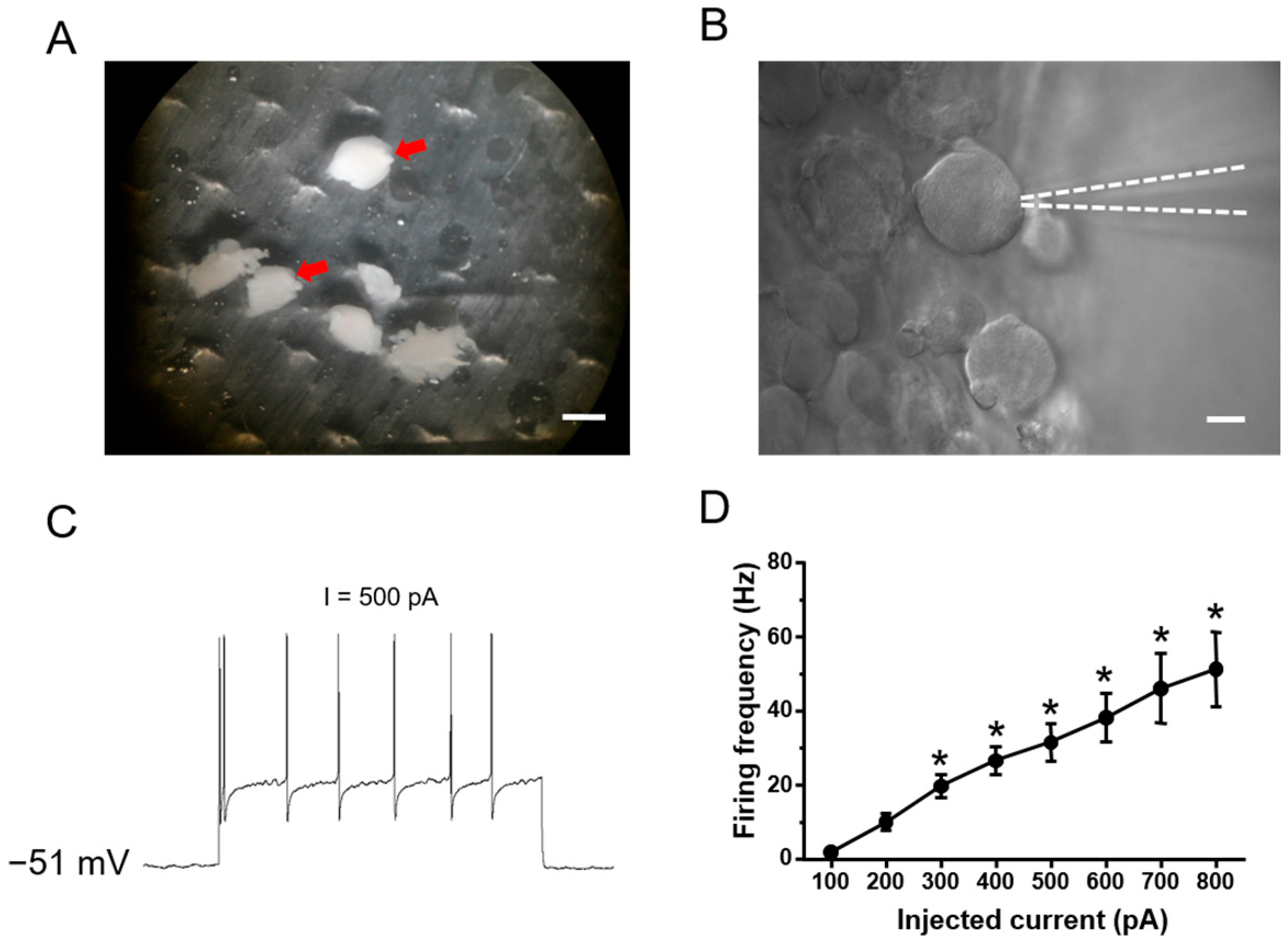
4.4. Intact Whole Mount DRG Preparations
4.5. Whole Cell Patch-Clamp Recording
4.6. Ih Current Analysis
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ih | Hyperpolarization-Activated Cation Currents |
| DRG | Dorsal Root Ganglion |
| CCD | Chronic Compression of Dorsal Root Ganglion |
| IB4 | Isolectin B4 |
| INa,P | Persistent Sodium Current |
References
- Maxwell, D.J.; Réthelyi, M. Ultrastructure and synaptic connections of cutaneous afferent fibres in the spinal cord. Trends Neurosci. 1987, 10, 117–123. [Google Scholar] [CrossRef]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.; Hokfelt, T.; Brodin, E.; Fahrenkrug, J.; Fischer, J.A.; Frey, P.; Elde, R.P.; Brown, J.C. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res. 1987, 247, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rivero-Melian, C.; Robertson, B.; Grant, G. Transganglionic transport and binding of the isolectin B4 from Griffonia simplicifolia I in rat primary sensory neurons. Neuroscience 1994, 62, 539–551. [Google Scholar] [CrossRef]
- Li, C.L.; Li, K.C.; Wu, D.; Chen, Y.; Luo, H.; Zhao, J.R.; Wang, S.S.; Sun, M.M.; Lu, Y.J.; Zhong, Y.Q.; et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 2016, 26, 967. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Ringkamp, M.; Campbell, J.; Raja, S. Peripheral mechanisms of cutaneous nociception. In Wall & Melzack’s Textbook of Pain; Elsevier: Amsterdam, The Netherlands, 2006; pp. 3–34. [Google Scholar]
- Li, C.; Wang, S.; Chen, Y.; Zhang, X. Somatosensory Neuron Typing with High-Coverage Single-Cell RNA Sequencing and Functional Analysis. Neurosci. Bull. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L. The local electric changes associated with repetitive action in a non-medullated axon. J. Physiol. 1948, 107, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M. Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting; MIT Press: Cambridge, MA, USA, 2007; Volume 25. [Google Scholar]
- Hsiao, C.F.; Gougar, K.; Asai, J.; Chandler, S.H. Intrinsic membrane properties and morphological characteristics of interneurons in the rat supratrigeminal region. J. Neurosci. Res. 2007, 85, 3673–3686. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.F.; Kaur, G.; Vong, A.; Bawa, H.; Chandler, S.H. Participation of Kv1 channels in control of membrane excitability and burst generation in mesencephalic V neurons. J. Neurophysiol. 2009, 101, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Tateno, T.; Harsch, A.; Robinson, H.P. Threshold firing frequency-current relationships of neurons in rat somatosensory cortex: Type 1 and type 2 dynamics. J. Neurophysiol. 2004, 92, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xing, J.L.; Wu, N.P.; Liu, Y.H.; Zhang, C.Z.; Kuang, F.; Han, V.Z.; Hu, S.J. Membrane current-based mechanisms for excitability transitions in neurons of the rat mesencephalic trigeminal nuclei. Neuroscience 2009, 163, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Xing, J.L. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain 1998, 77, 15–23. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.M.; Xie, R.G.; Yue, Z.F.; Song, X.J.; Hu, S.J.; Xing, J.L. Evoked bursting in injured Abeta dorsal root ganglion neurons: A mechanism underlying tactile allodynia. Pain 2012, 153, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Na, H.S.; Kim, S.H.; Han, H.C.; Yoon, Y.W.; Sung, B.; Nam, H.J.; Shin, S.L.; Hong, S.K. Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience 1998, 86, 301–309. [Google Scholar] [CrossRef]
- Liu, C.N.; Wall, P.D.; Ben-Dor, E.; Michaelis, M.; Amir, R.; Devor, M. Tactile allodynia in the absence of C-fiber activation: Altered firing properties of DRG neurons following spinal nerve injury. Pain 2000, 85, 503–521. [Google Scholar] [CrossRef]
- Zhang, J.M.; Song, X.J.; LaMotte, R.H. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J. Neurophysiol. 1999, 82, 3359–3366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Henry, J.L. Excitability of Abeta sensory neurons is altered in an animal model of peripheral neuropathy. BMC Neurosci. 2012, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.X.; Zhuang, Z.Y.; Woolf, C.J.; Ji, R.R. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [PubMed]
- Liu, C.N.; Michaelis, M.; Amir, R.; Devor, M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: Relation to neuropathic pain. J. Neurophysiol. 2000, 84, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.S.; Huang, Q.H.; Zhang, F.X.; Bao, L.; Lu, Y.J.; Guo, C.; Yang, L.; Huang, W.J.; Fu, G.; Xu, S.H.; et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl. Acad. Sci. USA 2002, 99, 8360–8365. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Fjell, J.; Cummins, T.R.; Zheng, Z.; Fried, K.; LaMotte, R.; Black, J.A.; Waxman, S.G. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain 1999, 83, 591–600. [Google Scholar] [CrossRef]
- Zheng, J.H.; Walters, E.T.; Song, X.J. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J. Neurophysiol. 2007, 97, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M. Neural excitability, spiking and bursting. Int. J. Bifurc. Chaos 2000, 10, 1171–1266. [Google Scholar] [CrossRef]
- Rinzel, J.; Ermentrout, G.B. Analysis of neural excitability and oscillations. In Methods in Neuronal Modeling; Christof, K., Idan, S., Eds.; MIT Press: Cambridge, MA, USA, 1989; pp. 135–169. [Google Scholar]
- Chaplan, S.R.; Guo, H.Q.; Lee, D.H.; Luo, L.; Liu, C.; Kuei, C.; Velumian, A.A.; Butler, M.P.; Brown, S.M.; Dubin, A.E. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J. Neurosci. 2003, 23, 1169–1178. [Google Scholar] [PubMed]
- Luo, L.; Chang, L.; Brown, S.M.; Ao, H.; Lee, D.H.; Higuera, E.S.; Dubin, A.E.; Chaplan, S.R. Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience 2007, 144, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Young, G.T.; Berrocoso, E.M.; Chen, L.; McNaughton, P.A. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 2011, 333, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Biel, M.; Wahl-Schott, C.; Michalakis, S.; Zong, X. Hyperpolarization-activated cation channels: From genes to function. Physiol. Rev. 2009, 89, 847–885. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Smith, T.; Sathish, J.; Djouhri, L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current Ih in C- but not Adelta-nociceptors. Pain 2012, 153, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-L.; Wang, X.; Chu, W.-G.; Lu, N.; Han, W.-J.; Du, Y.-K.; Hu, S.-J.; Bai, Z.-T.; Wu, S.-X.; Xie, R.-G.; et al. Chronic cervical radiculopathic pain is associated with increased excitability and hyperpolarization-activated current (Ih) in large-diameter dorsal root ganglion neurons. Mol. Pain 2017, 13, 1744806917707127. [Google Scholar] [CrossRef] [PubMed]
- Scroggs, R.S.; Todorovic, S.M.; Anderson, E.G.; Fox, A.P. Variation in IH, IIR, and ILEAK between acutely isolated adult rat dorsal root ganglion neurons of different size. J. Neurophysiol. 1994, 71, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.C.; Mishra, S.K.; Maric, D.; Kaszas, K.; Gonnella, G.L.; Clokie, S.J.; Kominsky, H.D.; Gross, J.R.; Keller, J.M.; Mannes, A.J.; et al. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J. Pain 2014, 15, 1338–1359. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Crow, M.; Richards, N.; Davey, G.I.; Levine, E.; Kelleher, J.H.; Agley, C.C.; Denk, F.; Harridge, S.D.; McMahon, S.B. Defining the nociceptor transcriptome. Front. Mol. Neurosci. 2014, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Barrett, L.B.; Williams, E.K.; Strochlic, D.E.; Lee, S.; Weyer, A.D.; Lou, S.; Bryman, G.S.; Roberson, D.P.; Ghasemlou, N.; et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lonnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.H.; Wang, W.T.; Chen, J.Y.; Xie, R.G.; Hu, S.J. Gabapentin selectively reduces persistent sodium current in injured type-A dorsal root ganglion neurons. Pain 2009, 143, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Lu, N.; Han, W.J.; Chen, R.G.; Cong, R.; Xie, R.G.; Zhang, Y.F.; Kong, W.W.; Hu, S.J.; Luo, C. Upregulation of Ih expressed in IB4-negative Adelta nociceptive DRG neurons contributes to mechanical hypersensitivity associated with cervical radiculopathic pain. Sci. Rep. 2015, 5, 16713. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.H.; Xing, J.L.; Duan, J.H.; Hu, S.J. Effects of gabapentin on spontaneous discharges and subthreshold membrane potential oscillation of type A neurons in injured DRG. Pain 2005, 116, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ingram, S.L.; Williams, J.T. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J. Physiol. 1996, 492 Pt 1, 97–106. [Google Scholar] [CrossRef]
- Xie, R.G.; Zheng, D.W.; Xing, J.L.; Zhang, X.J.; Song, Y.; Xie, Y.B.; Kuang, F.; Dong, H.; You, S.W.; Xu, H.; et al. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS ONE 2011, 6, e18681. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Miao, B.; Wang, X.C.; Duan, J.H.; Wang, W.T.; Kuang, F.; Xie, R.G.; Xing, J.L.; Xu, H.; Song, X.J.; et al. Reduced conduction failure of the main axon of polymodal nociceptive C-fibres contributes to painful diabetic neuropathy in rats. Brain 2012, 135, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.G.; Gao, Y.J.; Park, C.K.; Lu, N.; Luo, C.; Wang, W.T.; Wu, S.X.; Ji, R.R. Spinal CCL2 Promotes Central Sensitization, Long-Term Potentiation, and Inflammatory Pain via CCR2: Further Insights into Molecular, Synaptic, and Cellular Mechanisms. Neurosci. Bull. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.R.; Oertel, D. Hyperpolarization-activated currents regulate excitability in stellate cells of the mammalian ventral cochlear nucleus. J. Neurophysiol. 2006, 95, 76–87. [Google Scholar] [CrossRef] [PubMed]



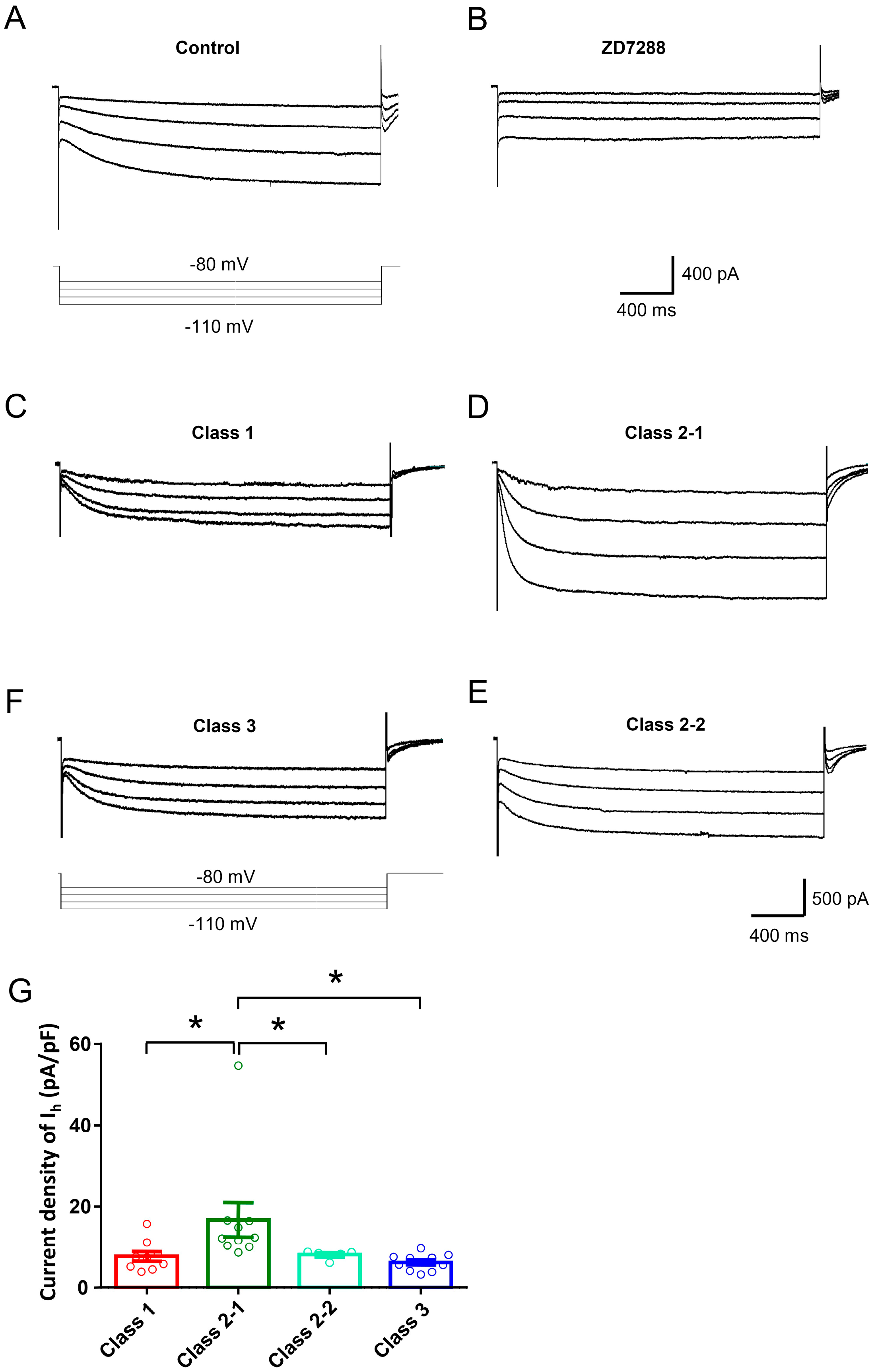
| Type | RMP (mV) | Cm (GOhms) | Rm (MOhms) |
|---|---|---|---|
| Class 1 | −55.38 ± 2.05 | 102.67 ± 15.54 | 0.088 ± 0.02 |
| Class 2-1 | −57.64 ± 2.25 | 97.23 ± 10.55 | 0.099 ± 0.02 |
| Class 2-2 | −53.98 ± 1.13 | 79.69 ± 2.48 | 0.071 ± 0.02 |
| Class 3 | −59.61 ± 1.11 | 92.36 ± 7.41 | 0.079 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, R.-G.; Chu, W.-G.; Hu, S.-J.; Luo, C. Characterization of Different Types of Excitability in Large Somatosensory Neurons and Its Plastic Changes in Pathological Pain States. Int. J. Mol. Sci. 2018, 19, 161. https://doi.org/10.3390/ijms19010161
Xie R-G, Chu W-G, Hu S-J, Luo C. Characterization of Different Types of Excitability in Large Somatosensory Neurons and Its Plastic Changes in Pathological Pain States. International Journal of Molecular Sciences. 2018; 19(1):161. https://doi.org/10.3390/ijms19010161
Chicago/Turabian StyleXie, Rou-Gang, Wen-Guang Chu, San-Jue Hu, and Ceng Luo. 2018. "Characterization of Different Types of Excitability in Large Somatosensory Neurons and Its Plastic Changes in Pathological Pain States" International Journal of Molecular Sciences 19, no. 1: 161. https://doi.org/10.3390/ijms19010161